Simultaneous Assay of CA 72-4, CA 19-9, CEA and CA 125 in Biological Samples Using Needle Three-Dimensional Stochastic Microsensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Boron- and Nitrogen-Decorated Graphenes
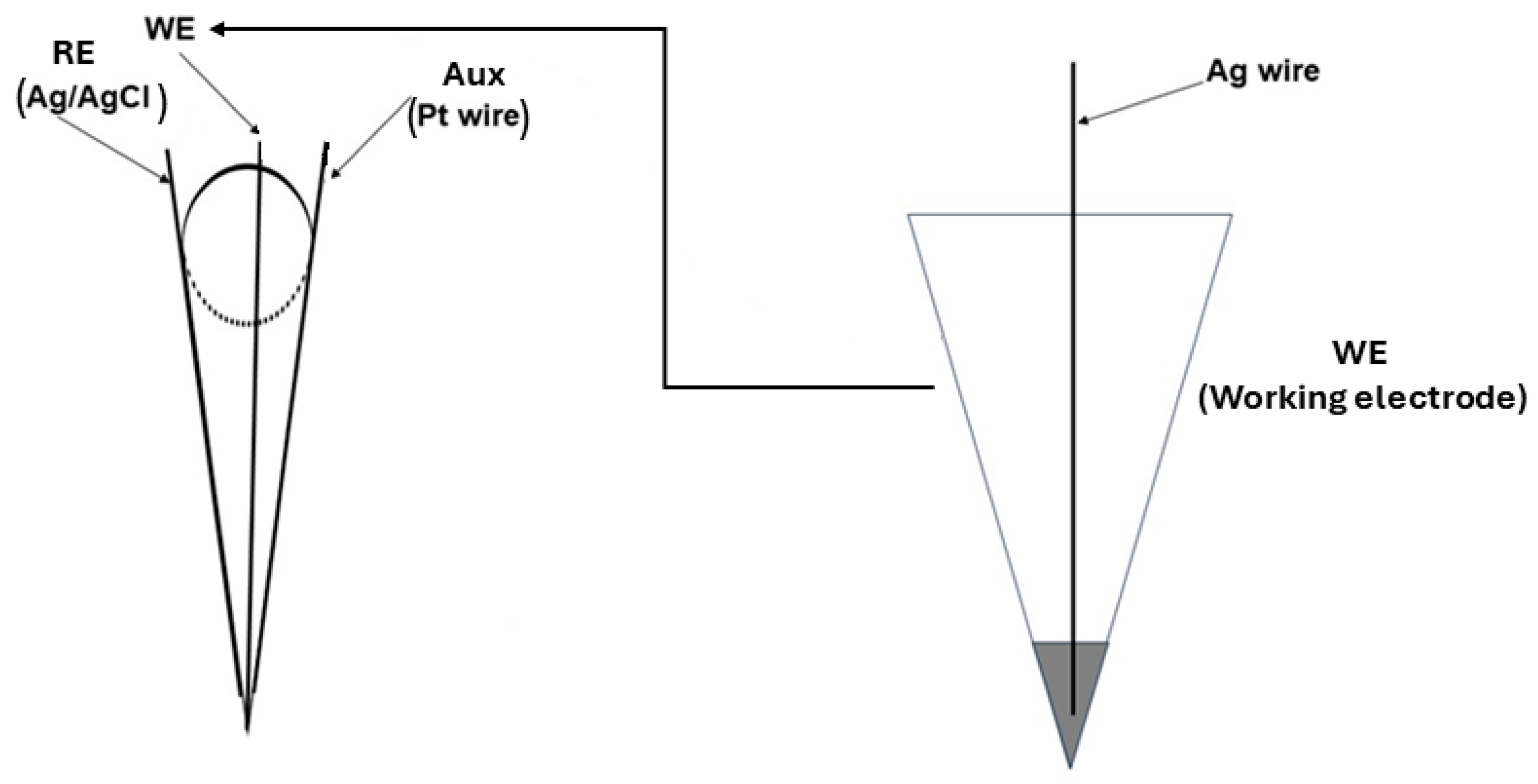
2.3. Design of the Stochastic Microsensors
2.4. Apparatus
2.5. Stochastic Method
2.6. Samples
3. Results and Discussion
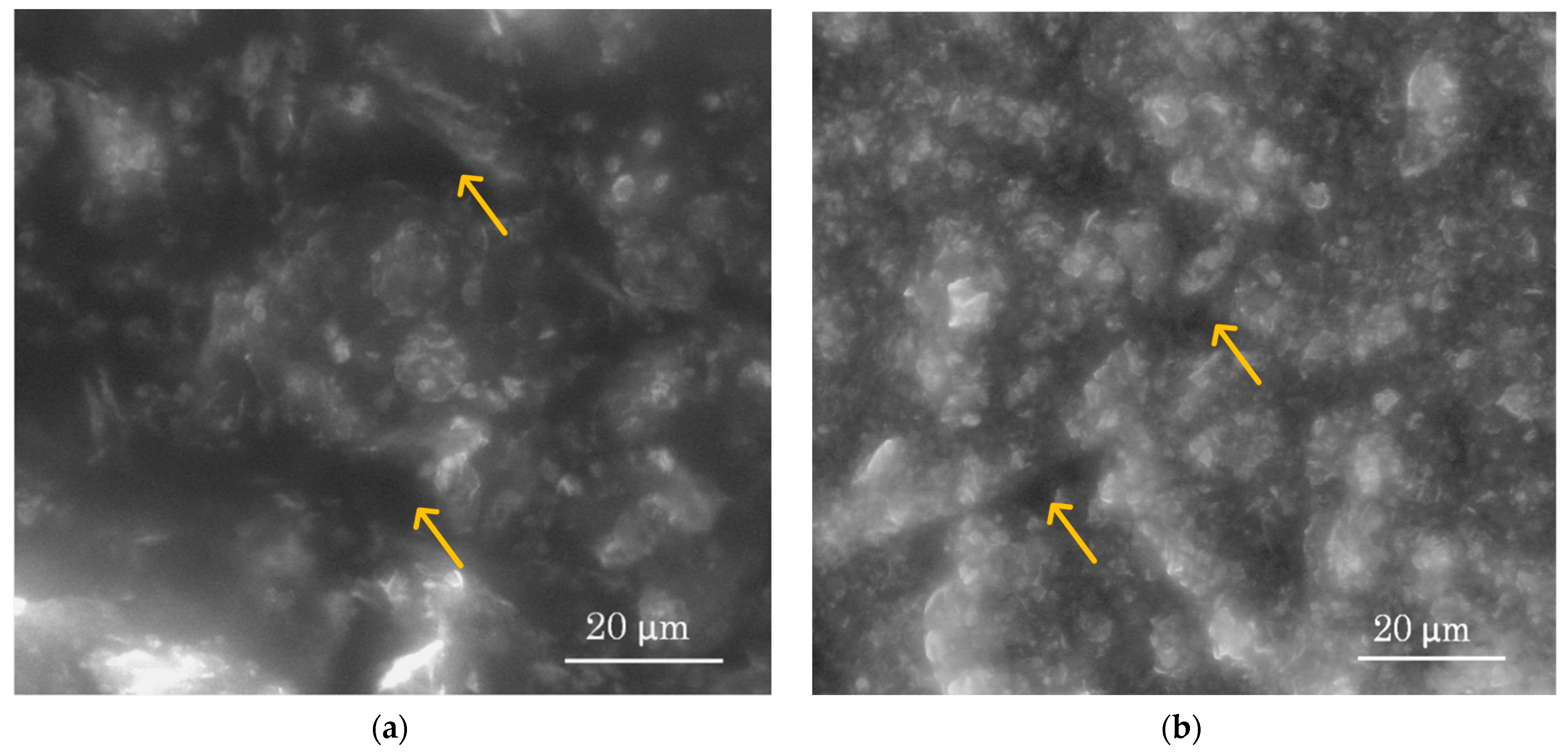
3.1. Morphology of the Active Surface of the Needle 3D Stochastic Microsensors
3.2. Response Characteristics of the Two Stochastic Microsensors Used for the Assay of the Four Biomarkers (CA 19-9, CA 72-4, CA125 and CEA)
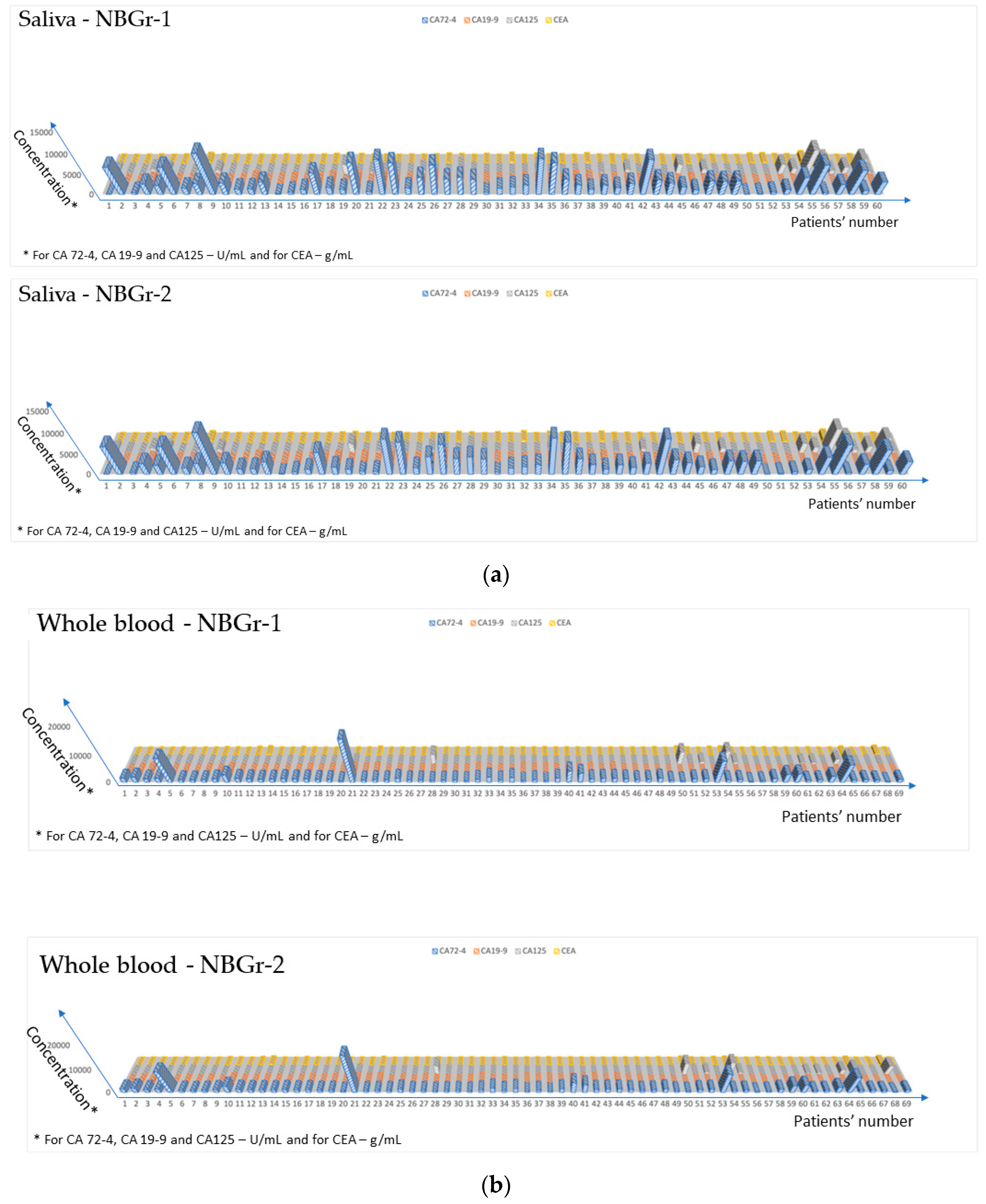
3.3. Ultrasensitive Determination of the Four Biomarkers in All Four Biological Fluids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Ding, Y.; Bai, Y.; Zhou, Q.; Lee, H.; Li, X.; Teng, L. Identification of serum circulating MicroRNAs as novel diagnostic biomarkers of gastric cancer. Front. Genet. 2021, 11, 591515. [Google Scholar] [CrossRef]
- Tsujiura, M.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Otsuji, E. Liquid biopsy of gastric cancer patients: Circulating tumor cells and cell-free nucleic acids. World J. Gastroenterol. 2014, 20, 3265–3286. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Zheng, T.H.; Zhao, J.L.; Guleng, B. Advances in molecular biomarkers for gastric cancer. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 299–305. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Q.; Chen, K.; Liu, H.P.; Chen, X. Prognostic value of combination of inflammatory and tumor markers in respectable gastric cancer. J. Gastrointest. Surg. 2021, 25, 2470–2483. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Xu, L.; Zhang, P.J.; Han, L. Evaluation of the value of multiparameter combined analysis of serum markers in the early diagnosis of gastric cancer. World J. Gastrointest. Oncol. 2020, 12, 483–491. [Google Scholar] [CrossRef]
- Colcher, D.; Hand, P.H.; Nuti, M.; Schlom, J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3199–3203. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Clausen, H.; Hirohashi, S.; Ogawa, T.; Iijima, H.; Hakomori, S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2-6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988, 48, 2214–2220. [Google Scholar]
