Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors
Abstract
:1. Introduction
2. Polymer Field-Effect Transistors (PFETs)
2.1. Classification and Working Mechanism of PFETs
2.2. Conjugated Polymers
2.3. Crystallisation and Orientation Regulation
3. Application of P3HT in Gas Detection
3.1. P3HT Nanowire Structure in Gas Detection
3.1.1. Preparation of P3HT Nanowires by Photoinduction and Solvent Evaporation

3.1.2. P3HT Nanowires Prepared by External Environment Control Method
3.2. P3HT Nanoporous Structure in Gas Detection
3.2.1. P3HT Nanoporous Structures Prepared by Photolithography and Spin Coating
3.2.2. The Pore Structure of P3HT Prepared by Respiration Pattern and Doping Method
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [PubMed]
- Hou, S.; Fan, H.; Wu, M.; Yu, X.; Yu, J. Microstructure control of organic semiconductors via UV-ozone for high-sensitivity NO2 detection. Sci. China Technol. Sci. 2021, 64, 1057–1064. [Google Scholar]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 7, 4471–4568. [Google Scholar]
- Rahmanudin, A.; Tate, D.J.; Marcial-Hernandez, R.; Bull, N.; Garlapati, S.K.; Zamhuri, A.; Khan, R.U.; Faraji, S.; Gollu, S.R.; Persaud, K.C.; et al. Robust high-capacitance polymer gate dielectrics for stable low-voltage organic field-effect transistor sensors. Adv. Bioelectron. Mater. 2020, 31, 2212–2240. [Google Scholar]
- Sun, S.; Salim, T.; Wong, L.H.; Foo, Y.L.; Boey, F.; Lam, Y.M. A new insight into controlling poly(3-hexylthiophene) nanofiber growth through a mixed-solvent approach for organic photovoltaics applications. J. Mater. Chem. 2011, 21, 377–386. [Google Scholar]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic gas removal-metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 541930. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Tsao, H.N.; Feng, X.; Müllen, K. Patterned graphene electrodes from solution-processed graphite oxide films for organic field-effect transistors. Adv. Mater. 2009, 21, 3488–3491. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar]
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 594462. [Google Scholar]
- Li, H.; Shi, W.; Song, J.; Jang, H.J.; Dailey, J.; Yu, J.; Katz, H.E. Chemical and biomolecule sensing with organic field-effect transistors. Chem. Rev. 2019, 119, 3–35. [Google Scholar]
- Zhang, S.; Zhao, Y.; Du, X.; Chu, Y.; Zhang, S.; Huang, J. Gas sensors based on nano/microstructured organic field-effect transistors. Small 2019, 15, e1805196. [Google Scholar] [PubMed]
