Spectroscopy of Laser-Induced Dielectric Breakdown Plasma in Mixtures of Air with Inert Gases Ar, He, Kr, and Xe
Abstract
:1. Introduction
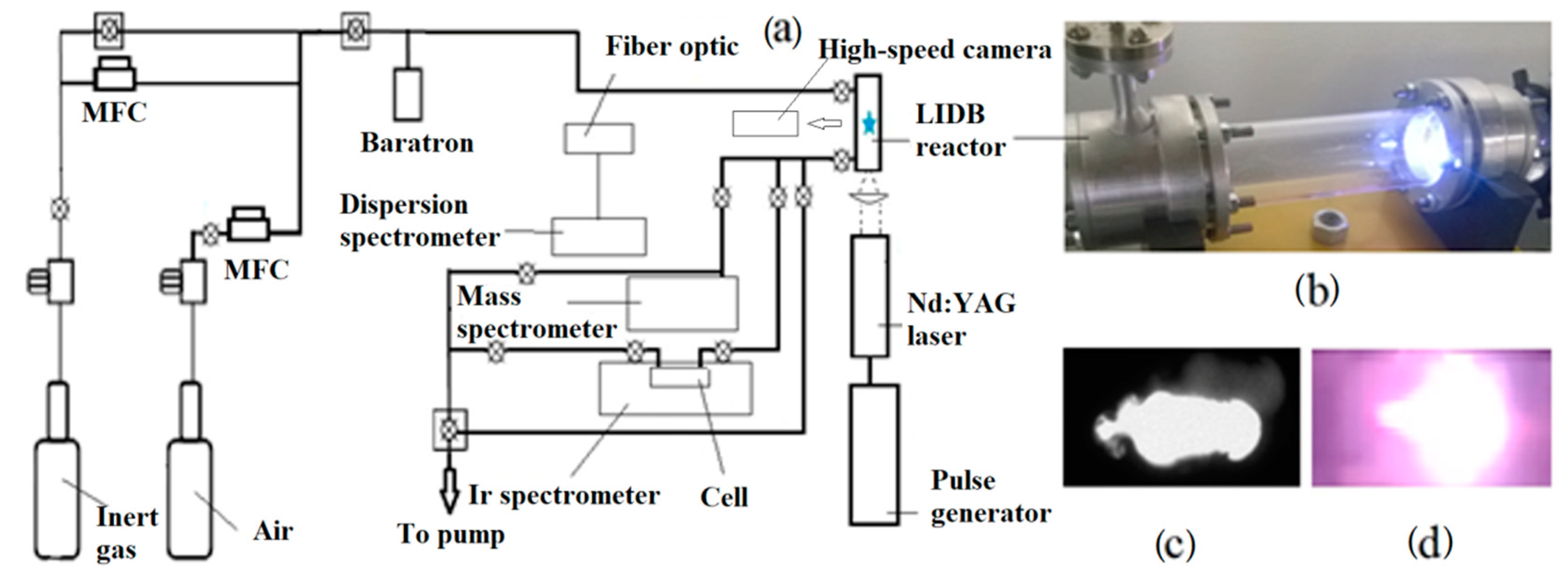
2. Materials and Methods
2.1. Emission Spectroscopy
2.2. IR Spectroscopy
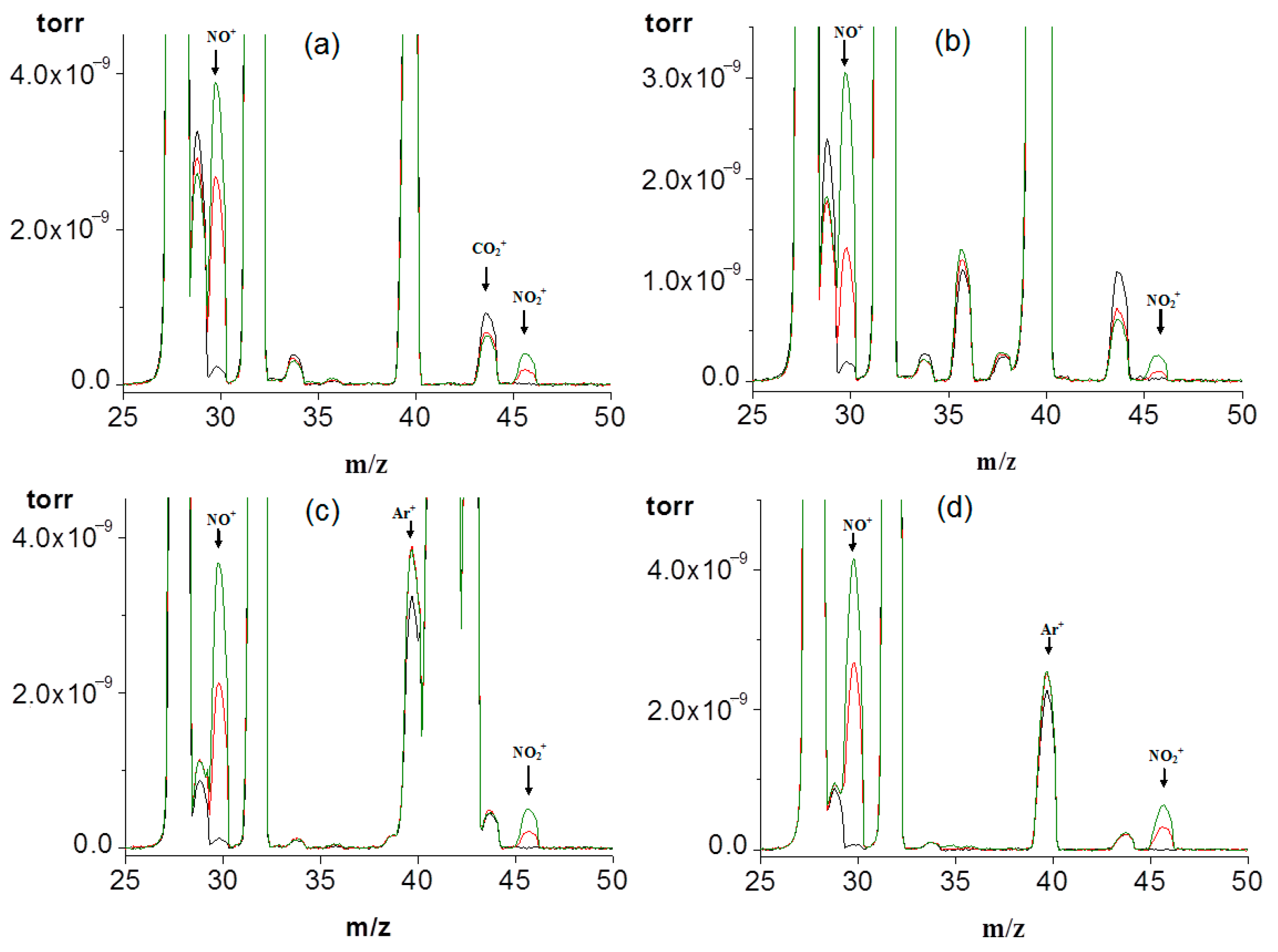
2.3. Mass Spectrometry of Gases
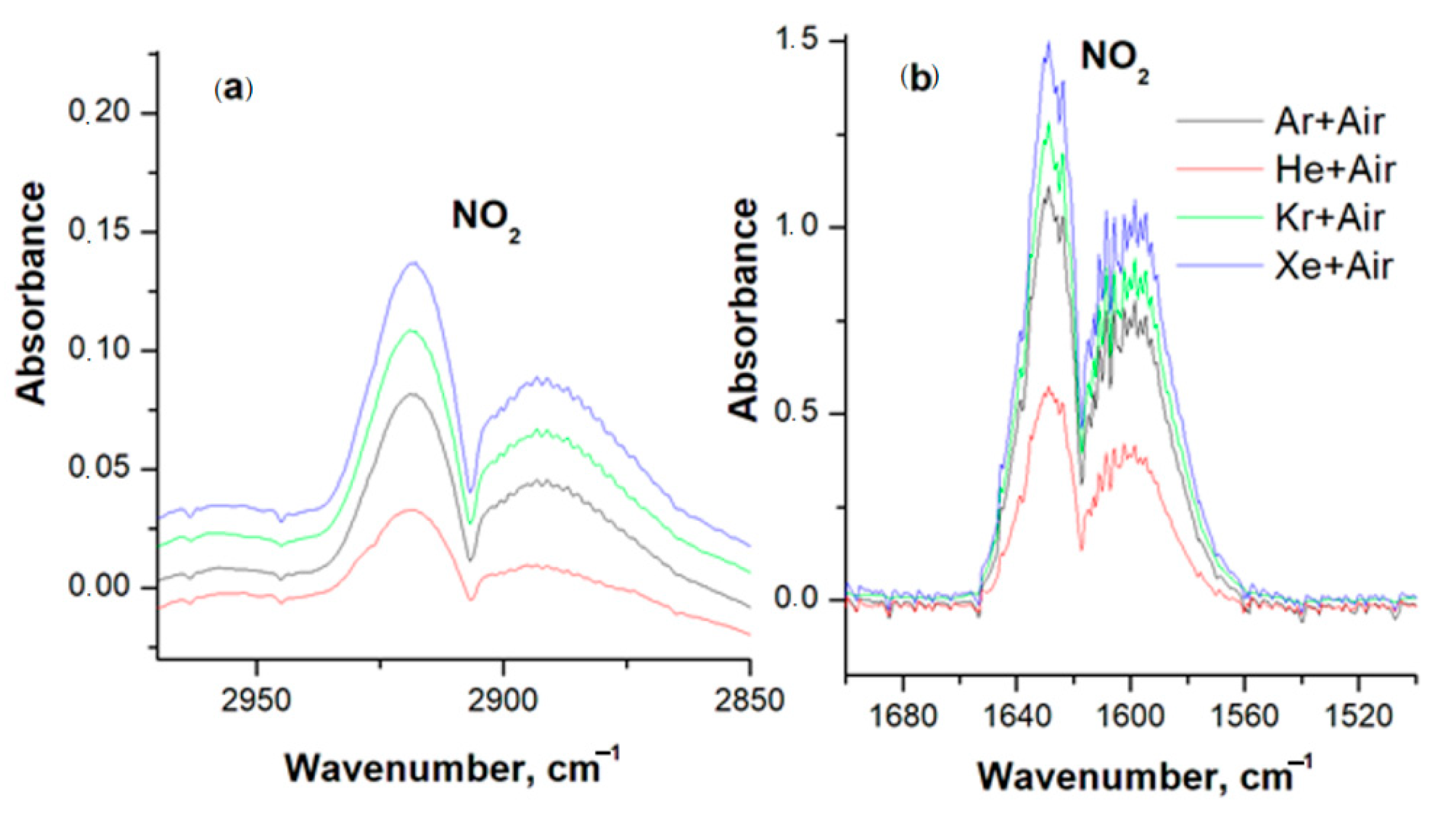
2.4. Spectrophotometry of Gases
2.5. Thermodynamic Analysis of the System Air + Ar (Kr, Xe)
3. Results and Discussion
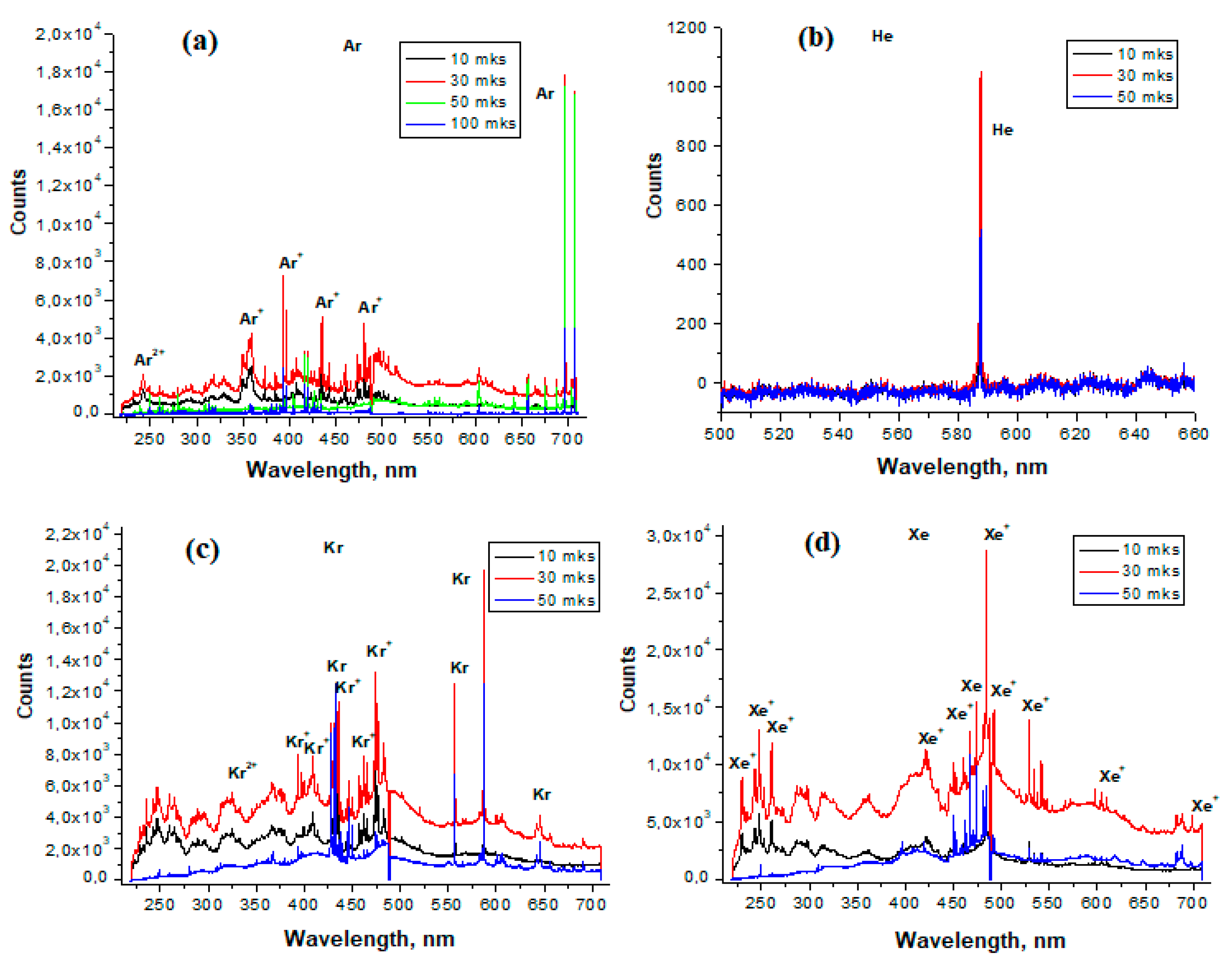
3.1. Emission Spectra of Ar, He, Kr, Xe in LIDB Plasma
3.2. Thermodynamics
4. Conclusions
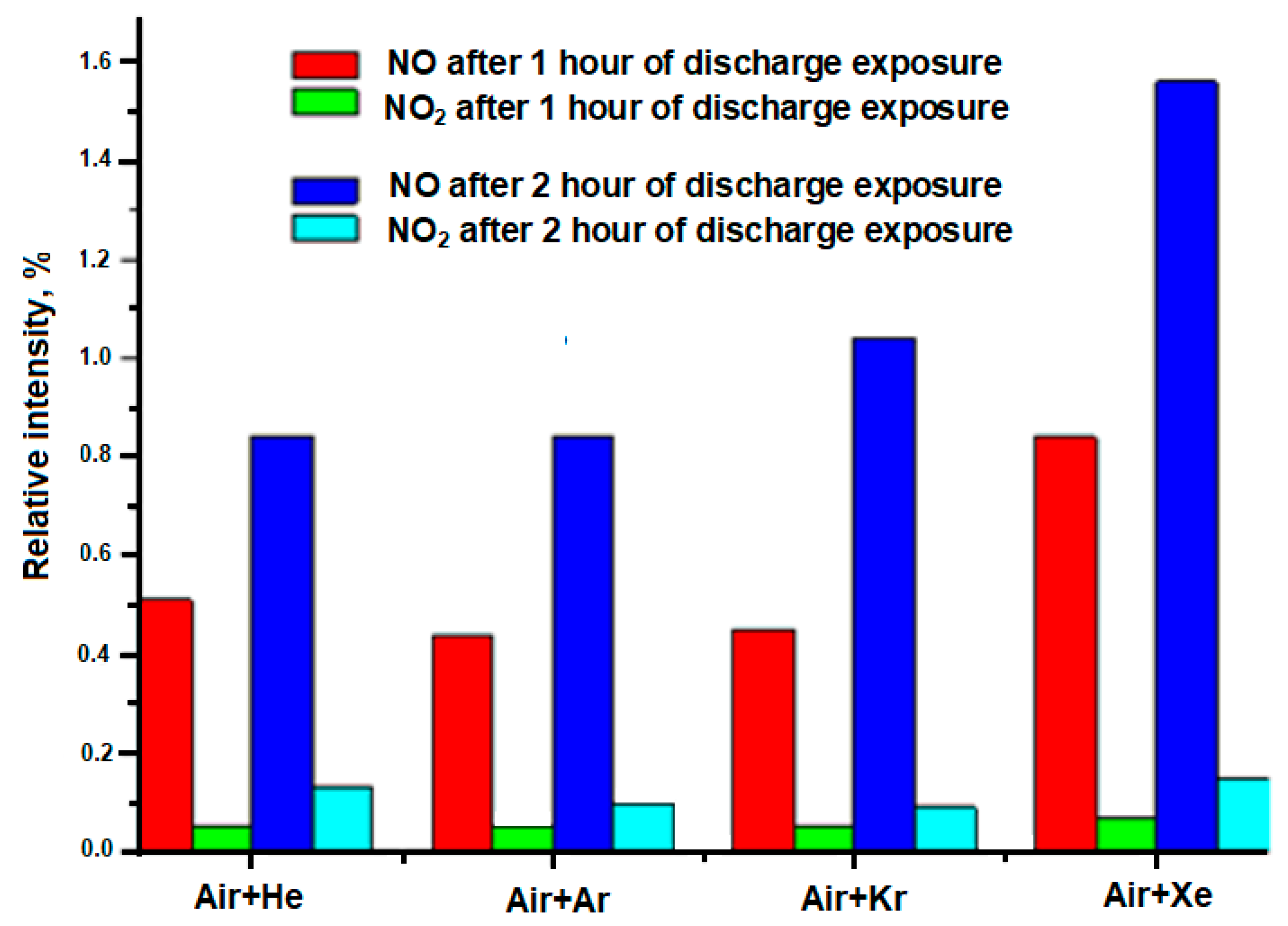
- The formation of NO and NO2 in LIDB plasma noticeably depends on the type of inert gas.
- An increase in the concentration of NO and NO2 is affected by the complication of the electronic configuration of an inert gas, as well as a decrease in its ionization potential.
