Evaluation of a Commercial Device Based on Reflection Spectroscopy as an Alternative to Resonance Raman Spectroscopy in Measuring Skin Carotenoid Levels: Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet Intervention Study Design
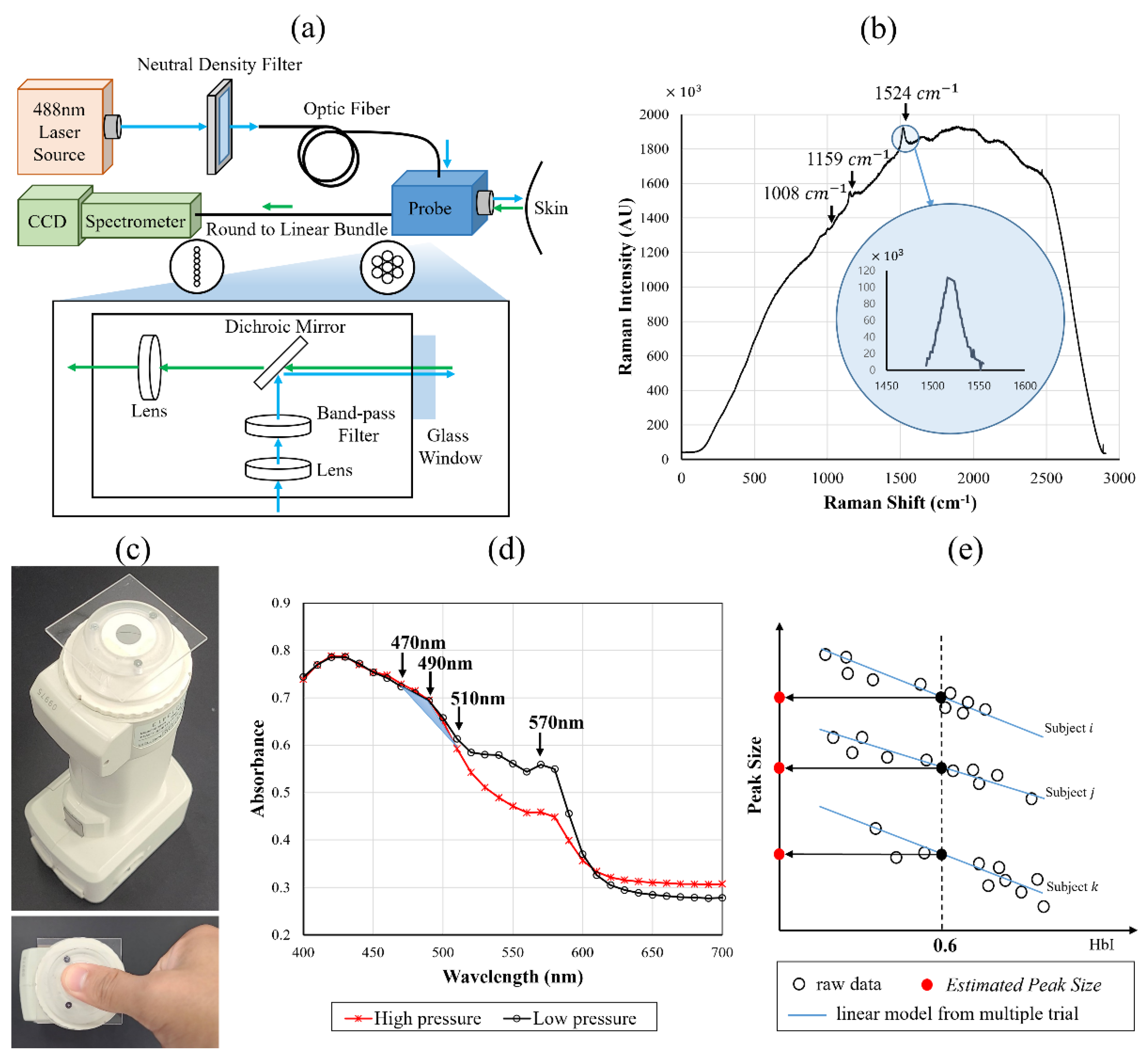
2.2. RRS-Based Measurement
2.3. RS-Based Measurement
2.4. Statistical Analysis and Evaluation
2.5. Visualization Using a Difference Vector
2.6. Hit Rate
2.7. Subgroup Classification
2.8. Sample Size
3. Results
3.1. Participants’ Characteristics
3.2. Correlation between RRS and RS
3.3. Change Rate between RRS and RS
3.4. Vector Field between RRS and RS
3.5. Trend of Angles between RRS and RS
3.6. Hit Rates of RRS and RS
4. Discussion
4.1. Principal Findings
4.2. The Strengths of This Study
4.3. Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Ermakov, I.V.; Gellermann, W. Optical detection methods for carotenoids in human skin. Arch. Biochem. Biophys. 2015, 572, 101–111. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Matsumoto, M. Skin carotenoid level as an alternative marker of serum total carotenoid concentration and vegetable intake correlates with biomarkers of circulatory diseases and metabolic syndrome. Nutrients 2020, 12, 1825. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—A review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.R.; McClane, R.W.; Gellermann, W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt. Lett. 2001, 26, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Lin, S.; Quaroni, L.; White, W.S.; Cotton, T.; Chumanov, G. Localization of carotenoids in plasma low-density lipoproteins studied by surface-enhanced resonance Raman Spectroscopy. Biopolymers 2000, 57, 249–256. [Google Scholar] [CrossRef]
- Hata, T.R.; Scholz, T.A.; Ermakov, I.V.; McClane, R.W.; Khachik, F.; Gellermann, W.; Pershing, L.K. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J. Investig. Dermatol. 2000, 115, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.M.; Peng, Y.S.; Lin, Y.; Moon, T.; Roe, D.J.; Ritenbaugh, C. Concentrations and plasma-tissue-diet relationships of carotenoids, retinoids, and tocopherols in humans. Nutr. Cancer 1995, 23, 233–246. [Google Scholar] [CrossRef]
- Sholola, M.J.; Cooperstone, J.L. Carotenoid extraction and analysis from blood plasma/serum. Methods Enzymol. 2022, 670, 423–457. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.D.; Pitts, S.J.; Jahns, L.; Firnhaber, G.C.; Loofbourrow, B.M.; Zeng, A.; Scherr, R.E. Criterion-Related Validity of Spectroscopy-Based Skin Carotenoid Measurements as a Proxy for Fruit and Vegetable Intake: A Systematic Review. Adv. Nutr. 2020, 11, 1282–1299. [Google Scholar] [CrossRef]