- Kim, J.; Kweon, H.; Park, H.W.; Go, P.; Hwang, H.; Lee, J.; Choi, S.J.; Kim, D.H. Interpenetrating polymer semiconductor nanonetwork channel for ultrasensitive, selective, and fast recovered chemodetection. ACS Appl. Mater. Interfaces 2020, 12, 55107. [Google Scholar] [PubMed]
- Yang, Y.; Liu, Z.; Chen, L.; Yao, J.; Lin, G.; Zhang, X.; Zhang, G.; Zhang, D. Conjugated semiconducting polymer with thymine groups in the side chains: Charge mobility enhancement and application for selective field-effect transistor sensors toward CO and H2S. Chem. Mater. 2019, 31, 1800–1807. [Google Scholar] [CrossRef]
- Lu, C.F.; Liao, S.F.; Chen, I.F.; Chen, C.T.; Chao, C.Y.; Su, W.F. Detecting minute chemical vapors via chemical interactions between analyte and fluorinated thiophene-isoindigo conjugated polymer transistor. ACS Appl. Electron. Mater. 2019, 1, 1873–1880. [Google Scholar] [CrossRef]
- Kwon, E.H.; Kim, M.; Lee, C.Y.; Kim, M.; Park, Y.D. Metal-organic-framework-decorated carbon nanofibers with enhanced gas sensitivity when incorporated into an organic semiconductor-based gas sensor. ACS Appl. Mater. Interfaces 2022, 14, 10637–10647. [Google Scholar] [CrossRef] [PubMed]
- Cranston, R.R.; Vebber, M.C.; Berbigier, J.F.; Rice, N.A.; Tonnele, C.; Comeau, Z.J.; Boileau, N.T.; Brusso, J.L.; Shuhendler, A.J.; Castet, F.; et al. Thin-film engineering of solution-processable n-type silicon phthalocyanines for organic thin-film transistors. ACS Appl. Mater. Interfaces 2021, 13, 1008–1020. [Google Scholar]
- Liu, Q.; Kanahashi, K.; Matsuki, K.; Manzhos, S.; Feron, K.; Bottle, S.E.; Tanaka, K.; Nanseki, T.; Takenobu, T.; Tanaka, H.; et al. Triethylene glycol substituted diketopyrrolopyrrole and isoindigo-dye based donor-acceptor copolymers for organic light-emitting electrochemical cells and transistors. Adv. Electron. Mater. 2020, 6, 1901414. [Google Scholar]
- Yin, X.; Yang, J.; Wang, H. Vertical phase separation structure for high-performance organic thin-film transistors: Mechanism, optimization strategy, and large-area fabrication toward flexible and stretchable electronics. Adv. Funct. Mater. 2022, 32, 22020. [Google Scholar]
- Kanimozhi, C.; Yaacobi-Gross, N.; Chou, K.W.; Amassian, A.; Anthopoulos, T.D.; Patil, S. Diketopyrrolopyrrole-diketopyrrolopyrrole-based conjugated copolymer for high-mobility organic field-effect transistors. J. Am. Chem. Soc. 2012, 134, 16532–16535. [Google Scholar]
