ESPRESS.0: Eustachian Tube-Inspired Tactile Sensor Exploiting Pneumatics for Range Extension and SenSitivity Tuning
Abstract
:1. Introduction
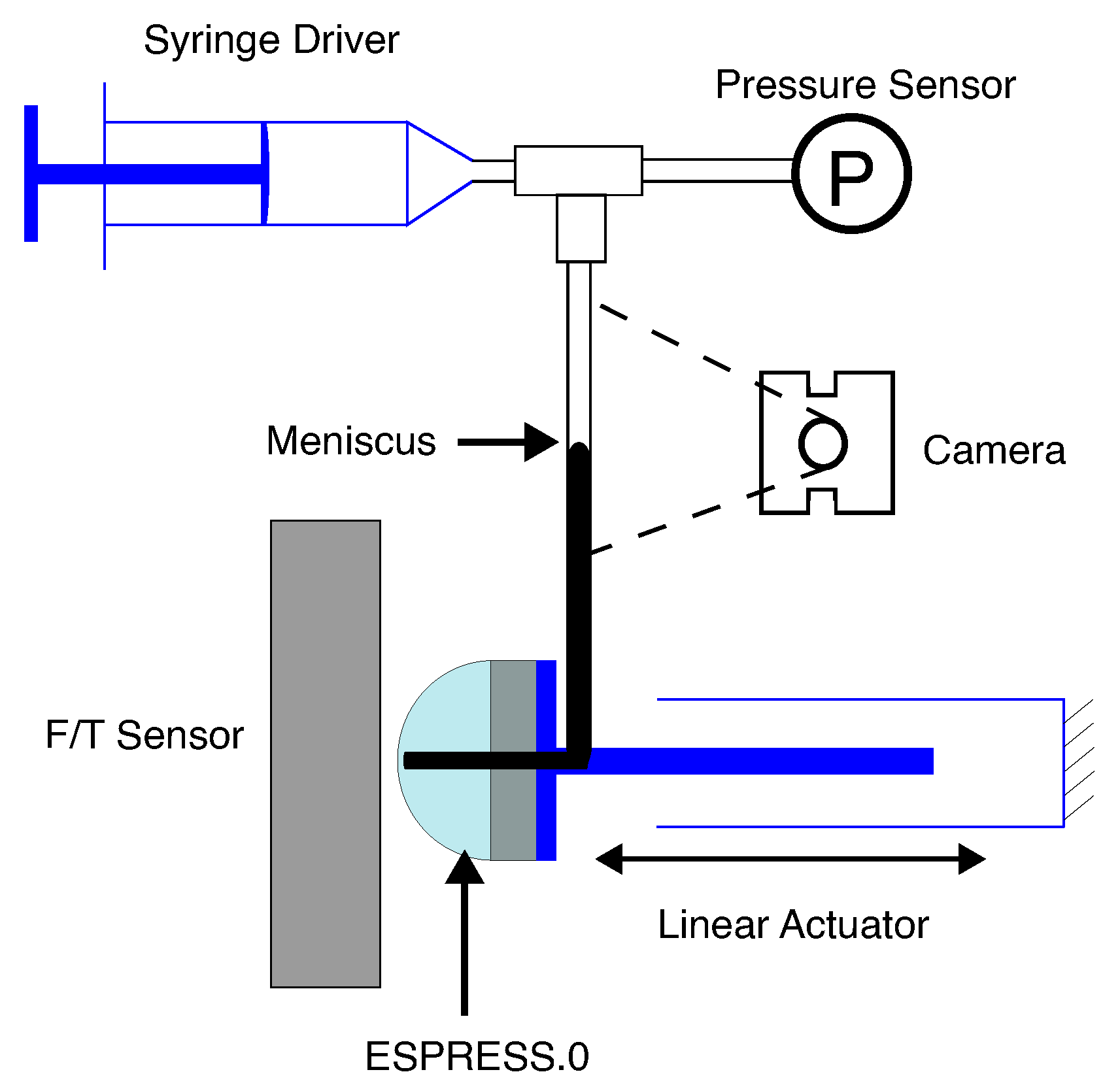
2. Methods
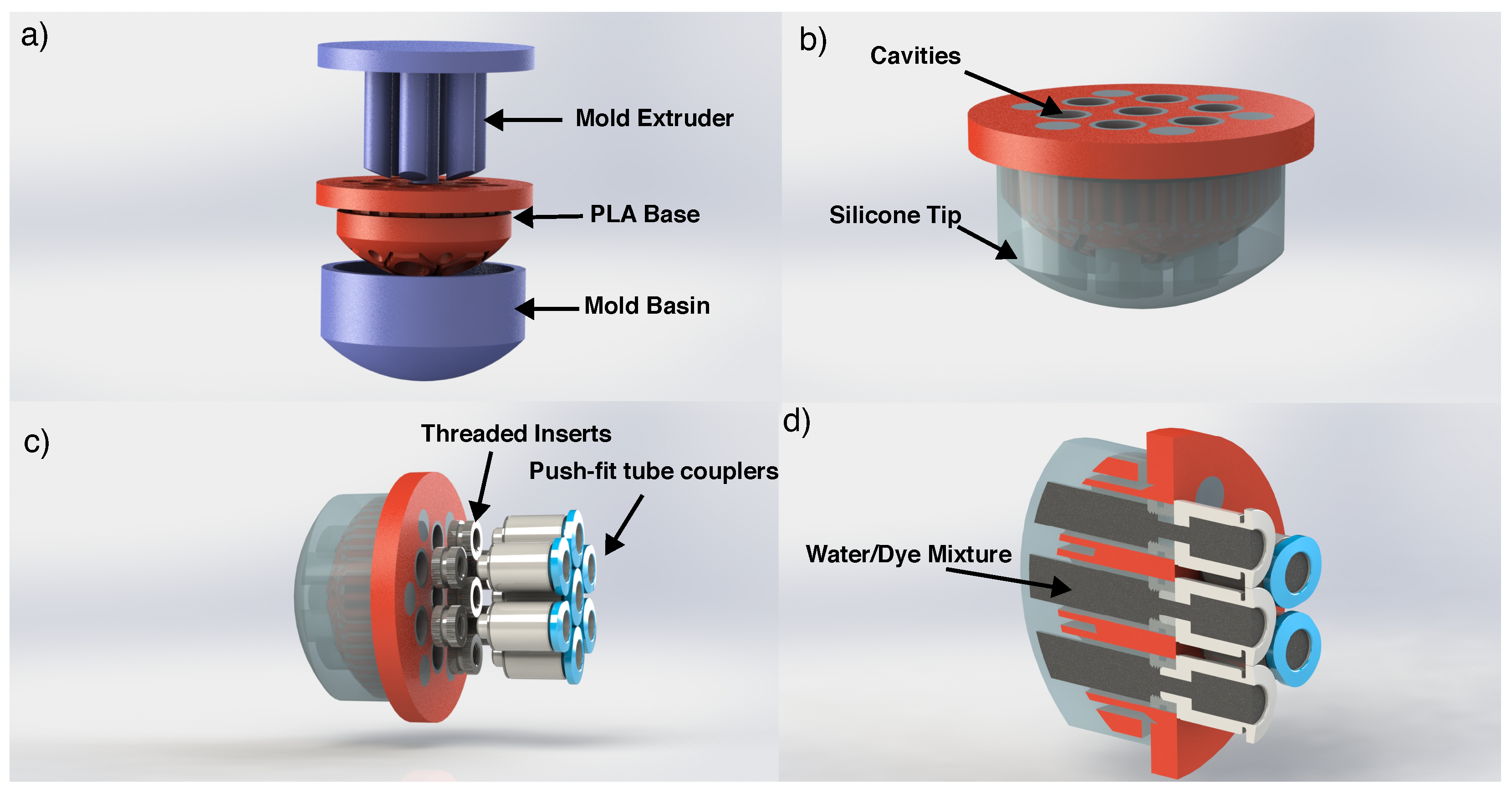
2.1. Design and Fabrication
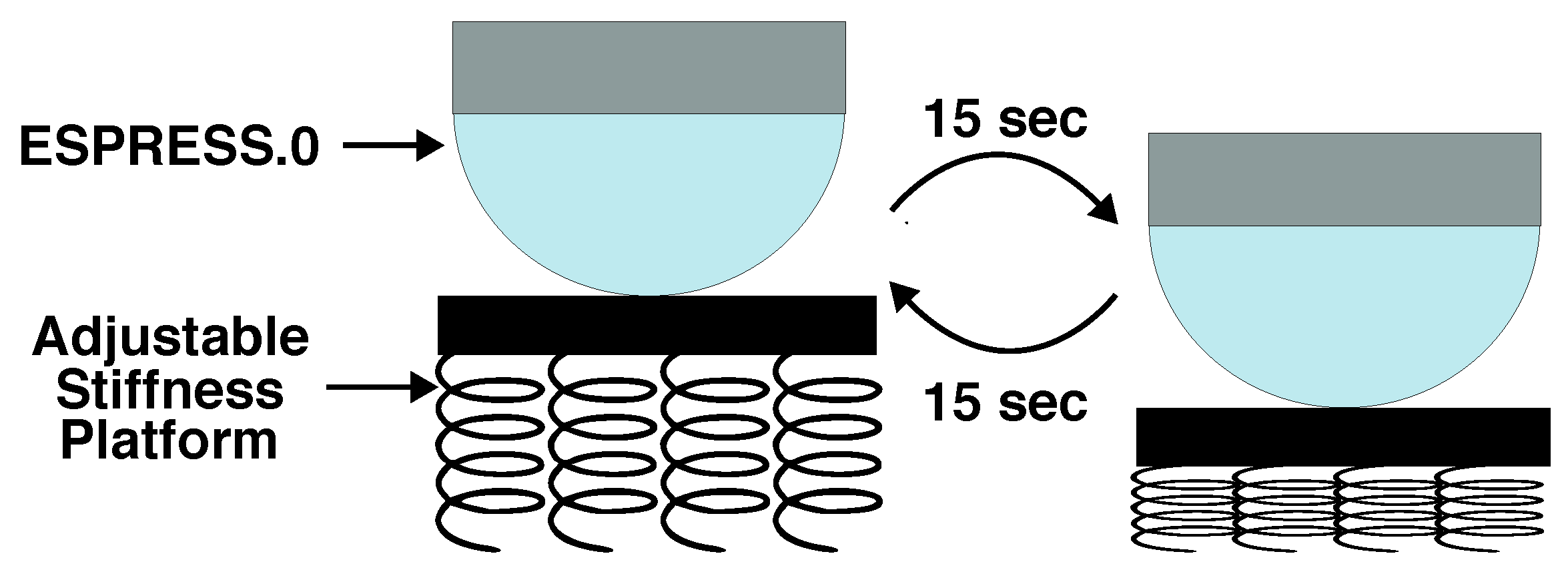
2.1.1. ESPRESS.0
2.1.2. Untethered Version
2.2. Image Processing
3. Characterisation and Modelling
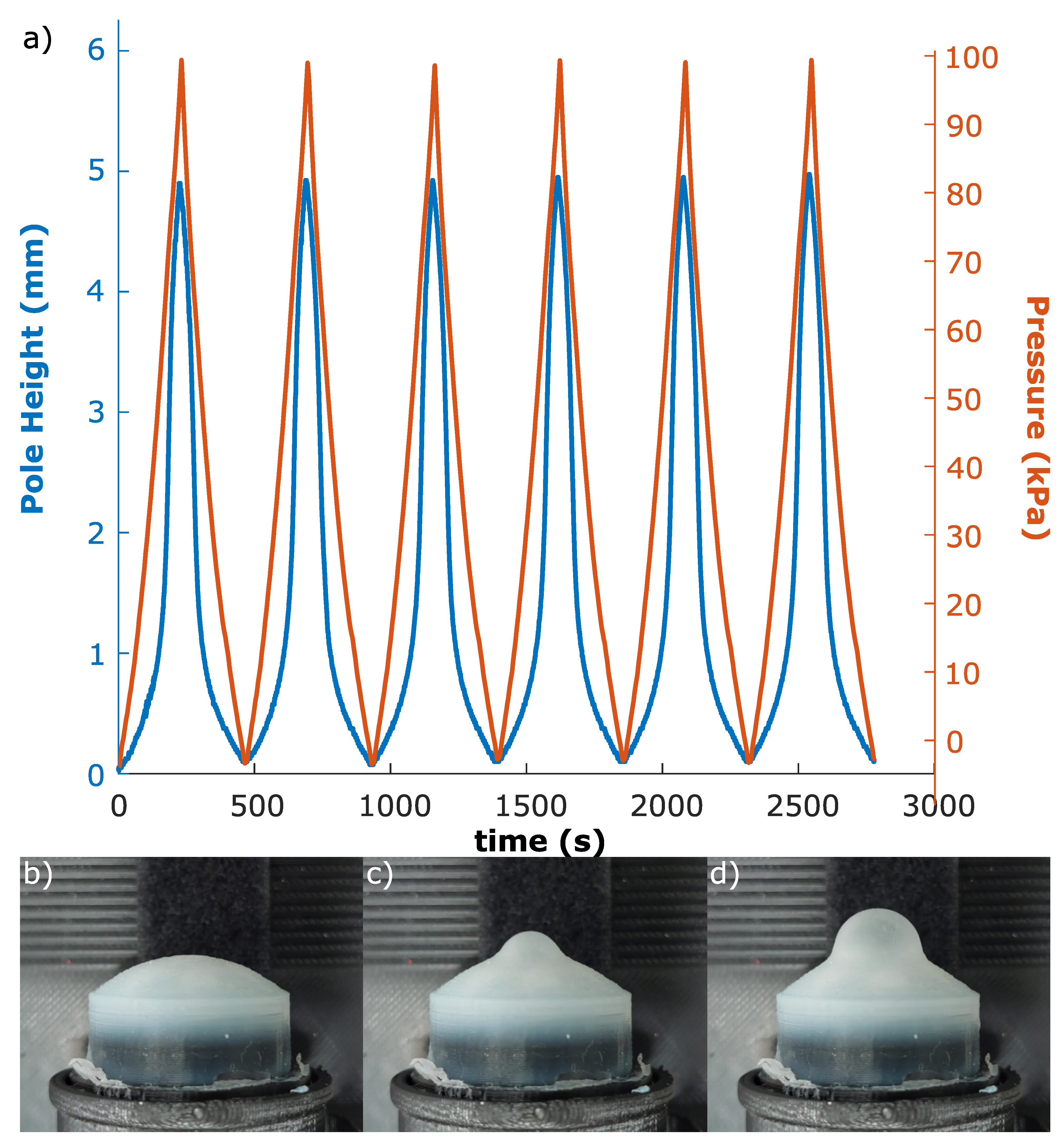
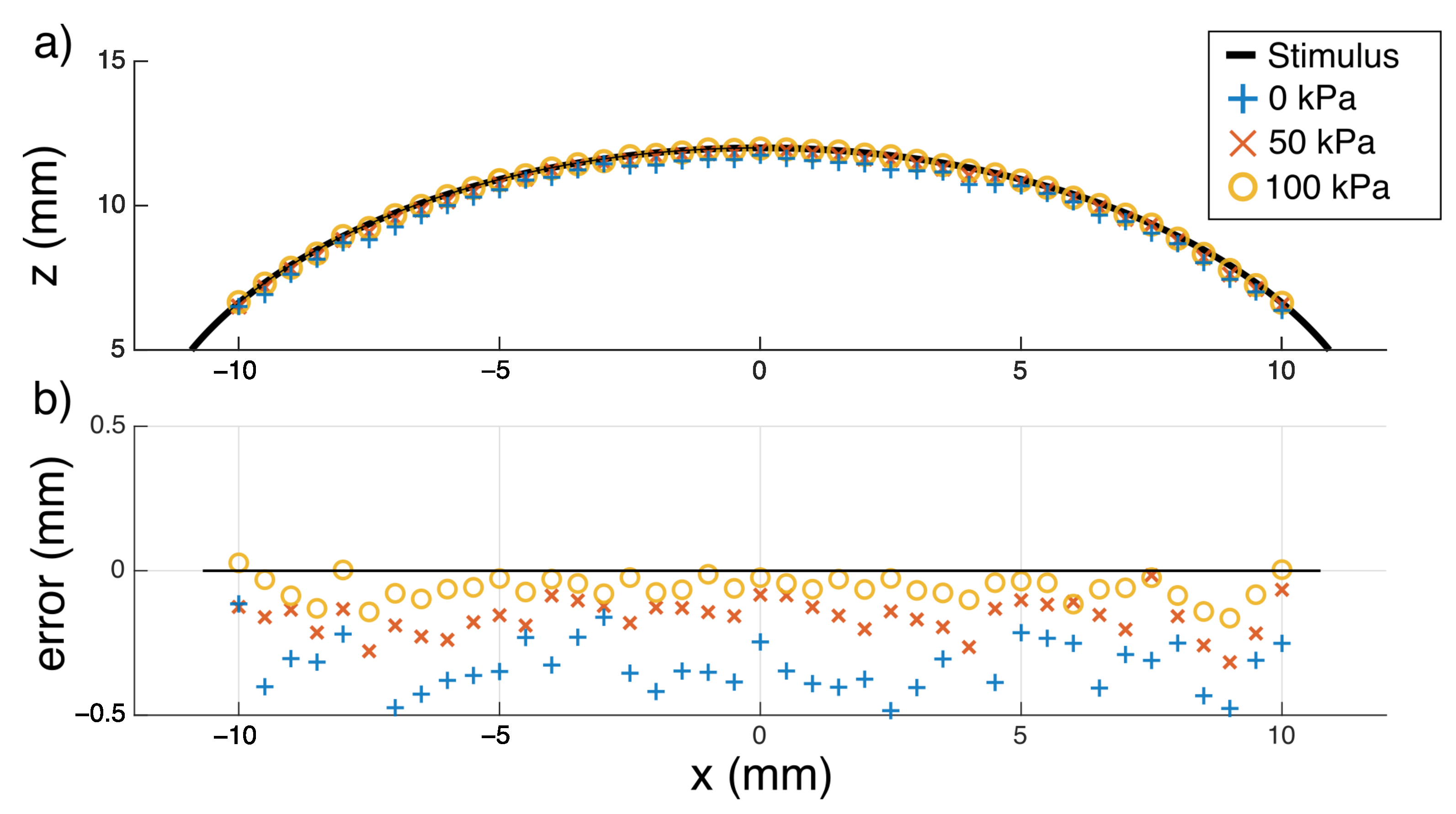
3.1. Mechanical Impression
3.2. Sensitivity Recovery
3.3. Noise Characterisation
3.4. Mechanical Modelling of Membrane
4. Experimental Evaluation
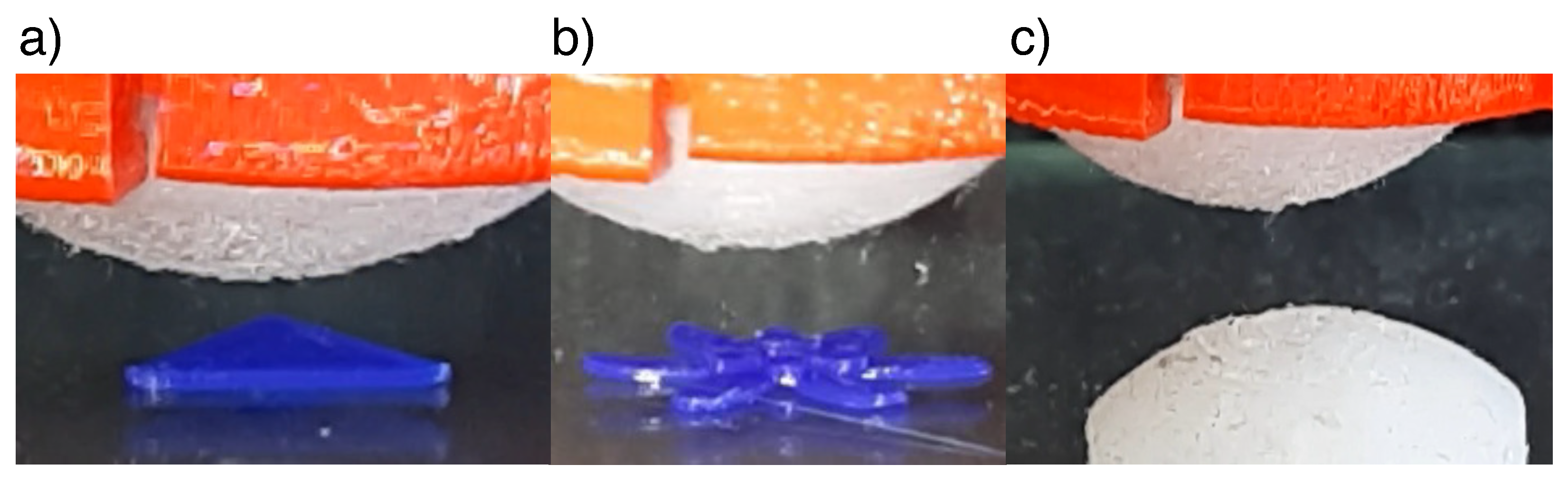
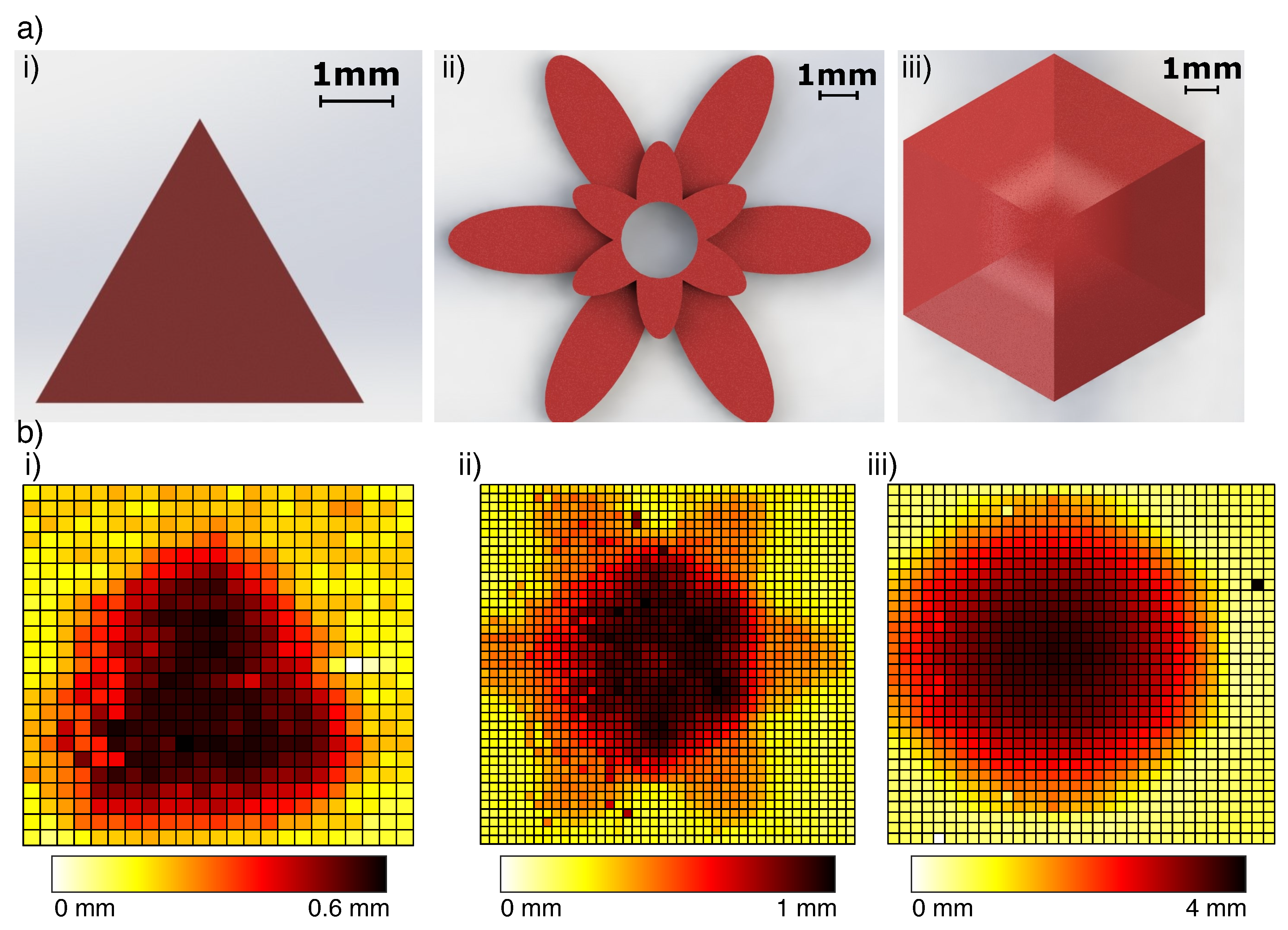
4.1. Shape Reconstruction
4.2. Stiffness Classification
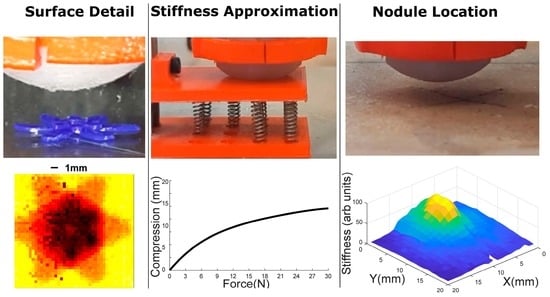
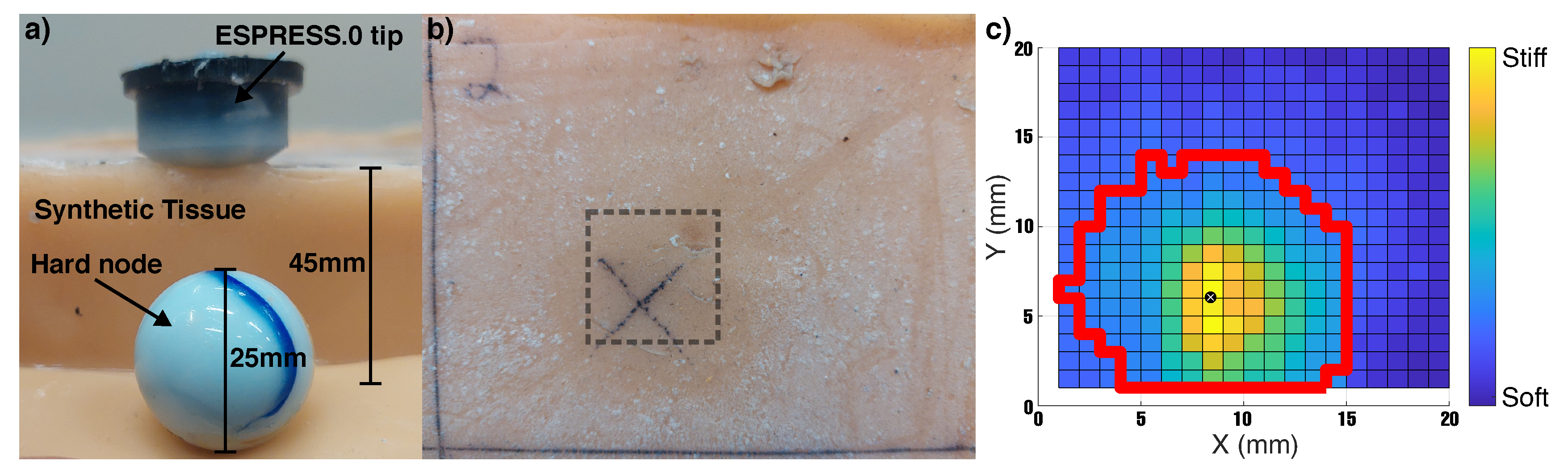
4.3. 3D Tactile Map of Synthetic Tissue
5. Discussion
5.1. Shape Reconstruction and Super Resolution
5.2. Stiffness Classification
5.3. 3D Tactile Map of Synthetic Tissue
5.4. Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogan, N. Impedance Control An Approach to Manipulation Part 1—Theory. J. Dyn. Syst. Meas. Control. Trans. ASME 1985, 107, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Sornkarn, N.; Dasgupta, P.; Nanayakkara, T. Morphological computation of haptic perception of a controllable stiffness probe. PLoS ONE 2016, 11, e0156982. [Google Scholar] [CrossRef] [Green Version]
- Gwilliam, J.C.; Pezzementi, Z.; Jantho, E.; Okamura, A.M.; Hsiao, S. Human vs. robotic tactile sensing: Detecting lumps in soft tissue. In Proceedings of the 2010 IEEE Haptics Symposium, HAPTICS 2010, Waltham, MA, USA, 25–26 March 2010; pp. 21–28. [Google Scholar] [CrossRef] [Green Version]