- Henning, T.; Wagner, P.; Gedat, E.; Kochlik, B.; Kusch, P.; Sowoidnich, K.; Vastag, M.; Gleim, J.; Braune, M.; Maiwald, M.; et al. Evaluation of Modern Approaches for the Assessment of Dietary Carotenoids as Markers for Fruit and Vegetable Consumption. Nutrients 2023, 15, 1665. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Gellermann, W. Validation model for Raman based skin carotenoid detection. Arch. Biochem. Biophys. 2010, 504, 40–49. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Henebery, K.; Peracchio, H.; Cartmel, B.; Lin, H.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Duffy, V.B.; Mayne, S.T. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur. J. Clin. Nutr. 2012, 66, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.S.; Wengreen, H.J.; Lefevre, M.; Madden, G.J.; Gast, J. Skin carotenoids: A biomarker of fruit and vegetable intake in children. J. Acad. Nutr. Diet. 2014, 114, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, I.V.; Ermakova, M.; Sharifzadeh, M.; Gorusupudi, A.; Farnsworth, K.; Bernstein, P.S.; Stookey, J.; Evans, J.; Arana, T.; Tao-Lew, L.; et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018, 646, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. One-year study on the variation of carotenoid antioxidant substances in living human skin; influence of dietary supplementation and stress factors. J. Biomed. Opt. 2008, 13, 044028. [Google Scholar] [CrossRef]
- Buckley, W.R.; Grum, F. Absorption characteristics and color of human skin. Arch. Dermatol. 1964, 89, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Jungmann, H.; von Laar, J.; Schietzel, M.; Sies, H.; Tronnier, H. Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlate with serum levels in women ingesting betatene. J. Nutr. 1998, 128, 903–907. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Gellermann, W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics 2012, 5, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, I.V.; Whigham, L.D.; Redelfs, A.H.; Jahns, L.; Stookey, J.; Bernstein, P.S.; Gellermann, W. Skin carotenoids as biomarker for vegetable and fruit intake: Validation of the reflection-spectroscopy based “Veggie Meter”. FASEB J. 2016, 30, 409. [Google Scholar] [CrossRef]
- Jilcott Pitts, S.B.; Jahns, L.; Wu, Q.; Moran, N.E.; Bell, R.A.; Truesdale, K.P.; Laska, M.N. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 2018, 21, 1664–1670. [Google Scholar] [CrossRef]
- Jilcott Pitts, S.B.; Moran, N.E.; Wu, Q.; Harnack, L.; Craft, N.E.; Hanchard, N.; Bell, R.; Moe, S.G.; Johnson, N.; Obasohan, J.; et al. Pressure-mediated reflection spectroscopy criterion validity as a biomarker of fruit and vegetable intake: A 2-site cross-sectional study of 4 racial or ethnic groups. J. Nutr. 2022, 152, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sandhagen, C.; Koecher, W.; Sterry, W.; Lademann, J.; Meinke, M.C. Comparison of two methods for noninvasive determination on carotenoids in human and animal skin: Raman spectroscopy versus reflection spectroscopy. J. Biophotonics 2012, 5, 550–558. [Google Scholar] [CrossRef]