- Yao, Y.; Zhang, L.; Leydecker, T.; Samori, P. Direct photolithography on molecular crystals for high performance organic optoelectronic devices. J. Am. Chem. Soc. 2018, 140, 6984–6990. [Google Scholar]
- Tao, J.; Liu, D.; Jing, J.; Dong, H.; Liu, L.; Xu, B.; Tian, W. Organic single crystals with high photoluminescence quantum yields close to 100% and high mobility for optoelectronic devices. Adv. Mater. 2021, 33, 2105466. [Google Scholar]
- Zhang, Q.; Lei, L.; Zhu, S. Gas-responsive polymers. ACS Macro Lett. 2017, 6, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, Y.; Yan, Q. Polymeric partners breathe together: Using gas to direct polymer self-assembly via gas-bridging chemistry. Sci. China Chem. 2022, 65, 1401–1410. [Google Scholar]
- Porrazzo, R.; Bellani, S.; Luzio, A.; Lanzarini, E.; Caironi, M.; Antognazza, M.R. Improving mobility and electrochemical stability of a water-gated polymer field-effect transistor. Org. Electron. 2014, 15, 2126–2134. [Google Scholar]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dotz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar]
- Besar, K.; Yang, S.; Guo, X.; Huang, W.; Rule, A.M.; Breysse, P.N.; Kymissis, I.J.; Katz, H.E. Printable ammonia sensor based on organic field effect transistor. Org. Electron. 2014, 15, 3221–3230. [Google Scholar]
- Wu, M.; Hou, S.; Yu, X.; Yu, J. Recent progress in chemical gas sensors based on organic thin film transistors. J. Mater. Chem. C 2020, 8, 13482–13500. [Google Scholar]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar]
- Yang, G.G.; Kim, D.H.; Samal, S.; Choi, J.; Roh, H.; Cunin, C.E.; Lee, H.M.; Kim, S.O.; Dincă, M.; Gumyusenge, A. Polymer-based thermally stable chemiresistive sensor for real-time monitoring of NO2 gas emission. ACS Sens. 2023. [Google Scholar] [CrossRef]
- Su, Y.W.; Lin, Y.C.; Wei, K.H. Evolving molecular architectures of donor-acceptor conjugated polymers for photovoltaic applications: From one-dimensional to branched to two-dimensional structures. J. Mater. Chem. A 2017, 5, 24051–24075. [Google Scholar]
- Samitsu, S.; Shimomura, T.; Heike, S.; Hashizume, T.; Ito, K. Field-effect carrier transport in poly(3-alkylthiophene) nanofiber networks and isolated nanofibers. Macromolecules 2010, 43, 7891–7894. [Google Scholar]
- Guo, Y.; Jiang, L.; Ma, X.; Hu, W.; Su, Z. Poly(3-hexylthiophene) monolayer nanowhiskers. Polym. Chem. 2013, 4, 4308. [Google Scholar]
- Aiyar, A.R.; Hong, J.I.; Nambiar, R.; Collard, D.M.; Reichmanis, E. Tunable crystallinity in regioregular poly(3-hexylthiophene) thin films and its impact on field effect mobility. Adv. Funct. Mater. 2011, 21, 2652–2659. [Google Scholar]