- Karadogan, E.; Williams, R.L.; Howell, J.N.; Conatser, R.R. A stiffness discrimination experiment including analysis of palpation forces and velocities. Simul. Healthc. 2010, 5, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Sen, S.; Kapadia, R.; Jen, Y.; McKinley, S.; Miller, L.; Goldberg, K. Tumor localization using automated palpation with Gaussian Process Adaptive Sampling. In Proceedings of the IEEE International Conference on Automation Science and Engineering, Fort Worth, TX, USA, 21–25 August 2016; pp. 194–200. [Google Scholar] [CrossRef]
- Nichols, K.A.; Okamura, A.M. Autonomous robotic palpation: Machine learning techniques to identify hard inclusions in soft tissues. In Proceedings of the IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 4384–4389. [Google Scholar] [CrossRef]
- Yamamoto, T.; Vagvolgyi, B.; Balaji, K.; Whitcomb, L.L.; Okamura, A.M. Tissue property estimation and graphical display for teleoperated robot-assisted surgery. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 4239–4245. [Google Scholar] [CrossRef]
- Bishara, A.M.; Succi, M.D.; Hadiwidjana, F.P.W.; Ezra, E. Method and Apparatus for Medical Diagnosis Based on the Tissue Stiffness. U.S. Patent US20180000348A1, 4 January 2018. [Google Scholar]
- Pacchierotti, C.; Prattichizzo, D.; Kuchenbecker, K.J. Cutaneous feedback of fingertip deformation and vibration for palpation in robotic surgery. IEEE Trans. Biomed. Eng. 2016, 63, 278–287. [Google Scholar] [CrossRef]
- Trejos, A.L.; Jayender, J.; Perri, M.T.; Naish, M.D.; Patel, R.V.; Malthaner, R.A. Robot-assisted tactile sensing for minimally invasive tumor localization. Int. J. Robot. Res. 2009, 28, 1118–1133. [Google Scholar] [CrossRef]