- The complication of the electronic configuration of an inert gas, as well as a decrease in its ionization potential, leads to the suppression of the lines of nitrogen and oxygen ions. Thus, it can be assumed that the main mechanism affecting the chemical transformations in these mixtures and in particular, the formation of NO and NO2 is atomic.
- The formation of ozone occurs outside the gas discharge zone by the photolytic reaction.
- Using the method of thermodynamic analysis, it has been established that the equilibrium concentration of NO in in 1:1 mixtures of air with inert gases does not depend on the choice of an inert gas. On the contrary, the equilibrium concentration of the NO+ ion decreases as the electronic configuration of the inert gas becomes more complex.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoppe, P.; Praml, G.; Rabe, G.; Lindner, J.; Fruhmann, G.; Kessel, R. Environmental Ozone Field Study on Pulmonary and Subjective Responses of Assumed Risk Groups. Environ. Res. 1995, 71, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Baysan, A.; Lynch, E. The Use of Ozone in Dentistry and Medicine. Prim. Dent. Care 2005, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Belianin, I.I.; Shmelev, E.I. The use of an ozonised sorbent in treating patients with progressive pulmonary tuberculosis combined with hepatitis. Ter. Arkh. 1994, 66, 29–32. [Google Scholar] [PubMed]
- Carpendale, M.T.; Freeberg, J.; Griffiss, J.M. Does Ozone Alleviate AIDS Diarrhea? J. Clin. Gastr. 1993, 17, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Martusevich, A.A.; Peretyagin, S.P.; Martusevich, A.K. Molecular and cellular mechanisms of the action of singlet oxygen on biological systems. M. Tech. Med. 2012, 2, 128–134. [Google Scholar]
- Martusevich, A.A.; Solovieva, A.G.; Martusevich, A.K. Influence of singlet oxygen inhalation on the state of blood pro- and antioxidant systems and energy metabolism. Bull. Exper. Biol. Med. 2013, 156, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Martusevich, A.K.; Peretyagin, S.P.; Martusevich, A.A.; Peretyagin, P.V. Effect of ROS inhalations on systemic and local hemodynamics in rats. Bull. Exp. Boil. Med. 2016, 161, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. The Contribution of Singlet Oxygen to Insulin Resistance. Oxid. Med. Cell. Longev. 