- Chang, M.; Lee, J.; Kleinhenz, N.; Fu, B.; Reichmanis, E. Photoinduced anisotropic supramolecular assembly and enhanced charge transport of poly(3-hexylthiophene) thin films. Adv. Funct. Mater. 2014, 24, 4457–4469. [Google Scholar] [CrossRef]
- Lu, J.; Liu, D.; Zhou, J.; Chu, Y.; Chen, Y.; Wu, X.; Huang, J. Porous organic field-effect transistors for enhanced chemical sensing performances. Adv. Funct. Mater. 2017, 27, 1700018. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.; Zhou, Y.; Huang, J.; Wu, M.; Jiang, Y.; Tai, H. Thin film transistors gas sensors based on reduced graphene oxide poly(3-hexylthiophene) bilayer film for nitrogen dioxide detection. Chem. Phys. Lett. 2014, 614, 275–281. [Google Scholar] [CrossRef]
- Pernites, R.B.; Foster, E.L.; Felipe, M.J.; Robinson, M.; Advincula, R.C. Patterned surfaces combining polymer brushes and conducting polymer via colloidal template electropolymerization. Adv. Mater. 2011, 23, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kappl, M.; Liebewirth, I.; Muller, M.; Kirchhoff, K.; Pisula, W.; Mullen, K. Organic field-effect transistors based on highly ordered single polymer fibers. Adv. Mater. 2012, 24, 417–420. [Google Scholar] [PubMed]
- Park, T.; Lee, S.; Kang, M.; Yu, S.H.; Nam, G.H.; Sim, K.M.; Chung, D.S. Nanowire-embedded polymer photomultiplication photodiode with EQE over 250,000%. Chem. Eng. J. 2021, 21, 1385–4187. [Google Scholar]
- Flagg, L.Q.; Bischak, C.G.; Onorato, J.W.; Rashid, R.B.; Luscombe, C.K.; Ginger, D.S. Polymer crystallinity controls water uptake in glycol side-chain polymer organic electrochemical transistors. J. Am. Chem. Soc. 2019, 141, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jung, H.T.; Ahn, C.W.; Jeon, H.J. Simultaneously induced self-assembly of poly(3-hexylthiophene) (P3HT) nanowires and thin-film fabrication via solution-floating method on a water substrate. Adv. Mater. Interfaces 2017, 4, 1700342. [Google Scholar]
- Nawrocki, R.A.; Pavlica, E.; Ćelić, N.; Orlov, D.; Valant, M.; Mihailović, D.; Bratina, G. Fabrication of poly(3-hexylthiophene) nanowires for high-mobility transistors. Org. Electron. 2016, 30, 92–98. [Google Scholar]
- Qiu, L.; Lee, W.H.; Wang, X.; Kim, J.S.; Lim, J.A.; Kwak, D.; Lee, S.; Cho, K. Organic thin-film transistors based on polythiophene nanowires embedded in insulating polymer. Adv. Mater. 2009, 21, 1349–1353. [Google Scholar]
