Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules
Abstract
:1. Introduction
2. Colorimetric Sensors
2.1. Chemical Sensors
2.2. Biological Sensors
3. AuNPs
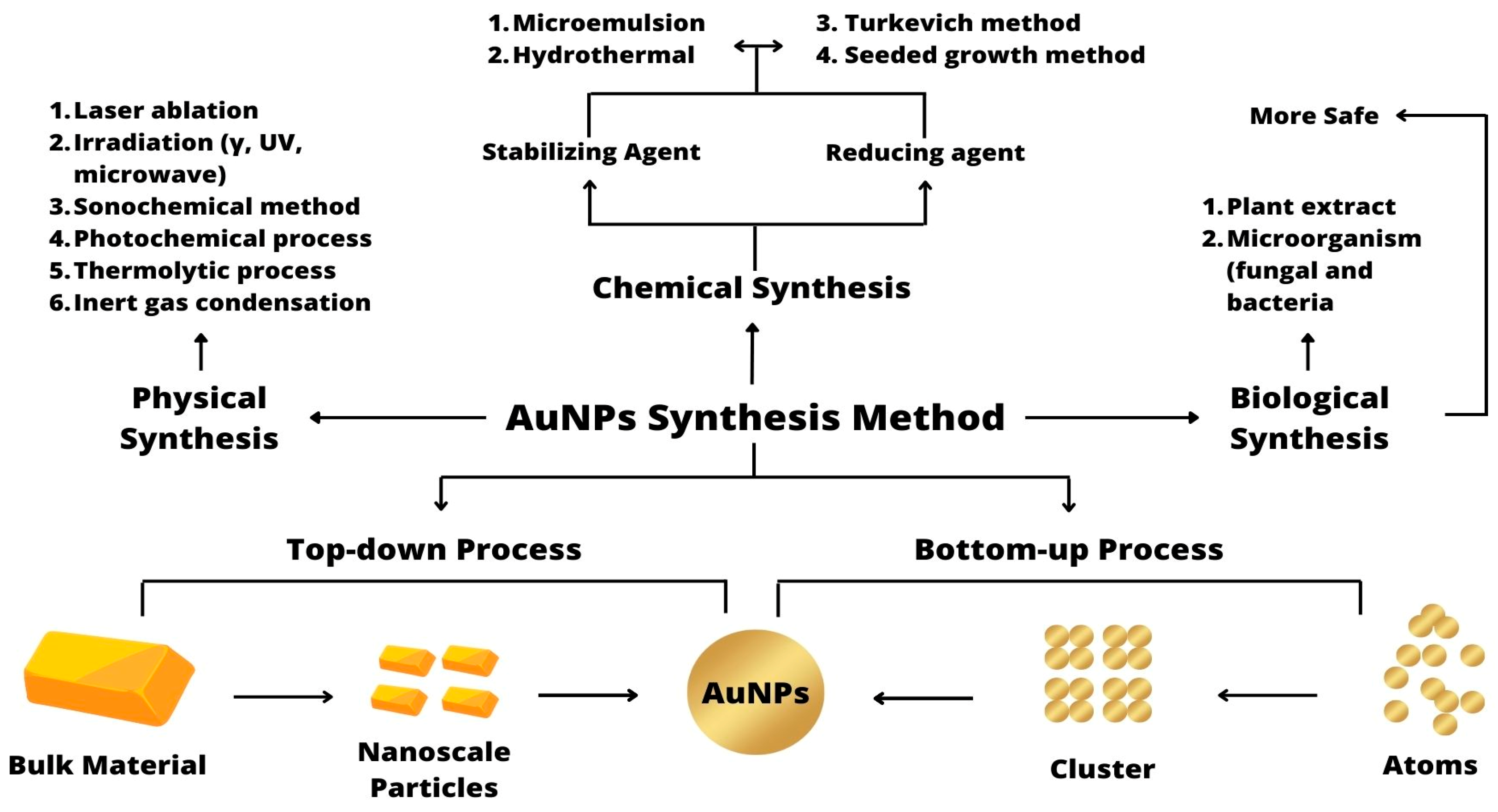
3.1. Synthesis
3.1.1. Physical Synthesis
3.1.2. Chemical Synthesis
3.1.3. Biological Synthesis
3.2. Properties/Characteristics
3.3. Conjugation of AuNPs
3.3.1. DNA-Conjugated AuNPs
3.3.2. Protein-Conjugated AuNPs
3.3.3. Aptamer-Conjugated AuNPs
4. Application of Detection Strategies Based on AuNPs
4.1. Application of AuNPs for the Detection of Heavy Metals
4.2. Application of AuNPs for the Detection of Biological Molecules
4.3. Application of AuNPs for the Detection of Pharmaceutical Compounds
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef] [PubMed]
- Borse, V.; Jain, P.; Sadawana, M.; Srivastava, R. “Turn-on” fluorescence assay for inorganic phosphate sensing. Sens. Actuators B Chem. 2016, 225, 340–347. [Google Scholar] [CrossRef]
- Kim, J.H.; Mun, S.; Ko, H.U.; Yun, G.Y.; Kim, J. Disposable chemical sensors and biosensors made on cellulose paper. Nanotechnology 2014, 25, 092001. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver nanoparticles as colorimetric sensors for water pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M.; Mangi, J.D. Applications of copper nanoparticles for colorimetric detection of dithiocarbamate pesticides. J. Nanostruct. Chem. 2019, 9, 77–93. [Google Scholar] [CrossRef]
- Grigorchuk, N.I. Size and shape effect on optical conductivity of metal nanoparticles. Europhys. Lett. 2018, 121, 67003. [Google Scholar] [CrossRef]
- Abdussalam-mohammed, W. Comparison of Chemical and Biological Properties of Metal Nanoparticles (Au, Ag), with Metal Oxide Nanoparticles (ZnO-NPs) and their Applications. Adv. J. Chem. A 2020, 3, 192–210. [Google Scholar] [CrossRef]
- Fan, J.; Sun, M. Nanoplasmonic Nanorods/Nanowires from Single to Assembly: Syntheses, Physical Mechanisms and Applications. Chem. Rec. 2020, 20, 1043–1073. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef]
- Borse, V.; Thakur, M.; Sengupta, S.; Srivastava, R. N-doped multi-fluorescent carbon dots for ‘turn off-on’ silver-biothiol dual sensing and mammalian cell imaging application. Sens. Actuators B Chem. 2017, 248, 481–492. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Banerjee, S.; Ghosh, S.S.; Chattopadhyay, A. Simultaneous RGB emitting Au nanoclusters in chitosan nanoparticles for anticancer gene theranostics. ACS Appl. Mater. Interfaces 2014, 6, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Alex, S.; Tiwari, A. Functionalized gold nanoparticles: Synthesis, properties and applications—A review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef] [PubMed]
- Amanulla, B.; Perumal, K.N.; Ramaraj, S.K. Chitosan functionalized gold nanoparticles assembled on sulphur doped graphitic carbon nitride as a new platform for colorimetric detection of trace Hg2+. Sens. Actuators B Chem. 2019, 281, 281–287. [Google Scholar] [CrossRef]
- Sengan, M.; Kamlekar, R.K.; Veerappan, A. Highly selective rapid colorimetric sensing of Pb2+ ion in water samples and paint based on metal induced aggregation of N-decanoyltromethamine capped gold nanoparticles. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 239, 118485. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; El-farargy, A.F.M.; Abd-Elaal, A.A. Enhancement the detection of Ni2+ and Zn2+ ions using nanostructure of synthesized dithiol surfactants with gold nanoparticles. J. Ind. Eng. Chem. 2014, 20, 3905–3912. [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; Zheng, Z.; Li, Y.; Wang, J. Highly sensitive and selective colorimetric and SERS dual-mode detection of arsenic (III) based on glutathione functionalized gold nanoparticles. Sens. Actuators Rep. 2020, 2, 100013. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Gul, A.R.; Kailasa, S.K.; Kim, K.W.; Lee, J.S.; Park, H.; Park, T.J. Functionalization of gold nanoparticles using guanidine thiocyanate for sensitive and selective visual detection of Cd2+. Sens. Actuators B Chem. 2021, 334, 129685. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Conjugation of cysteamine functionalized nanodiamond to gold nanoparticles for pH enhanced colorimetric detection of Cr3+ ions demonstrated by real water sample analysis. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2023, 286, 121962. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.A.; Bhamore, J.R.; Singhal, R.K.; Kailasa, S.K. Microwave assisted synthesis of tyrosine protected gold nanoparticles for dual (colorimetric and fluorimetric) detection of spermine and spermidine in biological samples. Biosens. Bioelectron. 2017, 88, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, C.; Gowthaman, N.S.K.; Abraham John, S. Chain-like 2-amino-4-thiazoleacetic acid tethered AuNPs as colorimetric and spectrophotometric probe for organophosphate pesticide in water and fruit samples. Microchem. J. 2021, 168, 106495. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Chai, K.; Hu, X.; Hu, Y.; Shi, S.; Yao, T. A turn-on unlabeled colorimetric biosensor based on aptamer-AuNPs conjugates for amyloid-β oligomer detection. Talanta 2023, 260, 124649. [Google Scholar] [CrossRef]
- Chen, X.; Ji, J.; Wang, D.; Gou, S.; Xue, Z.; Zhao, L.; Feng, S. Highly sensitive and selective colorimetric sensing of histidine by NAC functionalized AuNPs in aqueous medium with real sample application. Microchem. J. 2021, 160, 105661. [Google Scholar] [CrossRef]
- Ajay Piriya, V.S.; Joseph, P.; Kiruba Daniel, S.G.C.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Memon, S.S.; Nafady, A.; Solangi, A.R.; Al-Enizi, A.M.; Sirajuddin; Shah, M.R.; Sherazi, S.T.H.; Memon, S.; Arain, M.; Abro, M.I.; et al. Sensitive and selective aggregation based colorimetric sensing of Fe3+ via interaction with acetyl salicylic acid derived gold nanoparticles. Sens. Actuators B Chem. 2018, 259, 1006–1012. [Google Scholar] [CrossRef]
- Gong, N.; Chen, S.; Jin, S.; Zhang, J.; Wang, P.C.; Liang, X.-J. Effects of the physicochemical properties of gold nanostructures on cellular internalization. Regen. Biomater. 2015, 2, 273–280. [Google Scholar] [CrossRef]
- da Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinski, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Al Mamun, M.S.; Habib, M.A.; Islam, A.B.M.N.; Mahiuddin, M.; Karim, K.M.R.; Naime, J.; Saha, P.; Dey, S.K.; Ara, M.H. A review on gold nanoparticles: Biological synthesis, characterizations, and analytical applications. Results Chem. 2022, 4, 100478. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, Z.; Safiabadi Tali, S.H.; Hajimiri, H.; Al-Kassawneh, M.; Jahanshahi-Anbuhi, S. Gold Nanoparticles-Based Colorimetric Assays for Environmental Monitoring and Food Safety Evaluation. Crit. Rev. Anal. Chem. 2022, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Chemical and biological sensors for food-quality monitoring and smart packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.M.; Mondal, S. Structural Properties and Sensing Characteristics of Sensing Materials; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, ISBN 9780080965338. [Google Scholar]
- Lo, S.H.; Wu, M.C.; Venkatesan, P.; Wu, S.P. Colorimetric detection of chromium(III) using O-phospho-l-serine dithiocarbamic acid functionalized gold nanoparticles. Sens. Actuators B Chem. 2015, 220, 772–778. [Google Scholar] [CrossRef]
- Baetsen-Young, A.M.; Vasher, M.; Matta, L.L.; Colgan, P.; Alocilja, E.C.; Day, B. Direct colorimetric detection of unamplified pathogen DNA by dextrin-capped gold nanoparticles. Biosens. Bioelectron. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Swager, T.M.; Mirica, K.A. Introduction: Chemical Sensors. Chem. Rev. 2019, 119, 1–2. [Google Scholar] [CrossRef]
- Sun, H.; Yu, L.; Chen, H.; Xiang, J.; Zhang, X.; Shi, Y.; Yang, Q.; Guan, A.; Li, Q.; Tang, Y. A colorimetric lead (II) ions sensor based on selective recognition of G-quadruplexes by a clip-like cyanine dye. Talanta 2015, 136, 210–214. [Google Scholar] [CrossRef]
- Farmani, M.R.; Peyman, H.; Roshanfekr, H. Blue luminescent graphene quantum dot conjugated cysteamine functionalized-gold nanoparticles (GQD-AuNPs) for sensing hazardous dye Erythrosine B. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 229, 117960. [Google Scholar] [CrossRef]
- Qazi, Z.; Hassan Baig, M.; Mabood Husain, F.; Yousef Alomar, S.; Dong, J.J.; Sajid Khan, M. Biological reaction mediated engineered AuNPs facilitated delivery encore the anticancer, antiglycation, and antidiabetic potential of garcinol. J. King Saud Univ.-Sci. 2023, 35, 102524. [Google Scholar] [CrossRef]