- Cho, J.; Kim, K.M.; Kim, H.C.; Lee, W.Y.; Kang, W.K.; Park, Y.S.; Ha, S.Y. The prognostic role of tumor associated glycoprotein 72 (TAG-72) in stage II and III colorectal adenocarcinoma. Pathol. Res. Pract. 2019, 215, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, F.; Roselli, M.; Amato, T.; Cosimelli, M.; Perri, P.; Casale, V.; Carlini, M.; Santoro, E.; Cavaliere, R.; Greiner, J.W. CA 72-4 measurement of tumor-associated glycoprotein 72 (TAG-72) as a serum marker in the management of gastric carcinoma. Cancer Res. 1992, 52, 1222–1227. [Google Scholar] [PubMed]
- Chen, X.Z.; Zhang, W.K.; Yang, K.; Wang, L.L.; Liu, J.; Wang, L.; Hu, J.K.; Zhang, B.; Chen, Z.X.; Chen, J.P.; et al. Correlation between serum CA724 and gastric cancer: Multiple analyses based on Chinese population. Mol. Biol. Rep. 2012, 39, 9031–9039. [Google Scholar] [CrossRef]
- Wada, N.; Kurokawa, Y.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer cases. Surg. Today 2017, 47, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Natsugoe, S.; Hokita, S.; Che, X.; Tokuda, K.; Nakajo, A.; Iwashige, H.; Tokushige, M.; Watanabe, T.; Takao, S.; et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J. Clin. Gastroenterol. 2001, 32, 41–44. [Google Scholar] [CrossRef]
- Song, Y.; Huang, X.; Gao, P.; Sun, J.; Chen, X.W.; Yang, Y.C.; Zhang, C.; Liu, H.P.; Wang, H.C.; Wang, Z.N. Clinicopathologic and prognostic value of serum carbohydrate antigen 19-9 in gastric cancer: A meta-analysis. Dis. Markers 2015, 2015, 549843. [Google Scholar] [CrossRef]
- Numabe, K. Serum and immunohistological examination of CA19-9 positive gastric cancer (in Japanese with English abstract). Showa Univ. J. Med. Sci. 1993, 53, 146–155. [Google Scholar]
- Reiter, M.; Costello, J.; Schwope, R.B.; Lisanti, C.J.; Osswald, M.B. Review of commonly used serum tumor markers and their relevance for image interpretation. J. Comput. Assist. Tomogr. 2015, 39, 825–834. [Google Scholar] [CrossRef]