- Rawlings, D.; Thomas, E.M.; Segalman, R.A.; Chabinyc, M.L. Controlling the doping mechanism in poly(3-hexylthiophene) thin-film transistors with polymeric ionic liquid dielectrics. Chem. Mater. 2019, 31, 8820–8829. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B 2016, 225, 10–15. [Google Scholar]
- Khim, D.; Ryu, G.S.; Park, W.T.; Kim, H.; Lee, M.; Noh, Y.Y. Precisely controlled ultrathin conjugated polymer films for large area transparent transistors and highly sensitive chemical sensors. Adv. Mater. 2016, 28, 2752–2759. [Google Scholar] [PubMed]
- Giridharagopal, R.; Guo, J.; Kong, J.; Ginger, D.S. Nanowire architectures improve ion uptake kinetics in conjugated polymer electrochemical transistors. ACS Appl. Mater. Interfaces 2021, 13, 34616–34624. [Google Scholar]
- Mun, S.; Park, Y.; Lee, Y.K.; Sung, M.M. Highly sensitive ammonia gas sensor based on single-crystal poly(3-hexylthiophene) (P3HT) organic field effect transistor. Langmuir 2017, 33, 13554–13560. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331. [Google Scholar]
- Tang, K.; Huang, L.; Lim, J.; Zaveri, T.; Azoulay, J.D.; Guo, S. Chemical doping of well-dispersed P3HT thin-film nanowire networks. ACS Appl. Polym. Mater. 2019, 11, 2943–2950. [Google Scholar] [CrossRef]
- Bastianini, F.; Pérez, G.E.; Hobson, A.R.; Rogers, S.E.; Parnell, A.J.; Grell, M.; Gutiérrez, A.F.; Dunbar, A.D.F. In-situ monitoring poly(3-hexylthiophene) nanowire formation and shape evolution in solution via small angle neutron scattering. Sol. Energy Mater. Sol. Cells 2019, 258, 110128. [Google Scholar]
- Shin, S.Y.; Jang, M.; Cheon, H.J.; Go, S.; Yoon, H.; Chang, M. Nanostructure-assisted solvent vapor annealing of conjugated polymer thin films for enhanced performance in volatile organic compound sensing. Sens. Actuators B 2022, 351, 130951. [Google Scholar]
- Tchutchulashvili, G.; Korona, K.P.; Mech, W.; Chusnutdinow, S.; Sobanska, M.; Klosek, K.; Zytkiewicz, Z.R.; Sadowski, W. Hybrid P3HT: PCBM/GaN nanowire/Si cascade heterojunction for photovoltaic application. J. Nanopart. Res. 2020, 22, 84. [Google Scholar]
- Kuo, C.G.; Chen, J.H.; Chao, Y.C.; Chen, P.L. Fabrication of a P3HT-ZnO nanowires gas sensor detecting ammonia gas. Sensors 2017, 18, 37. [Google Scholar] [CrossRef]
- Hart, A.S.; Andersen, T.R.; Griffith, M.J.; Fahy, A.; Vaughan, B.; Belcher, W.J.; Dastoor, P.C. Roll-to-roll solvent annealing of printed P3HT: IC(X)A devices. RSC Adv. 2019, 9, 42294. [Google Scholar]