- Chen, N.; Liu, H.; Zhang, Y.; Zhou, Z.; Fan, W.; Yu, G.; Shen, Z.; Wu, A. A colorimetric sensor based on citrate-stabilized AuNPs for rapid pesticide residue detection of terbuthylazine and dimethoate. Sens. Actuators B Chem. 2018, 255, 3093–3101. [Google Scholar] [CrossRef]
- Reena, A.; Karpagavalli, S.G.; Rajendran, L.; Manimegalai, B.; Swaminathan, R. Theoretical analysis of putrescine enzymatic biosensor with optical oxygen transducer in sensitive layer using Akbari–Ganji method. Int. J. Electrochem. Sci. 2023, 18, 100113. [Google Scholar] [CrossRef]
- Pei, R.; Ye, L.; Jing, C. Enzyme-based electrochemical biosensor for antimonite detection in water. Biosens. Bioelectron. 2023, 229, 115244. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Y.; Cui, X.; Yan, H.; Cao, L.; Gao, L.; Yin, H. Investigation of the effect of antibiotics on 5-formylcytosine content in mazie seedling tissues based on photoelectrochemical biosensor. J. Hazard. Mater. 2022, 436, 129146. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Sui, M.; Huang, Y.; Tang, Y.; Luo, J.; Dong, Y.; Guo, Y.; Ma, Y.; Gu, W.; Guo, M.; Huang, J.; et al. A high-sensitivity AuNPs/MWCNTs-MB/DNA-GCE quadruplex biosensor for Pb detection in medicinal teas through in-situ monitoring microstructure and conformational switch by SECM. Sens. Actuators B Chem. 2023, 393, 134193. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, H.; Wang, X. Thermal self-regulatory intelligent biosensor based on carbon-nanotubes-decorated phase-change microcapsules for enhancement of glucose detection. Biosens. Bioelectron. 2022, 195, 113586. [Google Scholar] [CrossRef]
- Zhang, J.; He, F. Mycobacterium tuberculosis piezoelectric sensor based on AuNPs-mediated enzyme assisted signal amplification. Talanta 2022, 236, 122902. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Liu, S.; Shi, X. Highly sensitive detection of L.monocytogenes using an electrochemical biosensor based on Si@MB / AuNPs modified glassy carbon electrode. Microchem. J. 2023, 194, 109357. [Google Scholar] [CrossRef]
- Sun, R.; Lv, R.; Li, Y.; Du, T.; Chen, L.; Zhang, Y.; Zhang, X.; Zhang, L.; Ma, H.; Sun, H.; et al. Simple and sensitive electrochemical detection of sunset yellow and Sudan I in food based on AuNPs/Zr-MOF-Graphene. Food Control 2023, 145, 109491. [Google Scholar] [CrossRef]
- Bäcker, M.; Rakowski, D.; Poghossian, A.; Biselli, M.; Wagner, P.; Schöning, M.J. Chip-based amperometric enzyme sensor system for monitoring of bioprocesses by flow-injection analysis. J. Biotechnol. 2013, 163, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Diesel, E.; Schreiber, M.; Van Der Meer, J.R. Development of bacteria-based bioassays for arsenic detection in natural waters. Anal. Bioanal. Chem. 2009, 394, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.; Sun, Y.; Chen, B.; Hu, F.; Guo, C.; Yang, T. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Tzianni, E.I.; Hrbac, J.; Christodoulou, D.K.; Prodromidis, M.I. A portable medical diagnostic device utilizing free-standing responsive polymer film-based biosensors and low-cost transducer for point-of-care applications. Sens. Actuators B Chem. 2020, 304, 127356. [Google Scholar] [CrossRef]
- Hagness, D.E.; Yang, Y.; Tilley, R.D.; Gooding, J.J. The application of an applied electrical potential to generate electrical fields and forces to enhance affinity biosensors. Biosens. Bioelectron. 2023, 238, 115577. [Google Scholar] [CrossRef]
- Arslan, M.; Zareef, M.; Tahir, H.E.; Guo, Z.; Rakha, A.; Xuetao, H.; Shi, J.; Zhihua, L.; Xiaobo, Z.; Khan, M.R. Discrimination of rice varieties using smartphone-based colorimetric sensor arrays and gas chromatography techniques. Food Chem. 2022, 368, 130783. [Google Scholar] [CrossRef]
- Braun, M.; Rýglová, Š.; Suchý, T. Determination of glycosaminoglycans in biological matrices using a simple and sensitive reversed-phase HPLC method with fluorescent detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1173, 122626. [Google Scholar] [CrossRef]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Starodub, N.F.; Katzev, A.M.; Starodub, V.M.; Levkoetz, I.A.; Goncharuk, V.V.; Klimenko, N.A. Biosensor for Water Quality Monitoring; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Al-Radadi, N.S. Ephedra mediated green synthesis of gold nanoparticles (AuNPs) and evaluation of its antioxidant, antipyretic, anti-asthmatic, and antimicrobial properties. Arab. J. Chem. 2023, 16, 104353. [Google Scholar] [CrossRef]