- Kato, I.; Koganezawa, K.; Fujimoto, H.; Hirata, M. The Automatic Breast-cancer Palpation Robot: Wapro-4r. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Tokyo, Japan, 31 October–2 November 1988; pp. 73–78. [Google Scholar] [CrossRef]
- Lepora, N.F.; Aquilina, K.; Cramphorn, L. Exploratory Tactile Servoing with Active Touch. IEEE Robot. Autom. Lett. 2017, 2, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Abu-Dakka, F.J.; Saveriano, M. Variable Impedance Control and Learning—A Review. Front. Robot. AI 2020, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Gubenko, M.M.; Morozov, A.V.; Lyubicheva, A.N.; Goryacheva, I.G.; Dosaev, M.Z.; Ju, M.S.; Yeh, C.H.; Su, F.C. Video-tactile pneumatic sensor for soft tissue elastic modulus estimation. BioMed. Eng. Online 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Kow, J.; Jones, D.; de Boer, G.; Ghanbari, A.; Serjouei, A.; Culmer, P.; Alazmani, A. Adjustable compliance soft sensor via an elastically inflatable fluidic dome. Sensors 2021, 21, 1970. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.; Jenkinson, G.P.; Tzemanaki, A. Optical-Tactile Sensor for Lump Detection Using Pneumatic Control. Front. Robot. AI 2021, 8, 672315. [Google Scholar] [CrossRef] [PubMed]
- McInroe, B.W.; Chen, C.L.; Goldberg, K.Y.; Bajcsy, R.; Fearing, R.S. Towards a Soft Fingertip with Integrated Sensing and Actuation. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Madrid, Spain, 1–5 October 2018; pp. 6437–6444. [Google Scholar] [CrossRef]
- He, L.; Lu, Q.; Abad, S.A.; Rojas, N.; Nanayakkara, T. Soft Fingertips with Tactile Sensing and Active Deformation for Robust Grasping of Delicate Objects. IEEE Robot. Autom. Lett. 2020, 5, 2714–2721. [Google Scholar] [CrossRef] [Green Version]