2017, 2017, 8765972. [Google Scholar] [CrossRef] [Green Version]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Pfitzner, M.; Preuß, A.; Röder, B. A new level of in vivo singlet molecular oxygen luminescence measurements. Photodiag. Photodyn. Ther. 2020, 29, 101613. [Google Scholar] [CrossRef]
- Malik, M.A. Nitric Oxide Production by High Voltage Electrical Discharges for Medical Uses: A Review. Plasma Chem. Plasma Proc. 2016, 36, 737–766. [Google Scholar] [CrossRef]
- Eliasson, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D 1987, 20, 1421–1437. [Google Scholar] [CrossRef]
- Pietsch, G.J.; Gibalov, V.I. Dielectric barrier discharges and ozone synthesis. Pur. Appl. Chem. 1998, 70, 1169–1174. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.A.; Schoenbach, K.H.; Heller, R. Coupled surface dielectric barrier discharge reactor-ozone synthesis and nitric oxide conversion from air. Chem. Eng. J. 2014, 256, 222–229. [Google Scholar] [CrossRef]
- Li, M.; Zhu, B.; Yan, Y.; Li, T.; Zhu, Y. A High-Efficiency Double Surface Discharge and Its Application to Ozone Synthesis. Plasm. Chem. Plasm. Proc. 2018, 38, 1063–1080. [Google Scholar] [CrossRef]
- Pekárek, S. Non-Thermal Plasma Ozone Generation. Act. Polytech. 2003, 43, 47–51. [Google Scholar] [CrossRef]
- Garamoon, A.A.; Elakshar, F.F.; Nossair, A.M.; Kotp, E.F. Experimental study of ozone synthesis. Plasm. Sour. Sc. Tech. 2002, 11, 254–259. [Google Scholar] [CrossRef]
- Fuller, N.C.M.; Malyshev, M.V.; Donnelly, V.M.; Herman, I.P. Characterization of transformer coupled oxygen plasmas by trace rare gases-optical emission spectroscopy and Langmuir probe analysis. Plasm. Sour. Sc. Tech. 2000, 9, 116–127. [Google Scholar] [CrossRef]
- Cvelbar, U.; Krstulović, N.; Milošević, S.; Mozetič, M. Inductively coupled RF oxygen plasma characterization by optical emission spectroscopy. Vacuum 2007, 82, 224–227. [Google Scholar] [CrossRef]