- Jeong, G.; Cheon, H.J.; Shin, S.Y.; Wi, E.; Kyokunzire, P.; Cheon, H.; Van Tran, V.; Vu, T.T.; Chang, M. Improved NO2 gas sensing performance of nanoporous conjugated polymer (CP) thin films by incorporating preformed CP nanowires. Dyes Pigm. 2023, 214, 111235. [Google Scholar]
- Yu, S.H.; Girma, H.G.; Sim, K.M.; Yoon, S.; Park, J.M.; Kong, H.; Chung, D.S. Polymer-based flexible NO(x) sensors with ppb-level detection at room temperature using breath-figure molding. Nanoscale 2019, 11, 17709. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, G.; Mohammadi, E.; Mei, J.; Diao, Y. Solution-processed nanoporous organic semiconductor thin films: Toward health and environmental monitoring of volatile markers. Adv. Funct. Mater. 2017, 27, 1701117. [Google Scholar] [CrossRef]
- Boujnah, A.; Boubaker, A.; Pecqueur, S.; Lmimouni, K.; Kalboussi, A. An electronic nose using conductometric gas sensors based on P3HT doped with triflates for gas detection using computational techniques (PCA, LDA, and KNN). J. Mater. Sci. Mater. Electron. 2022, 33, 27132–27146. [Google Scholar]
- Wang, Y.; Zhang, J.; Zhang, S.; Huang, J. OFET chemical sensors: Chemical sensors based on ultrathin organic field-effect transistors. Polym. Int. 2020, 70, 414–425. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, W.; Li, L.; Li, H.; Huang, J.; Cheng, J.; Fu, Y. Research progress of organic field-effect transistor based chemical sensors. Small 2023, 4, 2302406. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro-and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar]
- Zhang, C.; Chen, P.; Hu, W. Organic field-effect transistor-based gas sensors. Chem. Soc. Rev. 2015, 44, 2087–2107. [Google Scholar]
- Cao, Z.; Huo, X.; Ma, Q.; Song, J.; Pan, Q.; Chen, L.; Lai, J.; Shan, X.; Gao, J. TFT-CN/P3HT blending active layer based two-component organic field-effect transistor for improved H2S gas detection. Sens. Actuators B 2023, 385, 133685. [Google Scholar]
- Guo, X.; Liu, L.; Zhuang, Z.; Chen, X.; Ni, M.; Li, Y.; Cui, Y.; Zhan, P.; Yuan, C.; Ge, H.; et al. A new strategy of lithography based on phase separation of polymer blends. Sci. Rep. 2015, 5, 15947. [Google Scholar] [CrossRef]
- Hulkkonen, H.H.; Salminen, T.; Niemi, T. Block copolymer patterning for creating porous silicon thin films with tunable refractive indices. ACS Appl. Mater. Interfaces 2017, 9, 31260–31265. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Zhu, X.; Zhou, X.; Chi, L. An ultrasensitive organic semiconductor NO2 sensor based on crystalline TIPS-pentacene films. Adv. Mater. 2017, 29, 1703192. [Google Scholar] [CrossRef]