- Herzig, N.; He, L.; Maiolino, P.; Abad, S.A.; Nanayakkara, T. Conditioned haptic perception for 3D localization of nodules in soft tissue palpation with a variable stiffness probe. PLoS ONE 2020, 15, e0237379. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Liu, J.; Bajcsy, R. A depth camera-based soft fingertip device for contact region estimation and perception-action coupling. In Proceedings of the IEEE International Conference on Robotics and Automation, Montreal, QC, Canada, 20–24 May 2019; pp. 8443–8449. [Google Scholar] [CrossRef]
- Takao, H.; Sawada, K.; Ishida, M. Monolithic silicon smart tactile image sensor with integrated strain sensor array on pneumatically swollen single-diaphragm structure. IEEE Trans. Electron Devices 2006, 53, 1250–1259. [Google Scholar] [CrossRef]
- Raitt, D.G.; Abad, S.A.; Homer-Vanniasinkam, S.; Wurdemann, H.A. Soft, Stiffness-Controllable Sensing Tip for On-Demand Force Range Adjustment With Angled Force Direction Identification. IEEE Sens. J. 2022, 22, 8418–8427. [Google Scholar] [CrossRef]
- Xiang, C.; Guo, J.; Rossiter, J. Soft-smart robotic end effectors with sensing, actuation, and gripping capabilities. Smart Mater. Struct. 2019, 28, 055034. [Google Scholar] [CrossRef]
- Kitahara, M.; Kodama, A.; Ozawa, H.; Izukura, H. Mechanism of hearing disturbance due to alteration in atmospheric pressure. Acta Oto-Laryngol. 1994, 114, 92–95. [Google Scholar] [CrossRef]
- Murakami, S.; Gyo, K.; Goode, R.L. Effect of middle ear pressure change on middle ear mechanics. Acta Oto-Laryngol. 1997, 117, 390–395. [Google Scholar] [CrossRef]