- Kilpatrick, E.; Lind, M. Appropriate requesting of serum tumour markers. BMJ 2009, 339, b3111. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Sisik, A.; Kaya, M.; Bas, G.; Basak, F.; Alimoglu, O. CEA and CA19-9 are Still Valuable Markers for the Prognosis of Colorectal and Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 2013, 14, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.; Freedman, S.O. Specific Carcinoembryonic Antigens of the Human Digestive System. J. Exp. Med. 1965, 122, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, T.; Nakafusa, Y.; Sumi, K.; Miyoshi, A.; Kitajima, Y.; Sato, S.; Noshiro, H.; Miyazaki, K. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann. Surg. Oncol. 2010, 17, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Van der Schouw, Y.T.; Verbeek, A.L.M.; Wobbes, T.; Segers, M.F.G.; Thomas, C.M.G. Comparison of four serum tumour markers in the diagnosis of colorectal carcinoma. Br. J. Cancer 1992, 66, 148–154. [Google Scholar] [CrossRef]
- George, P.K.; Loewenstein, M.S.; O’Brien, M.J.; Bronstein, B.; Koff, R.S.; Zamcheck, N. Circulating CEA Levels in Patients with Fulminant Hepatitis. Dig. Dis. Sci. 1982, 27, 139–142. [Google Scholar] [CrossRef]
- Tan, E.; Gouvas, N.; Nicholls, R.J.; Ziprin, P.; Xynos, E.; Tekkis, P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009, 18, 15–24. [Google Scholar] [CrossRef]
- Lin, Z.; Bian, H.; Chen, C.; Chen, W.; Li, Q. Application of serum pepsinogen and carbohydrate antigen 72-4 (CA72-4) combined with gastrin-17 (G-17) detection in the screening, diagnosis, and evaluation of early gastric cancer. J. Gastrointest. Oncol. 2021, 12, 1042–1048. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, X.; Ma, Y.; Chen, S.; Chen, Z.; Li, M. Diagnostic value of macrophage inhibitory cytokine 1 as a novel prognostic biomarker for early gastric cancer screening. J. Clin. Lab. Anal. 2021, 35, e23568. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Meng, H.; Hu, J.; Yu, Z.; Xu, J. Increased progastrin-releasing peptide expression is associated with progression in gastric cancer patients. Yonsei Med. J. 2020, 61, 15–19. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, H. Diagnostic and prognostic values of anti-helicobacter pylori antibody combined with serum CA724, CA19-9, and CEA for young patients with early gastric cancer. J. Clin. Lab. Anal. 2020, 34, e23268. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, S.; Zhao, Y.; Wang, Y.; Piao, H.Y.; Wu, Y.; Zhang, J. Circulating long non-coding RNA PCGEM1 as a novel biomarker for gastric cancer diagnosis. Pathol. Res. Pract. 2019, 215, 152569. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhou, Z.; Zhong, Y.; Sun, Y.; Wang, Y.; Zhu, Z.; Jiao, W.; Bai, M.; Sun, J.; Lu, J.; et al. Plasma activity of thioredoxin reductase as a novel biomarker in gastric cancer. Sci. Rep. 2019, 9, 19084. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, P.Y.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Li, P.; Zheng, C.H.; Huang, C.M. Circular RNA hsa_circ_0006848 related to ribosomal protein L6 acts as a novel biomarker for early gastric cancer. Dis. Markers 2019, 2019, 3863458. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Wang, P.; Wang, X.; Ye, H.; Song, C.; Dai, L.; Wang, K.; Jiang, B.; Zhang, J. Autoantibody against 14-3-3 zeta: A serological marker in detection of gastric cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, Y.; Zhang, X.; Yang, Y.; Zheng, X.; Li, X.; Liu, Y.; Zhang, Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer 2018, 17, 68. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Run, Z.C.; Feng, W.; Liu, W.; Zhang, P.J.; Li, Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World J. Gastroenterol. 2018, 24, 2269–2278. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Q.; Zhao, Q.; Liu, M.; Guo, J. Value of combined detection of serum CEA, CA72-4, CA19-9, CA15-3 and CA12-5 in the diagnosis of gastric cancer. Ann. Clin. Lab. Sci. 2017, 47, 260–263. [Google Scholar]
- Pan, Y.Q.; Ruan, Y.Y.; Peng, J.B.; Han, Q.Y.; Zhang, X.; Lin, A.; Yan, W.H. Diagnostic significance of soluble human leukocyte antigen-G for gastric cancer. Hum. Immunol. 2016, 77, 317–324. [Google Scholar] [CrossRef]
- Yin, L.K.; Sun, X.Q.; Mou, D.Z. Value of combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the diagnosis of gastric cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3867–3870. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I. Challenges in Biomedical Analysis—From Classical Sensors to Stochastic Sensors. ECS Sens. Plus 2022, 1, 011603. [Google Scholar] [CrossRef]