- Darvin, M.E.; Magnussen, B.; Lademann, J.; Kocher, W. Multiple spatially resolved reflection spectroscopy for in vivo determination of carotenoids in human skin and blood. Laser Phys. Lett. 2016, 13, 095601. [Google Scholar] [CrossRef]
- Meinke, M.C.; Schanzer, S.; Lohan, S.B.; Shchatsinin, I.; Darvin, M.E.; Vollert, H.; Magnussen, B.; Köcher, W.; Helfmann, J.; Lademann, J. Comparison of different cutaneous carotenoid sensors and influence of age, skin type, and kinetic changes subsequent to intake of a vegetable extract. J. Biomed. Opt. 2016, 21, 107002. [Google Scholar] [CrossRef]
- Andree, S.; Reble, C.; Helfmann, J.; Gersonde, I.; Illing, G. Evaluation of a novel noncontact spectrally and spatially resolved reflectance setup with continuously variable source-detector separation using silicone phantoms. J. Biomed. Opt. 2010, 15, 067009. [Google Scholar] [CrossRef]
- Lisenko, S.A.; Kugeiko, M.M. Noninvasive diagnostics of skin microphysical parameters based on spatially resolved diffuse reflectance spectroscopy. J. Appl. Spectrosc. 2013, 79, 934–943. [Google Scholar] [CrossRef]
- Doornbos, R.M.; Lang, R.; Aalders, M.C.; Cross, F.W.; Sterenborg, H.J. The determination of in vivo human tissue optical properties and absolute chromophore concentrations using spatially resolved steady-state diffuse reflectance spectroscopy. Phys. Med. Biol. 1999, 44, 967–981. [Google Scholar] [CrossRef]
- Kienle, A.; Lilge, L.; Patterson, M.S.; Hibst, R.; Steiner, R.; Wilson, B.C. Spatially resolved absolute diffuse reflectance measurements for noninvasive determination of the optical scattering and absorption coefficients of biological tissue. Appl. Opt. 1996, 35, 2304–2314. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.I.; Oh, T.H. Statistical Test of Agreement between Measurements in Method-comparison Study. J. Vet. Clin. 2011, 28, 108–112. [Google Scholar]
- Ministry of Health and Welfare; The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2020; Ministry of Health and Welfare: Sejong, Republic of Korea, 2020. [Google Scholar]
- Ahn, S.; Ahn, S.; Jang, H.; Eom, K.; Kim, Y.J.; Hwang, J.E.; Chung, J.I.; Park, J.Y.; Nam, S.; Choi, Y.H.; et al. Validation of resonance Raman spectroscopy-measured skin carotenoid status as a biomarker for fruit and vegetable intake in Korean adults. Br. J. Nutr. 2023, 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Yamashita, T.; Hirao, T.; Takahashi, M. An innovative method to measure skin pigmentation. Skin Res. Technol. 2009, 15, 224–229. [Google Scholar] [CrossRef]
- Yun, I.S.; Lee, W.J.; Rah, D.K.; Kim, Y.O.; Park, B.Y. Skin color analysis using a spectrophotometer in Asians. Skin Res. Technol. 2010, 16, 311–315. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power. Version 3.1.9.7. Available online: https://www.gpower.hhu.de (accessed on 1 September 2020).