- Kitahara, M.; Ozawa, H.; Kodama, A.; Izukura, H.; Inoue, S.; Uchida, K. Effect of atmospheric pressure on hearing in normal subjects. Acta Oto-Laryngol. 1994, 114, 87–91. [Google Scholar] [CrossRef]
- Soter, G.; Garrad, M.; Conn, A.T.; Hauser, H.; Rossiter, J. Skinflow: A soft robotic skin based on fluidic transmission. In Proceedings of the RoboSoft 2019—2019 IEEE International Conference on Soft Robotics, Seoul, Republic of Korea, 14–18 April 2019; pp. 355–360. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, G.P.; Conn, A.T.; Tzemanaki, A. A Pressure Controlled Membrane Mechanism for Optimising Haptic Sensing. In Towards Autonomous Robotic Systems—21st Annual Conference, TAROS 2020; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- He, L.; Herzig, N.; Nanayakkara, T.; Maiolino, P. 3D-Printed Soft Sensors for Adaptive Sensing with Online and Offline Tunable Stiffness. Soft Robot. 2022, 9, 1062–1073. [Google Scholar] [CrossRef]
- Jones, J.; Damian, D.D. A Soft Fluidic Sensor-Actuator for Active Sensing of Force and Displacement in Biomedical Applications. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2022), Kyoto, Japan, 23–27 October 2022; pp. 6913–6919. [Google Scholar]
- Konstantinova, J.; Cotugno, G.; Dasgupta, P.; Althoefer, K.; Nanayakkara, T. Palpation force modulation strategies to identify hard regions in soft tissue organs. PLoS ONE 2017, 12, e0171706. [Google Scholar] [CrossRef] [Green Version]
- Konstantinova, J.; Li, M.; Mehra, G.; Dasgupta, P.; Althoefer, K.; Nanayakkara, T. Characteristics of Manual Palpation to Localize Hard Nodules in Soft Tissues. IEEE Trans. Biomed. Eng. 2014, 61, 1651–1659. [Google Scholar] [CrossRef]