- Nxumalo, E.N.; Mamba, B.B.; Kuvarega, A.T.; Mhlanga, S.D.; Yokwana, K.; Khenfouch, M.; Ray, S.C. Facile Synthesis of Nitrogen Doped Graphene Oxide from Graphite Flakes and Powders: A Comparison of Their Surface Chemistry. J. Nanosci. Nanotechnol. 2018, 18, 5470–5484. [Google Scholar]
- Hibbert, D.B.; Korte, E.H.; Örnemark, U. IUPAC Recommendations. Metrological and quality concepts in analytical chemistry (IUPAC Recommendations 2021). Pure Appl. Chem. 2021, 93, 997–1048. [Google Scholar] [CrossRef]
- Karlický, F.; Kumara Ramanatha Datta, K.; Otyepka, M.; Zbořil, R. Halogenated graphenes: Rapidly growing family of graphene derivatives. ACS Nano 2013, 7, 6434–6464. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Vazquez, E.; Prato, M. Organic functionalization of graphene in dispersions. Acc. Chem. Res. 2013, 46, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Englert, J.M.; Dotzer, C.; Yang, G.; Schmid, M.; Papp, C.; Gottfried, J.M.; Steinrück, H.P.; Spiecker, E.; Hauke, F.; Hirsch, A. Covalent bulk functionalization of graphene. Nat. Chem. 2011, 3, 279–286. [Google Scholar] [CrossRef]
- Di Pietro, P.; Forte, G.; Snyders, R.; Satriano, C.; Bittencourt, C.; Thiry, D. Sulphur functionalizion of graphene oxide by radio-frequency plasma. Plasma Process. Polym. 2020, 17, 2000039. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, R.; Chen, H.; Nau, W. An ultrasensitive electrochemical immunosensor for CA 72-4 based on a signal amplification strategy of MoS2 nanoflower–supported Au nanoparticles. J. Saudi Chem. Soc. 2023, 27, 101612. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, C.; Luo, Y.; Pan, C.; Li, J. Electrochemical sensor for the simultaneous detection of CA 72-4 and CA 19-9 tumor markers using dual recognition via glycosyl imprinting and lectin-specific binding for accurate diagnosis of gastric cancer. Biosens. Bioelectron. 2022, 216, 114672. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Yang, J.; Liu, D.; Wang, H.; Xu, R.; Zhang, Y.; Wei, Q. An ultrasensitive split-type electrochemical immunosensor based on controlled–release strategy for detection of CA 19-9. Biosens. Bioelectron. 2023, 227, 115180. [Google Scholar] [CrossRef]