- Darshan, V.; Rajeev, V.R.; Unni, K.N.N. Enhanced performance of room temperature ammonia sensors using morphology-controlled organic field-effect transistors. Org. Electron. 2021, 98, 106280. [Google Scholar]
- Tran, V.V.; Jeong, G.; Kim, K.S.; Kim, J.; Jung, H.R.; Park, B.; Park, J.J.; Chang, M. Facile strategy for modulating the nanoporous structure of ultrathin π-conjugated polymer films for high-performance gas sensors. ACS Sens. 2022, 7, 175–185. [Google Scholar] [CrossRef]
- Bertoni, C.; Naclerio, P.; Viviani, E.; Dal Zilio, S.; Carrato, S.; Fraleoni-Morgera, A. Nanostructured P3HT as a promising sensingelement for real-time, dynamic detection of gaseous acetone. Sensors 2019, 19, 1296. [Google Scholar] [CrossRef]
- Hou, S.; Yu, J.; Zhuang, X.; Li, D.; Liu, Y.; Gao, Z.; Sun, T.; Wang, F.; Yu, X. Phase separation of P3HT/PMMA blend film for forming semiconducting and dielectric layers in organic thin-film transistors for high-sensitivity NO2 detection. ACS Appl. Mater. Interfaces 2019, 11, 44521–44527. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Chen, J.N.; Zan, H.W.; Lu, C.J.; Meng, H.F. Modulated gas sensor based on vertical organic diode with blended channel for ppb-regime detection. Sens. Actuators B 2016, 230, 223–230. [Google Scholar]
- Shalu, C.; Shukla, M.; Tiwari, A.; Agrawal, J.; Bilgaiyan, A.; Singh, V. Role of solvent used to cast P3HT thin films on the performance of ZnO/P3HT hybrid photo detector. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 115, 113694. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yeh, P.S.; Hsu, Y.T.; Tong, Z.H.; Chiang, C.H. Effective control of solution self-assembly of P3HT/zinc salt complex for in situ template synthesis of P3HT/ZnO nanohybrids. Polymer 2021, 215, 123385. [Google Scholar]
- Liu, C.; Wu, M.; Gao, L.; Liu, H.; Yu, J. Nanoporous polymer films based on breath figure method for stretchable chemiresistive NO2 gas sensors. Sens. Actuators B 2022, 371, 132540. [Google Scholar] [CrossRef]
- Jeong, G.; Shin, S.Y.; Kyokunzire, P.; Cheon, H.J.; Wi, E.; Woo, M.; Chang, M. High-performance nitric oxide gas sensors based on an ultrathin nanoporous poly(3-hexylthiophene) film. Biosensors 2023, 13, 132. [Google Scholar]
- Lee, Y.H.; Jang, M.; Lee, M.Y.; Kweon, O.Y.; Oh, J.H. Flexible field-effect transistor-type sensors based on conjugated molecules. Chem 2017, 35, 724–763. [Google Scholar]
- Sun, C.; Wang, X.; Auwalu, M.A.; Cheng, S.; Hu, W. Organic thin film transistors-based biosensors. EcoMat 2021, 3, e12094. [Google Scholar]