- Yahaya, M.L.; Zakaria, N.D.; Noordin, R.; Abdul Razak, K. Synthesis of large and stable colloidal gold nanoparticles (AuNPs) by seeding-growth method. Mater. Today Proc. 2022, 66, 2943–2947. [Google Scholar] [CrossRef]
- Stanglmair, C.; Scheeler, S.P.; Pacholski, C. Seeding growth approach to gold nanoparticles with diameters ranging from 10 to 80 nanometers in organic solvent. Eur. J. Inorg. Chem. 2014, 2014, 3633–3637. [Google Scholar] [CrossRef]
- Abdulateef, S.A.; Raypah, M.E.; Omar, A.F.; Mat Jafri, M.Z.; Ahmed, N.M.; Kaus, N.H.M.; Seeni, A.; Mail, M.H.; Tabana, Y.; Ahmed, M.; et al. Rapid synthesis of bovine serum albumin-conjugated gold nanoparticles using pulsed laser ablation and their anticancer activity on hela cells. Arab. J. Chem. 2023, 16, 104395. [Google Scholar] [CrossRef]
- Tran, M.; DePenning, R.; Turner, M.; Padalkar, S. Effect of citrate ratio and temperature on gold nanoparticle size and morphology. Mater. Res. Express 2016, 3, 105027. [Google Scholar] [CrossRef]
- Umamaheswari, K.; Abirami, M. Assessment of antifungal action mechanism of green synthesized gold nanoparticles (AuNPs) using Allium sativum on Candida species. Mater. Lett. 2023, 333, 133616. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Cele, T.; Maaza, M.; Gibaud, A. Synthesis of Platinum nanoparticles by Gamma Radiolysis. MRS Adv. 2018, 3, 2537–2557. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Khiabani, M.S.; Hamishehkar, H.; Mokarram, R.R.; Amjadi, M. Antioxidant, antimicrobial and cytotoxic activities of biosynthesized gold nanoparticles (AuNPs) from Chinese lettuce (CL) leave extract (Brassica rapa var. pekinensis). Mater. Today Commun. 2021, 29, 102831. [Google Scholar] [CrossRef]
- Cheng, X.L.; Fu, T.R.; Zhang, D.F.; Xiong, J.H.; Yang, W.Y.; Du, J. Biomass-assisted fabrication of rGO-AuNPs as surface-enhanced Raman scattering substrates for in-situ monitoring methylene blue degradation. Anal. Biochem. 2023, 667, 115087. [Google Scholar] [CrossRef]
- Prasannadevi, R.; Vigneshwaran, J.; Suthakaran, S.; Jose, S.P.; Dhanapandian, S.; Krishnakumar, N. Bio-inspired green synthesis of sunlight driven AuNPs/RGO nanocomposites with enhanced photocatalytic activity and their promising electrochemical performance. Curr. Appl. Phys. 2022, 43, 15–28. [Google Scholar] [CrossRef]
- Kanchi, S.; Kumar, G.; Lo, A.Y.; Tseng, C.M.; Chen, S.K.; Lin, C.Y.; Chin, T.S. Exploitation of de-oiled jatropha waste for gold nanoparticles synthesis: A green approach. Arab. J. Chem. 2018, 11, 247–255. [Google Scholar] [CrossRef]
- Al-Shamari, A.A.; Abdelghany, A.M.; Alnattar, H.; Oraby, A.H. Structural and optical properties of PEO/CMC polymer blend modified with gold nanoparticles synthesized by laser ablation in water. J. Mater. Res. Technol. 2021, 12, 1597–1605. [Google Scholar] [CrossRef]
- Ali Dheyab, M.; Abdul Aziz, A.; Jameel, M.S.; Moradi Khaniabadi, P.; Mehrdel, B. Sonochemical-assisted synthesis of highly stable gold nanoparticles catalyst for decoloration of methylene blue dye. Inorg. Chem. Commun. 2021, 127, 108551. [Google Scholar] [CrossRef]
- Correard, F.; Maximova, K.; Estève, M.A.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.V.; Braguer, D. Gold nanoparticles prepared by laser ablation in aqueous biocompatible solutions: Assessment of safety and biological identity for nanomedicine applications. Int. J. Nanomed. 2014, 9, 5415–5430. [Google Scholar] [CrossRef]