- Babarickij, A.I.; Bibikov, M.B.; Demkin, S.A.; Moskovskij, A.S.; Smirnov, R.V.; Cheban’kov, F.N. Okislenie azota v mikrovolnovyh razryadah atmosfernogo davleniya. Him. Vys. En. 2021, 55, 487–492. (In Russian) [Google Scholar]
- Stricker, J. Experimental investigation of electrical breakdown in nitrogen and oxygen induced by focused laser radiation at 1.064 μ. J. Appl. Phys. 1982, 53, 851. [Google Scholar] [CrossRef]
- Civisš, S.; Babaánkovaá, D.; Cihelka, J.; Sazama, P.; Juha, L. Spectroscopic Investigations of High-Power Laser-Induced Dielectric Breakdown in Gas Mixtures Containing Carbon Monoxide. J. Phys. Chem. A 2008, 112, 7162–7169. [Google Scholar] [CrossRef]
- Gornushkin, I.B.; Stevenson, C.L.; Galbacs, G.; Smith, B.W.; Winefordner, J.D. Measurement and Modeling of Ozone and Nitrogen Oxides Produced by Laser Breakdown in Oxygen–Nitrogen Atmospheres. Appl. Spectr. 2003, 57, 1442–1450. [Google Scholar] [CrossRef]
- Available online: www.uv-vis-spectral-atlas-mainz.org (accessed on 22 November 2022).
- Available online: https://cearun.grc.nasa.gov (accessed on 22 November 2022).