- Sun, C.; Li, R.; Song, Y.; Jiang, X.; Zhang, C.; Cheng, S.; Hu, W. Ultrasensitive and reliable organic field-effect transistor-based biosensors in early liver cancer diagnosis. Anal. Chem. 2021, 93, 6188–6194. [Google Scholar] [CrossRef]
- Jang, D.; Park, S.Y.; Lee, H.S.; Park, Y.D. Low-regioregularity polythiophene for a highly sensitive and stretchable gas sensor. ACS Appl. Mater. Interfaces 2023, 15, 32629–32636. [Google Scholar] [CrossRef]
- Surya, S.G.; Raval, H.N.; Ahmad, R.; Sonar, P.; Salama, K.N.; Rao, V.R. Organic field effect transistors (OFETs) in environmental sensing and health monitoring: A review. TrAC Trends Anal. Chem. 2019, 111, 27–36. [Google Scholar]
- Lienerth, P.; Fall, S.; Lévêque, P.; Soysal, U.; Heiser, T. Improving the selectivity to polar vapors of OFET-based sensors by using the transfer characteristics hysteresis response. Sens. Actuators B 2016, 225, 90–95. [Google Scholar] [CrossRef]
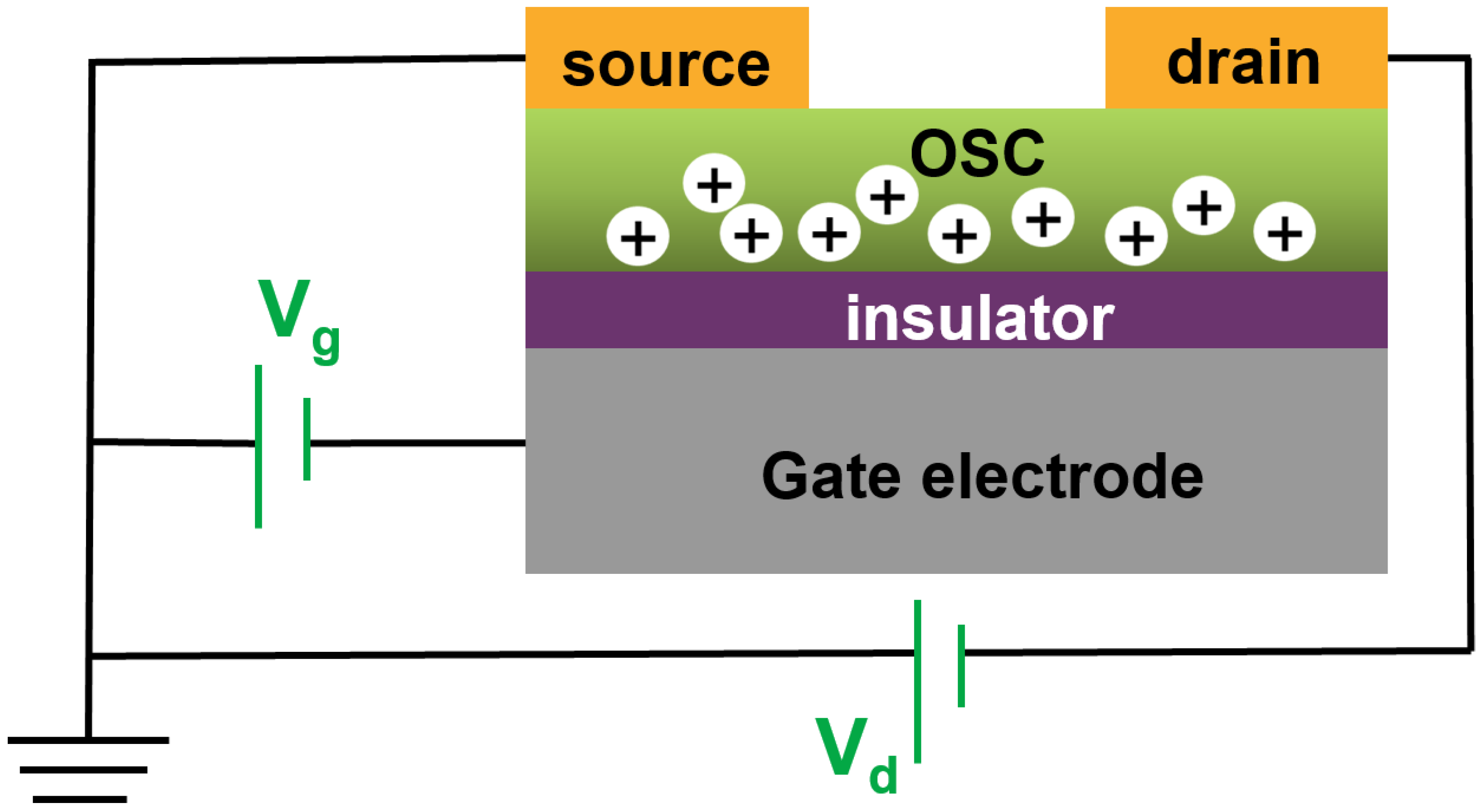



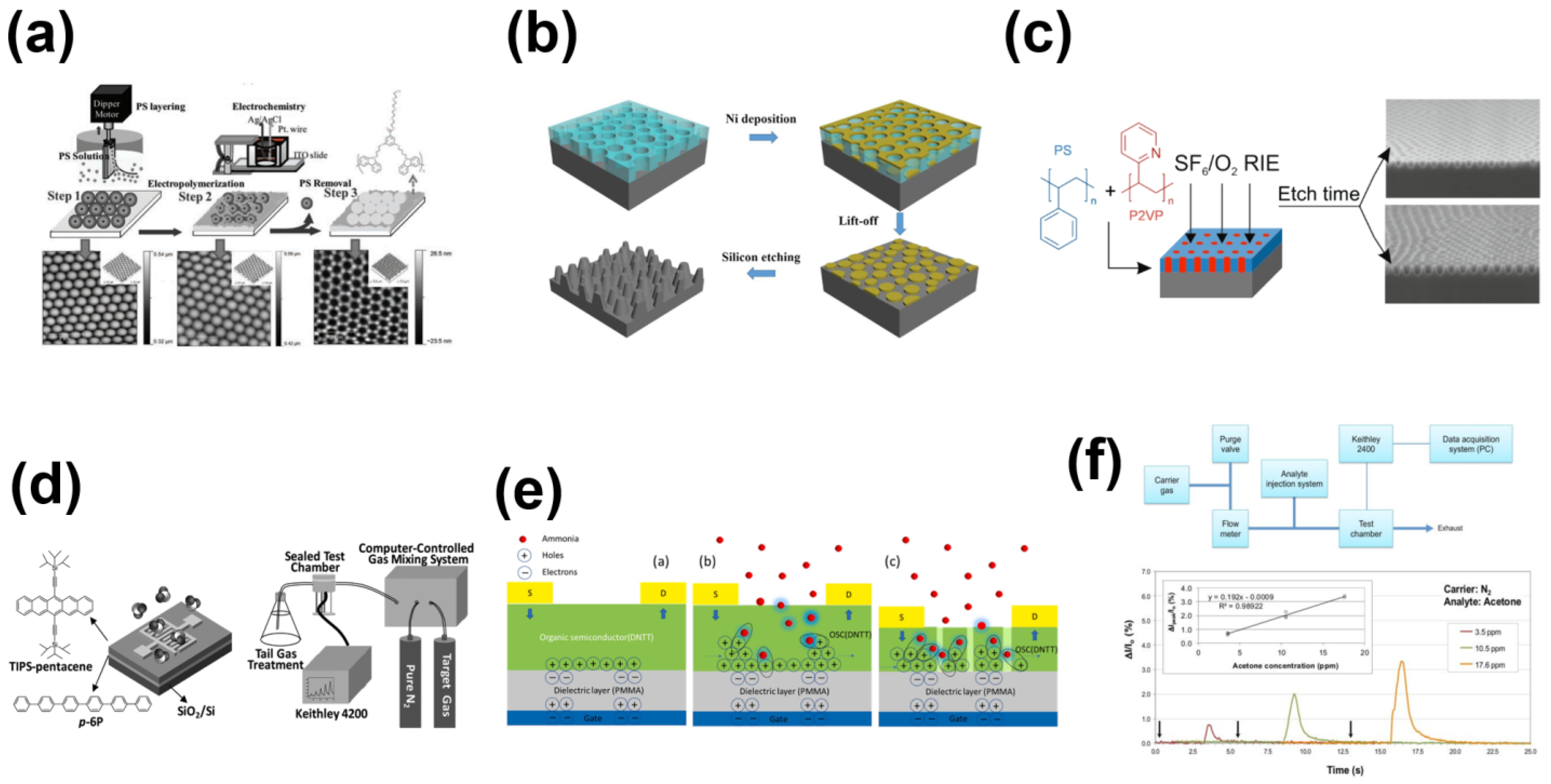

| Materials | Analytes | Property | Device | Reference |
|---|---|---|---|---|
| P3HT nanowires | NH3 | 5 ppm | BGTC | [44] |
| P3HT nanowires | NH3 | 25 ppm | BGTC | [47] |
| P3HT nanowires | NH3 | 11.58 ppm | BGTC | [53] |
| P3HT nanowires | NO2 | 38.2% | BGTC | [55] |
| P3HT nanopores | NH3 | 100 ppb | BGTC | [64] |
| P3HT nanopores | NH3 | 70.7% | TGTC | [65] |
| P3HT nanopores | Acetone | 3.5 pm | TGTC | [66] |
| P3HT nanopores | NO2 | 0.7 ppb | BGTC | [67] |
| P3HT nanopores | NO2 | 100 ppb | BGTC | [68] |
| P3HT nanopores | NH3 | 2.45 ppb | BGTC | [71] |
| P3HT nanopores | NO | 0.5 ppm | BGTC | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Wang, Y.; Zhang, R.; Wang, H.; Sun, C.; Wang, T. Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors. Sensors 2023, 23, 8309. https://doi.org/10.3390/s23198309
Cheng S, Wang Y, Zhang R, Wang H, Sun C, Wang T. Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors. Sensors. 2023; 23(19):8309. https://doi.org/10.3390/s23198309
Chicago/Turabian StyleCheng, Si, Yifan Wang, Ruishi Zhang, Hongjiao Wang, Chenfang Sun, and Tie Wang. 2023. "Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors" Sensors 23, no. 19: 8309. https://doi.org/10.3390/s23198309
APA StyleCheng, S., Wang, Y., Zhang, R., Wang, H., Sun, C., & Wang, T. (2023). Recent Progress in Gas Sensors Based on P3HT Polymer Field-Effect Transistors. Sensors, 23(19), 8309. https://doi.org/10.3390/s23198309