- Vislavath, S.; Kumar, M.P.; Balraj, G.; Sharath Babu, M.; Ayodhya, D. Synthesis of Schiff base stabilized AuNPs for enhanced catalytic degradation of pesticides, Cr(VI) detection, antioxidant, and antimicrobial activities. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Guo, C.; Liu, D.; Xu, W.; He, L.; Liu, S. Accelerating the peroxidase- and glucose oxidase-like activity of Au nanoparticles by seeded growth strategy and their applications for colorimetric detection of dopamine and glucose. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130555. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision gold nanoparticles using Turkevich method. KONA Powder Part. J. 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Sultana, N.; Abu Nayem, S.M.; Shah, S.S.; Kang, H.; Jafar Mazumder, M.A.; Awal, A.; Chandra Roy, S.; Uddin, J.; Aziz, M.A.; Ahammad, A.J.S. Synthesis and synergistic effect of positively charged jute carbon supported AuNPs coated polymer nanocomposite for selective determination of nitrite. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2023, 295, 116572. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.L.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization, purification, and stability of gold nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Iris, M.I.; Pohl, C.; Baldi, G.; Unger, R.E.; James, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 18. [Google Scholar] [CrossRef]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-Chemical Condition Optimization during Biosynthesis lead to development of Improved and Catalytically Efficient Gold Nano Particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, M.; Zare, D.; Davoodi, D. A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2012, 94, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.A.; Milosevic, A.M.; Bossert, D.; Crippa, F.; Moore, T.L.; Geers, C.; Balog, S.; Rothen-Rutishauser, B.; Petri-Fink, A. Taylor Dispersion of Inorganic Nanoparticles and Comparison to Dynamic Light Scattering and Transmission Electron Microscopy. Colloids Interface Sci. Commun. 2018, 22, 29–33. [Google Scholar] [CrossRef]
- Sakellari, G.I.; Hondow, N.; Gardiner, P.H.E. Factors Influencing the Surface Functionalization of Citrate Stabilized Gold Nanoparticles with Cysteamine, 3-Mercaptopropionic Acid or l-Selenocystine for Sensor Applications. Chemosensors 2020, 8, 80. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Oh, S.; Baba, R.; Zhou, H.; Hwang, S.; Lee, J.; Park, E.Y. Synthesis of Gold Nanoparticles with Buffer-Dependent Variations of Size and Morphology in Biological Buffers. Nanoscale Res. Lett. 2016, 11, 65. [Google Scholar] [CrossRef]
- Prasanna, S.B.; Bahajjaj, A.A.A.; Lee, Y.H.; Lin, Y.C.; Dhawan, U.; Sakthivel, R.; Chung, R.J. Highly responsive and sensitive non-enzymatic electrochemical sensor for the detection of β-NADH in food, environmental and biological samples using AuNP on polydopamine/titanium carbide composite. Food Chem. 2023, 426, 136609. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Q.; Xia, K.; Li, Z.; Chen, G.Y.; Zhang, D.; Zhou, X. An AuNPs/mesoporous NiO/nickel foam nanocomposite as a miniaturized electrode for heavy metal detection in groundwater. Engineering 2022, 27, 1–10. [Google Scholar] [CrossRef]
- De Bortoli, L.S.; Vanoni, C.R.; Jost, C.L.; Mezalira, D.Z.; Fredel, M.C. Stable and ligand-free gold nanoparticles produced by laser ablation as efficient electrocatalysts for electrochemical sensing of dopamine. J. Electroanal. Chem. 2023, 947, 117744. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S. Synthesis and optimization of the sonochemical method for functionalizing gold shell on Fe3O4 core nanoparticles using response surface methodology. Surf. Interfaces 2020, 21, 100647. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Excellent relaxivity and X-ray attenuation combo properties of Fe3O4@Au CSNPs produced via Rapid sonochemical synthesis for MRI and CT imaging. Mater. Today Commun. 2020, 25, 101368. [Google Scholar] [CrossRef]
- Su, L.; Xiong, Y.; Chen, Z.; Duan, Z.; Luo, Y.; Zhu, D.; Ma, X. MoO3 nanosheet-assisted photochemical reduction synthesis of Au nanoparticles for surface-enhanced Raman scattering substrates. Sens. Actuators B Chem. 2019, 279, 320–326. [Google Scholar] [CrossRef]
- Mirac Dizman, H.; Kazancioglu, E.O.; Shigemune, T.; Takahara, S.; Arsu, N. High sensitivity colorimetric determination of L-cysteine using gold nanoparticles functionalized graphene oxide prepared by photochemical reduction method. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2022, 264, 120294. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Huang, Y.; Liu, H.; Guan, K.; Chen, A.; Zhao, X.; Wang, S.; Zhang, L. A bifunctional AuNP probe-based enzyme-linked immunosorbent assay for facile and ultrasensitive detection of trace zearalenone in coix seed. Microchem. J. 2023, 184, 108152. [Google Scholar] [CrossRef]