- Sornkarn, N.; Nanayakkara, T. Can a Soft Robotic Probe Use Stiffness Control Like a Human Finger to Improve Efficacy of Haptic Perception? IEEE Trans. Haptics 2017, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.H.; Tanaka, Y.; Fujiwara, M. Tumor Depth and Size Perception Using a Pneumatic Tactile Display in Laparoscopic Surgery. IEEE Access 2021, 9, 167795–167811. [Google Scholar] [CrossRef]
- Konstantinova, J.; Jiang, A.; Althoefer, K.; Dasgupta, P.; Nanayakkara, T. Implementation of tactile sensing for palpation in robot-assisted minimally invasive surgery: A review. IEEE Sens. J. 2014, 14, 2490–2501. [Google Scholar] [CrossRef] [Green Version]
- Scimeca, L.; Maiolino, P.; Bray, E.; Iida, F. Structuring of tactile sensory information for category formation in robotics palpation. Auton. Robot. 2020, 44, 1377–1393. [Google Scholar] [CrossRef]
- Sangpradit, K.; Liu, H.; Dasgupta, P.; Althoefer, K.; Seneviratne, L.D. Finite-element modeling of soft tissue rolling indentation. IEEE Trans. Biomed. Eng. 2011, 58, 3319–3327. [Google Scholar] [CrossRef]
- Mance, M.; Bulić, K.; Antabak, A.; Milošević, M. The influence of size, depth and histologic characteristics of invasive ductal breast carcinoma on thermographic properties of the breast. EXCLI J. 2019, 18, 549–557. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [Green Version]
- Aleskandarany, M.A.; Vandenberghe, M.E.; Marchiò, C.; Ellis, I.O.; Sapino, A.; Rakha, E.A. Tumour Heterogeneity of Breast Cancer: From Morphology to Personalised Medicine. Pathobiology 2018, 85, 23–34. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor heterogeneity in breast cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, M.; Dong, Z.X.; Jiang, D.; Li, X.; Lin, S.; Chen, D.; Zou, X.; Zhang, X.D.; Luker, G.D. Heterogeneous microenvironmental stiffness regulates pro-metastatic functions of breast cancer cells. Acta Biomater. 2021, 131, 326–340. [Google Scholar] [CrossRef]