- Jahns, L.; Johnson, L.K.; Conrad, Z.; Bukowski, M.; Raatz, S.K.; Jilcott Pitts, S.; Wang, Y.; Ermakov, I.V.; Gellermann, W. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: A cohort study. Nutr. J. 2019, 18, 78. [Google Scholar] [CrossRef]
- Coyle, D.H.; Pezdirc, K.; Hutchesson, M.J.; Collins, C.E. Intake of specific types of fruit and vegetables is associated with higher levels of skin yellowness in young women: A cross-sectional study. Nutr. Res. 2018, 56, 23–31. [Google Scholar] [CrossRef]
- Ashton, L.M.; Pezdirc, K.B.; Hutchesson, M.J.; Rollo, M.E.; Collins, C.E. Is skin coloration measured by reflectance spectroscopy related to intake of nutrient-dense foods? A cross-sectional evaluation in Australian young adults. Nutrients 2018, 10, 11. [Google Scholar] [CrossRef]
- Madore, M.P.; Hwang, J.E.; Park, J.Y.; Ahn, S.; Joung, H.; Chun, O.K. A Narrative Review of Factors Associated with Skin Carotenoid Levels. Nutrients 2023, 15, 2156. [Google Scholar] [CrossRef]
- Pleuvry, J.P. Hysteresis in drug response. Anaesth. Intensive Care Med. 2008, 9, 372–373. [Google Scholar] [CrossRef]
- Jahns, L.; Johnson, L.K.; Mayne, S.T.; Cartmel, B.; Picklo, M.J.; Ermakov, I.V., Sr.; Gellermann, W.; Whigham, L.D. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: Uptake and depletion kinetics. Am. J. Clin. Nutr. 2014, 100, 930–937. [Google Scholar] [CrossRef]
- Marin, R.P.; Joan, K.F. Beta-carotene accumulation in serum and skin. Am. J. Clin. Nutr. 1993, 57, 175–181. [Google Scholar]
- Maretti, E.; Leo, E.; Rustichelli, C.; Truzzi, E.; Siligardi, C.; Iannuccelli, V. In vivo β-carotene skin permeation modulated by Nanostructured Lipid Carriers. Int. J. Pharm. 2021, 597, 120322. [Google Scholar] [CrossRef]
- Mehta, M.; Naffa, R.; Zhang, W.; Schreurs, N.M.; Martin, N.P.; Hickson, R.E.; Waterland, M.; Holmes, G. Raman spectroscopic detection of carotenoids in cattle skin. RSC Adv. 2020, 10, 22758–22765. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Caspers, P.; van der Pool, A.; Richter, H.; Patzelt, A.; Sterry, W.; Lademann, J. In vivo distribution of carotenoids in different anatomical locations of human skin: Comparative assessment with two different Raman spectroscopy methods. Exp. Dermatol. 2009, 18, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Roemmich, J.N.; Larson, K.J.; Hess, J.M.; Palmer, D.G.; Jahns, L. Sensitivity of Pressure-Mediated Reflection Spectroscopy to Detect Changes in Skin Carotenoids in Adults Without Obesity in Response to Increased Carotenoid Intake: A Randomized Controlled Trial. J. Nutr. 2023, 153, 588–597. [Google Scholar] [CrossRef] [PubMed]






| Comparison Factors | Previous Study | Additional Analysis in This Study |
|---|---|---|
| Cross-sectional value | Correlation coefficient | No additional analysis |
| Response to intervention | Change in average | Angle of difference vector |
| Visualization | Scatter plot (Cross-sectional) | Hit rate |
| Total | HG | CG | p Value b | ||
|---|---|---|---|---|---|
| (n = 80) | (n = 40) | (n = 40) | |||