- García-Caballero, V.; Mohammed-Ibrahim, H.K.; Giner-Casares, J.J.; Cano, M. Influence of the synthesis route on the electrocatalytic performance for ORR of citrate-stabilized gold nanoparticles. Electrochem. Commun. 2022, 142, 107364. [Google Scholar] [CrossRef]
- Iranmanesh, S.; Shahidi Bonjar, G.H.; Baghizadeh, A. Study of biosynthesis of gold nanoparticles by using several saprophytic fungi. SN Appl. Sci. 2020, 2, 1851. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; Cruz, N.D.; de Oliveira, G.R.; Sá, W.S.; de Oliveira, J.D.; Ribeiro, P.R.S.; Leite, S.G.F. Evaluation of the kinetics of gold biosorption processes and consequent biogenic synthesis of AuNPs mediated by the fungus Trichoderma harzianum. Environ. Technol. Innov. 2021, 21, 101238. [Google Scholar] [CrossRef]
- Chan, J.Z.; Rasit Ali, R.; Shameli, K.; Salleh, M.S.N.; Lee, K.X.; Mohamed Isa, E.D. Green Synthesis of Gold Nanoparticles using Aqueous Extract of Clitoria Ternatea Flower. IOP Conf. Ser. Mater. Sci. Eng. 2020, 808, 012036. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Ao, L.; Wang, F.; Jing, W.; Zhang, S.; Liu, Y. Colorimetric sensor array for proteins discrimination based on the tunable peroxidase-like activity of AuNPs-DNA conjugates. Sens. Actuators B Chem. 2017, 245, 66–73. [Google Scholar] [CrossRef]
- Yadav, K.; Garg, S.; Singh, A.K.; Singh, S.; Singh Parmar, A. Rosy Protein nano Dots conjugated AuNP, poly-Lysine biointerface for the selective voltammetric estimation of Melatonin in pharmaceutical and food samples. Microchem. J. 2022, 179, 107563. [Google Scholar] [CrossRef]
- Poklepovich-Caride, S.; Oestreicher, V.; Mercedes Zalduendo, M.; Bordoni, A.V.; Soler-Illia, G.J.A.A.; Angelomé, P.C. A versatile one-pot room temperature approach for the synthesis of gold nanoparticles with multiple sizes and shapes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128890. [Google Scholar] [CrossRef]
- Thatai, S.; Khurana, P.; Prasad, S.; Soni, S.K.; Kumar, D. Trace colorimetric detection of Pb2+ using plasmonic gold nanoparticles and silica-gold nanocomposites. Microchem. J. 2016, 124, 104–110. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Le, T.T.V.; Huynh, B.A.; Nguyen, N.V.; Le, V.T.; Doan, V.D.; Tran, V.A.; Nguyen, A.T.; Cao, X.T.; Vasseghian, Y. Novel biogenic gold nanoparticles stabilized on poly(styrene-co-maleic anhydride) as an effective material for reduction of nitrophenols and colorimetric detection of Pb(II). Environ. Res. 2022, 212, 113281. [Google Scholar] [CrossRef]
- Zhang, D.; Chu, S.; Wang, L.; Zhan, X.; Zhou, P.; Zhang, D. Dual-mode colorimetric determination of As(III) based on negatively-charged aptamer-mediated aggregation of positively-charged AuNPs. Anal. Chim. Acta 2022, 1221, 340111. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhao, Z.L.; Hao, T.T.; Li, X. A simply visual and rapidly colorimetric detection of Hg2+ in cosmetics based on gold nanoparticles modified by sulfadiazine. Opt. Mater. 2023, 137, 113622. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Selective colorimetric and electrochemical detections of Cr(III) pollutant in water on 3-mercaptopropionic acid-functionalized gold plasmon nanoparticles. Anal. Chim. Acta 2021, 1152, 338272. [Google Scholar] [CrossRef] [PubMed]
- Borah, N.; Kalita, A.J.; Guha, A.K.; Das, M.R.; Tamuly, C. Highly selective, rapid and simple colorimetric detection of Fe3+ in fortified foods by L-Cysteine modified AuNP. Microchem. J. 2022, 179, 107480. [Google Scholar] [CrossRef]
- Andreani, A.S.; Kunarti, E.S.; Hashimoto, T.; Hayashita, T.; Santosa, S.J. Fast and selective colorimetric detection of Fe3+ based on gold nanoparticles capped with ortho-hydroxybenzoic acid. J. Environ. Chem. Eng. 2021, 9, 105962. [Google Scholar] [CrossRef]
- Khan, Z.A.; Park, S. AuNPs- Aβ-Ni-HRP sandwich assay: A new sensitive colorimetric method for the detection of Aβ 1-40. Talanta 2022, 237, 122946. [Google Scholar] [CrossRef]
- Zhuang, X.; Hu, Y.; Wang, J.; Hu, J.; Wang, Q.; Yu, X. A colorimetric and SERS dual-readout sensor for sensitive detection of tyrosinase activity based on 4-mercaptophenyl boronic acid modified AuNPs. Anal. Chim. Acta 2021, 1188, 339172. [Google Scholar] [CrossRef]