- Wang, L. Early diagnosis of breast cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [Green Version]
- Bradski, G. The OpenCV Library. 2000. Available online: https://github.com/opencv/opencv (accessed on 18 November 2022).
- Ogden, R.W. Large deformation isotropic elasticity - on the correlation of theory and experiment for incompressible rubberlike solids. Proc. R. Soc. Lond. A Math. Phys. Sci. 1972, 326, 565–584. [Google Scholar] [CrossRef]
- Geer, R.K. Analyzing the Coiling Motion of Plant-Inspired Soft Actuators with Tilted Helix Fiber Reinforcement. Master’s Thesis, Clemson University, Clemson, SC, USA, 2019. [Google Scholar]
- Low, J.H.; Ang, M.H.; Yeow, C.H. Customizable soft pneumatic finger actuators for hand orthotic and prosthetic applications. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Singapore, 11–14 August 2015; pp. 380–385. [Google Scholar] [CrossRef]
- Hanon, M.; Dobos, J.; Zsidai, L. The influence of 3D printing process parameters on the mechanical performance of PLA polymer and its correlation with hardness. Procedia Manuf. 2021, 54, 244–249. [Google Scholar] [CrossRef]
- Smooth-On. Dragon Skin® Series. 2015. Available online: http://www.smooth-on.com/tb/files/DRAGON_SKIN_SERIES_TB.pdf (accessed on 18 November 2022).
- Sumith, Y. Fast Circle Fitting Using Landau Method. Matlab Central. 2019. Available online: https://ww2.mathworks.cn/matlabcentral/fileexchange/44219-fast-circle-fitting-using-landau-method (accessed on 18 November 2022).