- Griem, H.R. Principles of Plasma Spectroscopy; Cambridge University Press: Cambridge, UK, 1997; Volume 7. [Google Scholar]
- Cristoferetti, G.; Tognoni, E.; Gizzi, L.A. Thermodynamic equilibrium states in laser-induced plasmas: From the general case to laser-induced breakdown spectroscopy plasmas. Spectr. Act. B 2013, 90, 1–22. [Google Scholar] [CrossRef]
- Raizer, Y.P. Gas Discharge Physics; Springer: Berlin, Germany, 1991. [Google Scholar]
- Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 22 November 2022).
- Petruci, J.F.; Tutuncu, E.; Cardoso, A.A.; Mizaikoff, B. Real-time and simultaneous monitoring of NO, NO2, and N2O using substrate–integrated hollow waveguides coupled to a compact fourier transform Infrared (FT-IR) Spectrometer. Appl. Spectr. 2018, 73, 1–6. [Google Scholar] [CrossRef]



| Wavelength, µm | 1.064 |
| Radiation frequency, Hz | 10 |
| Energy per pulse, mJ | 820 |
| Pulse duration, ns | 15 |
| Area of a focal spot, cm2 | 10−2 |
| Pulse power, MW | 50 |
| Irradiance, GW/cm2 | 5 |
| Fluence, J/cm2 | 80 |
| Photon flux density, cm−2 s−1 | 2.9 × 1028 |
| Electric field strength, MV/cm | 1.4 |
| Inert Gas | Electronic Configuration | Ionization Energy, eV | Metastable States | |
|---|---|---|---|---|
| Excitation Energy, eV | Lifetime, s | |||
| Hе | 1s2 | 24.6 | 19.82 (23S1) | 6 × 105 |
| Ar | 1s22s22p63s23p6 | 15.8 | 11.55 (43P02) 11.72 | >1.3 - |
| Kr | 1s22s22p63s23p6 3d104s24p6 | 14.0 | 9.91 10.5 | - - |
| Xe | 1s22s22p63s23p6 3d104s24p64d105s25p6 | 12.1 | 8.32 9.4 | - - |
| Line (Band), nm | Transition | Air | Air/He = 9 | Air/Ar = 1 | Air/Kr = 1 | Air/Xe = 1 |
|---|---|---|---|---|---|---|
| N+ (399.5) | 1D2-1Po1 | strong | strong | strong | medium | weak |
| N+ (463.0) | 3P2-3Po2 | strong | strong | - | - | - |
| N+ (500.5) | 3D3-3Fo4 | strong | strong | strong | medium | - |
| N+(568.0) | 3D3-3Po2 | medium | medium | medium | medium | - |
| N+ (594.1) | 3Do3-3P2 | medium | medium | medium | - | - |
| O (615.8) | 5Do4-5P3 | weak | weak | weak | weak | - |
| O+ (407.6) | 4F9/2-4Do7/2 | strong | strong | strong | - | - |
| O2+ (334.0) | 3S1-3Po2 | medium | medium | medium | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martusevich, A.; Kornev, R.; Ermakov, A.; Gornushkin, I.; Nazarov, V.; Shabarova, L.; Shkrunin, V. Spectroscopy of Laser-Induced Dielectric Breakdown Plasma in Mixtures of Air with Inert Gases Ar, He, Kr, and Xe. Sensors 2023, 23, 932. https://doi.org/10.3390/s23020932
Martusevich A, Kornev R, Ermakov A, Gornushkin I, Nazarov V, Shabarova L, Shkrunin V. Spectroscopy of Laser-Induced Dielectric Breakdown Plasma in Mixtures of Air with Inert Gases Ar, He, Kr, and Xe. Sensors. 2023; 23(2):932. https://doi.org/10.3390/s23020932
Chicago/Turabian StyleMartusevich, Andrew, Roman Kornev, Artur Ermakov, Igor Gornushkin, Vladimir Nazarov, Lyubov Shabarova, and Vladimir Shkrunin. 2023. "Spectroscopy of Laser-Induced Dielectric Breakdown Plasma in Mixtures of Air with Inert Gases Ar, He, Kr, and Xe" Sensors 23, no. 2: 932. https://doi.org/10.3390/s23020932