- Mirsalari, M.; Elhami, S. Colorimetric detection of insulin in human serum using GO/AuNPs/TX-100 nanocomposite. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 240, 118617. [Google Scholar] [CrossRef]
- Dadmehr, M.; Mortezaei, M.; Korouzhdehi, B. Dual mode fluorometric and colorimetric detection of matrix metalloproteinase MMP-9 as a cancer biomarker based on AuNPs@gelatin/AuNCs nanocomposite. Biosens. Bioelectron. 2023, 220, 114889. [Google Scholar] [CrossRef]
- Cheng, C.; Qiao, J.; Zhang, H.; Zhao, Z.; Qi, L. Temperature modulating the peroxidase-mimic activity of poly(N-isopropyl acrylamide) protected gold nanoparticles for colorimetric detection of glutathione. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2022, 280, 121516. [Google Scholar] [CrossRef]
- Khurana, S.; Kukreti, S.; Kaushik, M. Designing a two-stage colorimetric sensing strategy based on citrate reduced gold nanoparticles: Sequential detection of Sanguinarine (anticancer drug) and visual sensing of DNA. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 246, 119039. [Google Scholar] [CrossRef]
- Zou, L.; Li, X.; Lai, Y. Colorimetric aptasensor for sensitive detection of kanamycin based on target-triggered catalytic hairpin assembly amplification and DNA-gold nanoparticle probes. Microchem. J. 2021, 162, 105858. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, Y.; Su, H.; Wang, L.; Li, L.; Ma, R.; Yan, X.; Sun, J.; Wang, S.; Mao, X. A label-free colorimetric aptasensor based on split aptamers-chitosan oligosaccharide-AuNPs nanocomposites for sensitive and selective detection of kanamycin. Talanta 2022, 238, 123032. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cheng, Y.; Qi, X.; Li, X.; Zhang, Z.; Chen, L.; Sun, T.; Gao, Z.; Zhu, M. Target-induced gold nanoparticles colorimetric sensing coupled with aptamer for rapid and high-sensitivity detecting kanamycin. Anal. Chim. Acta 2022, 1230, 340377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Jang, C.H. Ultrasensitive colorimetric detection of amoxicillin based on Tris-HCl-induced aggregation of gold nanoparticles. Anal. Biochem. 2022, 645, 114634. [Google Scholar] [CrossRef]
- Zhang, S.; Geng, Y.; Ye, N.; Xiang, Y. A simple and sensitive colorimetric sensor for determination of gentamicin in milk based on lysine functionalized gold nanoparticles. Microchem. J. 2020, 158, 105190. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Huang, P.; Wu, F.Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, J.; Sun, L.; An, S. A simple and sensitive AuNPs-based colorimetric aptasensor for specific detection of azlocillin. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2022, 271, 120924. [Google Scholar] [CrossRef]

| Physical Methods | Chemical Methods | Biological Methods |
|---|---|---|
| No use of toxic chemicals | Use of toxic chemicals | No use of toxic chemicals |
| Difficult to control size and shape | Easy to control size and shape | Easy to control size and shape |
| Low stability | High stability | Low stability |
| Synthesis | Method | Characteristics of AuNPs | Reference |
|---|---|---|---|
| Physical | Laser Ablation | Average size of 8 nm with spherical shape | [64] |
| Size ranging between 10 and 25 nm with spherical shape | [73] | ||
| Average size of 10 ± 2 with a predominantly spherical shape (some irregular shapes also observed) | [90] | ||
| Sonochemical | Size ranging between 13.6 and 22.3 nm with a spherical shape | [74] | |
| Average size of 8.7 nm with a spherical shape | [91] | ||
| Average size of 21 nm with a spherical shape | [92] | ||
| Photochemical | Average size of 10 nm with a spherical shape | [93] | |
| Average size of 11 nm with a dominantly spherical shape | [94] | ||
| Chemical | Seeded Growth | Average size of 27 nm with a spherical shape | [78] |
| Size ranging between 5.09 ± 17.22 nm with a spherical shape | [95] | ||
| Turkevich | Average size of 15 nm with a uniform spherical shape | [62] | |
| Size ranging between 15 and 30 nm with a spherical shape | [78] | ||
| Average size of 17.9 ± 3.6 nm with a similar quasi-spherical morphology | [96] | ||
| Biological | Microorganism | Size ranging between 20 and 40 nm with a spherical shape | [97] |
| Size ranging between 7 and 21 nm with a spherical shape | [66] | ||