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postep. Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef]
- Kitahara, M.; Suzuki, M.; Kodama, A. Equilibrium of inner and middle ear pressure. Acta Oto-Laryngol. 1994, 114, 113–115. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Saraf, R.F. Tactile imaging of an imbedded palpable structure for breast cancer screening. ACS Appl. Mater. Interfaces 2014, 6, 16368–16374. [Google Scholar] [CrossRef] [Green Version]
- Van Lier, M.G.; De Groot, J.E.; Muller, S.; Den Heeten, G.J.; Schilling, K.J. Pressure-based Compression Guidance of the Breast in Digital Breast Tomosynthesis Using Flexible Paddles Compared to Conventional Compression. J. Breast Imaging 2020, 2, 541–551. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Q.; Zhang, Q. Tactile Pattern Super Resolution with Taxel-Based Sensors. In Proceedings of the IEEE International Workshop on Intelligent Robots and Systems (IROS), Kyoto, Japan, 23–27 October 2022; pp. 3644–3650. [Google Scholar]
- Lepora, N.F.; Ward-Cherrier, B. Superresolution with an optical tactile sensor. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Hamburg, Germany, 28 September–2 October 2015; pp. 2686–2691. [Google Scholar] [CrossRef]
- He, L.; Herzig, N.; Lusignan, S.D.; Nanayakkara, T. An Abdominal Phantom with Tunable Stiffness Nodules and Force Sensing Capability for Palpation Training. IEEE Trans. Robot. 2020, 37, 1051–1064. [Google Scholar] [CrossRef]




| Palpation with small (0.3 mm) sample at 0 kPa | Palpation with large (2.1 mm) sample at 0 kPa | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of springs (0.0905 N/mm) classified | Number of springs (0.0905 N/mm) classified | |||||||||||||||||
| hard | 12 | 8 | 4 | 3 | 2 | 1 | hard | 12 | 8 | 4 | 3 | 2 | 1 | |||||
| Actual | hard | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Actual | hard | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 12 | 0.00 | 0.92 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 12 | 0.00 | 0.85 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 8 | 0.00 | 0.13 | 0.33 | 0.30 | 0.12 | 0.06 | 0.06 | 8 | 0.00 | 0.01 | 0.92 | 0.08 | 0.00 | 0.00 | 0.00 | |||
| 4 | 0.00 | 0.07 | 0.17 | 0.26 | 0.21 | 0.09 | 0.20 | 4 | 0.00 | 0.00 | 0.00 | 1.00 | 0.01 | −0.01 | 0.00 | |||
| 3 | 0.00 | 0.01 | 0.08 | 0.34 | 0.29 | 0.10 | 0.18 | 3 | 0.00 | 0.00 | 0.00 | 0.25 | 0.36 | 0.39 | 0.00 | |||
| 2 | 0.00 | 0.00 | 0.03 | 0.07 | 0.24 | 0.09 | 0.56 | 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.40 | 0.26 | 0.28 | |||
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 0.13 | 0.67 | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.86 | |||
| Palpation with small (0.3 mm) sample at 100 kPa | Palpation with large (2.1 mm) sample at 100 kPa | |||||||||||||||||
| Number of springs (0.0905 N/mm) classified | Number of springs (0.0905 N/mm) classified | |||||||||||||||||
| hard | 12 | 8 | 4 | 3 | 2 | 1 | hard | 12 | 8 | 4 | 3 | 2 | 1 | |||||
| Actual | hard | 0.91 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Actual | hard | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 12 | 0.02 | 0.88 | 0.05 | 0.07 | 0.00 | 0.00 | −0.01 | 12 | 0.00 | 0.77 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 8 | 0.03 | 0.04 | 0.63 | 0.29 | 0.01 | 0.01 | 0.00 | 8 | 0.00 | 0.08 | 0.89 | 0.03 | 0.00 | 0.00 | 0.00 | |||
| 4 | 0.00 | 0.02 | 0.03 | 0.49 | 0.30 | 0.15 | 0.01 | 4 | 0.00 | 0.00 | 0.00 | 0.80 | 0.20 | 0.00 | 0.00 | |||
| 3 | 0.00 | 0.02 | 0.00 | 0.20 | 0.69 | 0.06 | 0.02 | 3 | 0.00 | 0.00 | 0.00 | 0.06 | 0.86 | 0.08 | 0.00 | |||
| 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.44 | 0.54 | 0.18 | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.98 | 0.00 | |||
| 1 | 0.00 | 0.01 | 0.00 | 0.03 | 0.06 | 0.25 | 0.65 | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.93 | |||
| Study | Hard Node Depth |
|---|---|
| Gwilliam et al. (2010) [3] | 3.5 mm |
| Yamamoto et al. (2009) [7] | 5 mm |
| Konstantinova et al. (2017) [31] | 5 mm |
| Konstantinova et al. (2014) [32] | 5 mm |
| Garg et al. (2016) [5] | 8 mm |
| Sonrkarn et al. (2017) [33] | 8 mm |
| Herzig et al. (2020) [19] | 8 mm |
| Ly et al. 2021 [34] | 8 mm |
| Konstantinova et al. (2014) [35] | 11 mm |
| Scimeca et al. (2020) [36] | 15 mm |
| Sangpradit et al. (2011) [37] | 15 mm |
| This work | 20 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkinson, G.P.; Conn, A.T.; Tzemanaki, A. ESPRESS.0: Eustachian Tube-Inspired Tactile Sensor Exploiting Pneumatics for Range Extension and SenSitivity Tuning. Sensors 2023, 23, 567. https://doi.org/10.3390/s23020567
Jenkinson GP, Conn AT, Tzemanaki A. ESPRESS.0: Eustachian Tube-Inspired Tactile Sensor Exploiting Pneumatics for Range Extension and SenSitivity Tuning. Sensors. 2023; 23(2):567. https://doi.org/10.3390/s23020567
Chicago/Turabian StyleJenkinson, George P., Andrew T. Conn, and Antonia Tzemanaki. 2023. "ESPRESS.0: Eustachian Tube-Inspired Tactile Sensor Exploiting Pneumatics for Range Extension and SenSitivity Tuning" Sensors 23, no. 2: 567. https://doi.org/10.3390/s23020567
APA StyleJenkinson, G. P., Conn, A. T., & Tzemanaki, A. (2023). ESPRESS.0: Eustachian Tube-Inspired Tactile Sensor Exploiting Pneumatics for Range Extension and SenSitivity Tuning. Sensors, 23(2), 567. https://doi.org/10.3390/s23020567