- Gholamin, D.; Karami, P.; Pahlavun, Y.; Johari-Ahar, M. Highly sensitive photoelectrochemical immunosensor for detecting cancer marker CA 19-9 based on a new SnSe quantum dot. Microchim. Acta 2023, 190, 154. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, J.; Liu, S.; Sun, Y.; Dai, Y.; Luo, C.; Wang, X. A novel flower–shaped Ag@ZIF-67 chemiluminescence for sensitive detection of CEA. Talanta 2023, 253, 123938. [Google Scholar]
- Jin, Y.; Zheng, J.; Ci, Y.; Zhu, L.; Zhang, M.; Yin, X.B. Magnetic copper silicate and boronic acid–conjugated AuNCs@keratin–based electrochemical/fluorescent dual–sensing for carcinoembryonic antigen. Talanta 2024, 266, 125012. [Google Scholar] [CrossRef] [PubMed]
- Er, O.F.; Alpaslan, D.; Dudu, T.E.; Aktas, N.; Celik, S.; Kivrak, H. A novel carbohydrate antigen 125 electrochemical sensor based on sweet almond oil organo–hydrogels. Mat. Chem. Phys. 2023, 298, 127441. [Google Scholar] [CrossRef]
- Kamac, M.B.; Altun, M.; Yilmaz, M.; Sezginturk, M.K. A label–free dual immunosensor for the simultaneous electrochemical determination of CA 125 and HE4 biomarkers for the early diagnosis of ovarian cancer. Anal. Bioanal. Chem. 2023, 415, 1709–1718. [Google Scholar] [CrossRef] [PubMed]





| Needle 3D Stochastic Microsensor Based on | Biomarker | Signature toff (s) | Equation of Calibration, r | Sensitivity | Limit of Quantification | Linear Concentration Range |
|---|---|---|---|---|---|---|
| NBGr-2 | CA 72-4 1 | 1.6 | 1/ton = 0.04 + 1.06 × 10−5 C r = 0.9923 | 1.06 × 10−5 s−1 U−1 mL | 4.00 × 10−11 s−1 U−1 mL | 4.00 × 10−11–4.00 × 103 s−1 U−1 mL |
| CA 19-9 1 | 1.8 | 1/ton = 0.03 + 1.82 × 10−4 C r = 0.9973 | 1.82 × 10−4 s−1 U−1 mL | 3.28 × 10−9 s−1 U−1 mL | 3.28 × 10−9–5.00 × 102 s−1 U−1 mL | |
| CA 125 1 | 1.4 | 1/ton = 0.03 + 9.92 × 10−5 C r = 0.9994 | 9.92 × 10−5 s−1 U−1 mL | 8.36 × 10−6 s−1 U−1 mL | 8.36 × 10−6–837.43 s−1 U−1 mL | |
| CEA 2 | 1.1 | 1/ton = 0.03 + 8.13 × 105 C r = 0.9861 | 8.13 × 105 s−1 g−1 mL | 4.10 × 10−15 s−1 g−1 mL | 4.10 × 10−15–2.00 × 10−7 s−1 g−1 mL | |