| Average size of 15 nm with a spherical shape | [98] | ||
| Plant Extract | Size ranging between 1.3 and 15.6 nm with a spherical and triangle shape | [61] | |
| Size ranging between 2.65 and 16.25 nm with a circular shape | [67] | ||
| Average size of 32.96 ± 5.25 nm with a spherical shape | [99] |
| Analytes | Conjugated AuNPs | Size and Shape of AuNPs | LOD | Stability | Reference |
|---|---|---|---|---|---|
| Pb2+ | N-decanoyl-tromethamine | 29 ± 7 nm with a spherical shape | 350 nM | Highly stable | [17] |
| Poly(styrene-co-maleic anhydride) | ±50 nm with a spherical shape | 30 nM | Well stable | [104] | |
| Ni2+, Zn2+ | Dithiol surfactants | ±20 nm with a spherical shape | - | Well stable | [18] |
| As3+ | Glutathione | ±40 nm with a spherical shape | 0.11 ppb | Well stable | [19] |
| Cysteamine | ±43 nm with a spherical shape | 7.41 nM | Stable (6 days) | [105] | |
| Hg2+ | S-g-C3N4 | ±30 nm with a spherical shape | 0.275 nM | Highly stable | [16] |
| Sulfadiazine | ±15 nm with a spherical shape | 71 ppb | Stable | [106] | |
| Cd2+ | Guanidine thiocyanate | 17.5 ± 3.5 nm with a spherical shape | 10 nM | Highly stable | [20] |
| Cr3+ | Cysteamine | 18.3 ± 4.8 nm with a spherical shape | 0.236 nM | Highly stable | [21] |
| 3-merca-ptopropionic acid | 17.1 ± 2.5 nm with a spherical shape | 0.34 ppb | Stable | [107] | |
| Fe3+ | L-Cysteine | 22.02 ± 1.5 nm with a spherical shape | 220 nM | Highly stable | [108] |
| ortho-hydroxybenzoic acid | ±25.04 nm with a spherical, triangular, and hexagonal shape | 9190 nM | Highly stable | [109] |
| Analytes | AuNP Conjugates | Size and Shape of AuNPs | LOD | Stability | Reference |
|---|---|---|---|---|---|
| Spermine and spermidine | Tyrosine | 3.3 nm with a roughly spherical shape and was well-dispersed | 0.136 and 0.636 nM | - | [22] |
| Quinalphos | 2-Amino-4-thiazoleacetic acid (ATA) | 6.4 nm with a spherical shape | 48.2 nM | Highly stable | [23] |
| Amyloid-β (Aβ) oligomers | Aptamer–polythymine–poly-adenine | 13 nm and a spherical shape | 3.03 nM | Well stable | [24] |
| Aβ-Nickel-horseradish peroxidase (Aβ-Ni-HRP) | 28–35 nm with a spherical shape | 0.22 nM | - | [110] | |
| Histidine | N-Acetyl-cysteine | 6 nm with a spherical shape and was well-dispersed | 0.167 nM | Stable | [25] |
| Tyrosinase | 4-mercaptophenyl boronic acid (4-MPBA) | 30 nm with spherical shape | 0.001 U/mL | - | [111] |
| Insulin | Graphene oxide and triton X-100 | 15–20 nm with a dominant spherical shape | 0.1 ppb | Stable | [112] |
| Matrix metalloproteinase (MMPs) | Gelatin/AuNCs | 18 nm with a spherical shape | 0.25 ppb | Well stable | [113] |
| Glutathione (GSH) | Poly(N-isopropyl acrylamide) (PNIPAM) | 0.6 nm with a spherical shape | 800 nM | High stable | [114] |
| Analytes | AuNP Conjugates | Size and Shape of AuNPs | LOD | Stability | Reference |
|---|---|---|---|---|---|
| Sanguinarine | - | 25–30 nm with spherical shape | 46 nM | Stable | [115] |
| Kanamycin | DNA (hairpin probes H1, H2, H3) | 13 nm with almost spherical shape | 0.01 nM | - | [116] |
| Poly A-split aptamer kanamycin | 25.3 nm | 20.58 nM | High stable | [117] | |
| - | 13 nm with spherical shape | 4 nM | Stable | [118] | |
| Amoxicillin | Aptamer and Tris-HCl buffer | 13 nm with spherical shape | 0.067 nM | Well stable | [119] |
| Gentamicin | Lysine | 13 nm | 1.22 nM | Stable | [120] |
| Chloram-phenicol and tetracycline | - | 12 nm with spherical shape | 7 nM and 32.9 nM | Stable | [121] |
| Azlocillin | - | 13 nm | 11.6 nM | Stable | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusuma, S.A.F.; Harmonis, J.A.; Pratiwi, R.; Hasanah, A.N. Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors 2023, 23, 8172. https://doi.org/10.3390/s23198172
Kusuma SAF, Harmonis JA, Pratiwi R, Hasanah AN. Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors. 2023; 23(19):8172. https://doi.org/10.3390/s23198172
Chicago/Turabian StyleKusuma, Sri Agung Fitri, Jacko Abiwaqash Harmonis, Rimadani Pratiwi, and Aliya Nur Hasanah. 2023. "Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules" Sensors 23, no. 19: 8172. https://doi.org/10.3390/s23198172
APA StyleKusuma, S. A. F., Harmonis, J. A., Pratiwi, R., & Hasanah, A. N. (2023). Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors, 23(19), 8172. https://doi.org/10.3390/s23198172