| Skin carotenoid level (AU) by | |||||
| Resonance Raman spectroscopy (RRS) | 129.0 ± 47.3 | 127.9 ± 44.6 | 130.2 ± 50.4 | 0.83 | |
| Reflection spectroscopy (RS) c | 134.8 ± 45.4 | 133.6 ± 42.6 | 136.0 ± 48.5 | 0.82 | |
| Demographic and physical characteristics | |||||
| Age (years) | 32.0 ± 7.4 | 32.0 ± 7.4 | 32.1 ± 7.5 | 0.93 | |
| Male/Female (n) | 42/38 | 21/19 | 21/19 | >0.99 | |
| BMI (kg/m2) | 27.1 ± 3.2 | 27.0 ± 3.3 | 27.1 ± 3.3 | 0.85 | |
| Waist circumference (cm) | 91.2 ± 8.5 | 90.6 ± 8.5 | 91.8 ± 8.6 | 0.55 | |
| All Participants | HG | CG | |
|---|---|---|---|
| (n = 67) | (n = 35) | (n = 32) | |
| Week 1 b (n = 80) | 0.944 (0.914–0.964) | 0.942 (0.892–0.969) | 0.946 (0.899–0.971) |
| Week 1 | 0.933 (0.893–0.958) | 0.931 (0.866–0.965) | 0.936 (0.871–0.968) |
| Week 2 | 0.935 (0.895–0.959) | 0.926 (0.858–0.962) | 0.942 (0.883–0.972) |
| Week 3 | 0.933 (0.893–0.958) | 0.918 (0.842–0.958) | 0.953 (0.905–0.977) |
| Week 4 | 0.931 (0.89–0.957) | 0.914 (0.834–0.956) | 0.941 (0.882–0.971) |
| Week 5 | 0.932 (0.891–0.958) | 0.918 (0.842–0.958) | 0.928 (0.857–0.965) |
| Week 6 | 0.921 (0.874–0.951) | 0.857 (0.733–0.926) | 0.949 (0.897–0.975) |
| Week 7 | 0.885 (0.819–0.928) | 0.798 (0.633–0.894) | 0.898 (0.801–0.95) |
| Week 8 | 0.926 (0.882–0.954) | 0.868 (0.752–0.932) | 0.939 (0.878–0.97) |
| Week 9 | 0.927 (0.884–0.955) | 0.889 (0.79–0.943) | 0.934 (0.868–0.968) |
| Week 10 | 0.925 (0.88–0.953) | 0.867 (0.75–0.931) | 0.944 (0.888–0.973) |
| Week 11 | 0.916 (0.867–0.948) | 0.888 (0.788–0.942) | 0.939 (0.877–0.97) |
| All periods (Week 1~11) | 0.930 (0.919–0.939) | 0.919 (0.901–0.933) | 0.934 (0.919–0.946) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-E.; Park, J.-Y.; Jung, M.H.; Eom, K.; Moon, H.S.; Joung, H.; Kim, Y.J. Evaluation of a Commercial Device Based on Reflection Spectroscopy as an Alternative to Resonance Raman Spectroscopy in Measuring Skin Carotenoid Levels: Randomized Controlled Trial. Sensors 2023, 23, 7654. https://doi.org/10.3390/s23177654
Hwang J-E, Park J-Y, Jung MH, Eom K, Moon HS, Joung H, Kim YJ. Evaluation of a Commercial Device Based on Reflection Spectroscopy as an Alternative to Resonance Raman Spectroscopy in Measuring Skin Carotenoid Levels: Randomized Controlled Trial. Sensors. 2023; 23(17):7654. https://doi.org/10.3390/s23177654
Chicago/Turabian StyleHwang, Jeong-Eun, Jin-Young Park, Myoung Hoon Jung, Kunsun Eom, Hyun Seok Moon, Hyojee Joung, and Yoon Jae Kim. 2023. "Evaluation of a Commercial Device Based on Reflection Spectroscopy as an Alternative to Resonance Raman Spectroscopy in Measuring Skin Carotenoid Levels: Randomized Controlled Trial" Sensors 23, no. 17: 7654. https://doi.org/10.3390/s23177654
APA StyleHwang, J.-E., Park, J.-Y., Jung, M. H., Eom, K., Moon, H. S., Joung, H., & Kim, Y. J. (2023). Evaluation of a Commercial Device Based on Reflection Spectroscopy as an Alternative to Resonance Raman Spectroscopy in Measuring Skin Carotenoid Levels: Randomized Controlled Trial. Sensors, 23(17), 7654. https://doi.org/10.3390/s23177654