| NBGr-1 | CA 72-4 1 | 1.0 | 1/ton = 0.04 + 1.43 × 10−5 C r = 0.9977 | 1.43 × 10−5 s−1 U−1 mL | 4.00 × 10−3 s−1 U−1 mL | 4.00 × 10−3–4.00 × 103 s−1 U−1 mL |
| CA 19-9 1 | 1.4 | 1/ton = 0.04 + 5.88 × 10−3 C r = 0.9997 | 5.88 × 10−3 s−1 U−1 mL | 2.09 × 10−13 s−1 U−1 mL | 2.09 × 10−13–20.00 s−1 U−1 mL | |
| CA 125 1 | 1.2 | 1/ton = 0.04 + 1.98 × 10−5 C r = 0.9955 | 1.98 × 10−5 s−1 U−1 mL | 8.37 × 10−14 s−1 U−1 mL | 8.37 × 10−14–8.37 × 103 s−1 U−1 mL | |
| CEA 2 | 1.6 | 1/ton = 0.06 + 9.24 × 104 C r = 0.9946 | 9.24 × 104 s−1 g−1 mL | 1.28 × 10−11 s−1 g−1 mL | 1.28 × 10−11–1.00 × 10−6 s−1 g−1 mL |
| Needle 3D Stochastic Microsensor Based on | Biomarker | Whole Blood | Saliva | Urine | Tumoral Tissue |
|---|---|---|---|---|---|
| NBGr-2 | %, Recovery | ||||
| CA72-4 | 98.99 ± 0.03 | 99.43 ± 0.04 | 99.29 ± 0.03 | 97.25 ± 0.05 | |
| CA19-9 | 97.23 ± 0.05 | 98.21 ± 0.03 | 98.14 ± 0.03 | 98.01 ± 0.03 | |
| CA125 | 98.47 ± 0.02 | 99.12 ± 0.05 | 98.37 ± 0.04 | 97.15 ± 0.04 | |
| CEA | 99.05 ± 0.02 | 99.54 ± 0.05 | 98.11 ± 0.02 | 98.00 ± 0.04 | |
| NBGr-1 | CA72-4 | 99.09 ± 0.08 | 99.01 ± 0.01 | 99.12 ± 0.02 | 98.29 ± 0.08 |
| CA19-9 | 99.00 ± 0.02 | 97.59 ± 0.04 | 99.87 ± 0.07 | 97.97 ± 0.09 | |
| CA125 | 98.32 ± 0.05 | 98.06 ± 0.02 | 98.93 ± 0.04 | 98.19 ± 0.04 | |
| CEA | 98.27 ± 0.03 | 98.89 ± 0.05 | 98.90 ± 0.03 | 98.05 ± 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratei, A.-A.; Stefan-van Staden, R.-I.; Ilie-Mihai, R.-M.; Gheorghe, D.-C. Simultaneous Assay of CA 72-4, CA 19-9, CEA and CA 125 in Biological Samples Using Needle Three-Dimensional Stochastic Microsensors. Sensors 2023, 23, 8046. https://doi.org/10.3390/s23198046
Bratei A-A, Stefan-van Staden R-I, Ilie-Mihai R-M, Gheorghe D-C. Simultaneous Assay of CA 72-4, CA 19-9, CEA and CA 125 in Biological Samples Using Needle Three-Dimensional Stochastic Microsensors. Sensors. 2023; 23(19):8046. https://doi.org/10.3390/s23198046
Chicago/Turabian StyleBratei, Alexandru-Adrian, Raluca-Ioana Stefan-van Staden, Ruxandra-Maria Ilie-Mihai, and Damaris-Cristina Gheorghe. 2023. "Simultaneous Assay of CA 72-4, CA 19-9, CEA and CA 125 in Biological Samples Using Needle Three-Dimensional Stochastic Microsensors" Sensors 23, no. 19: 8046. https://doi.org/10.3390/s23198046






