A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions
Abstract
1. Introduction
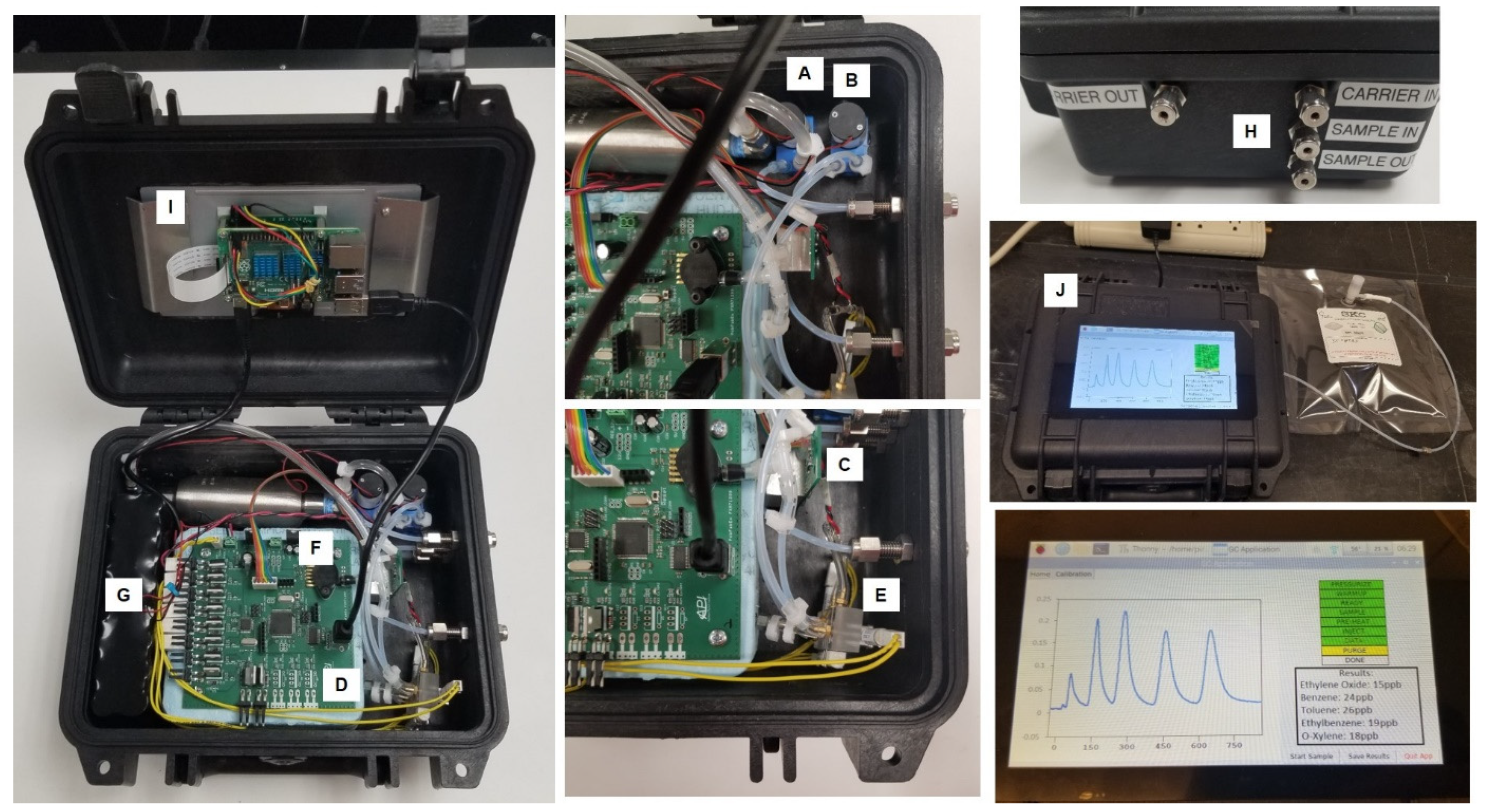
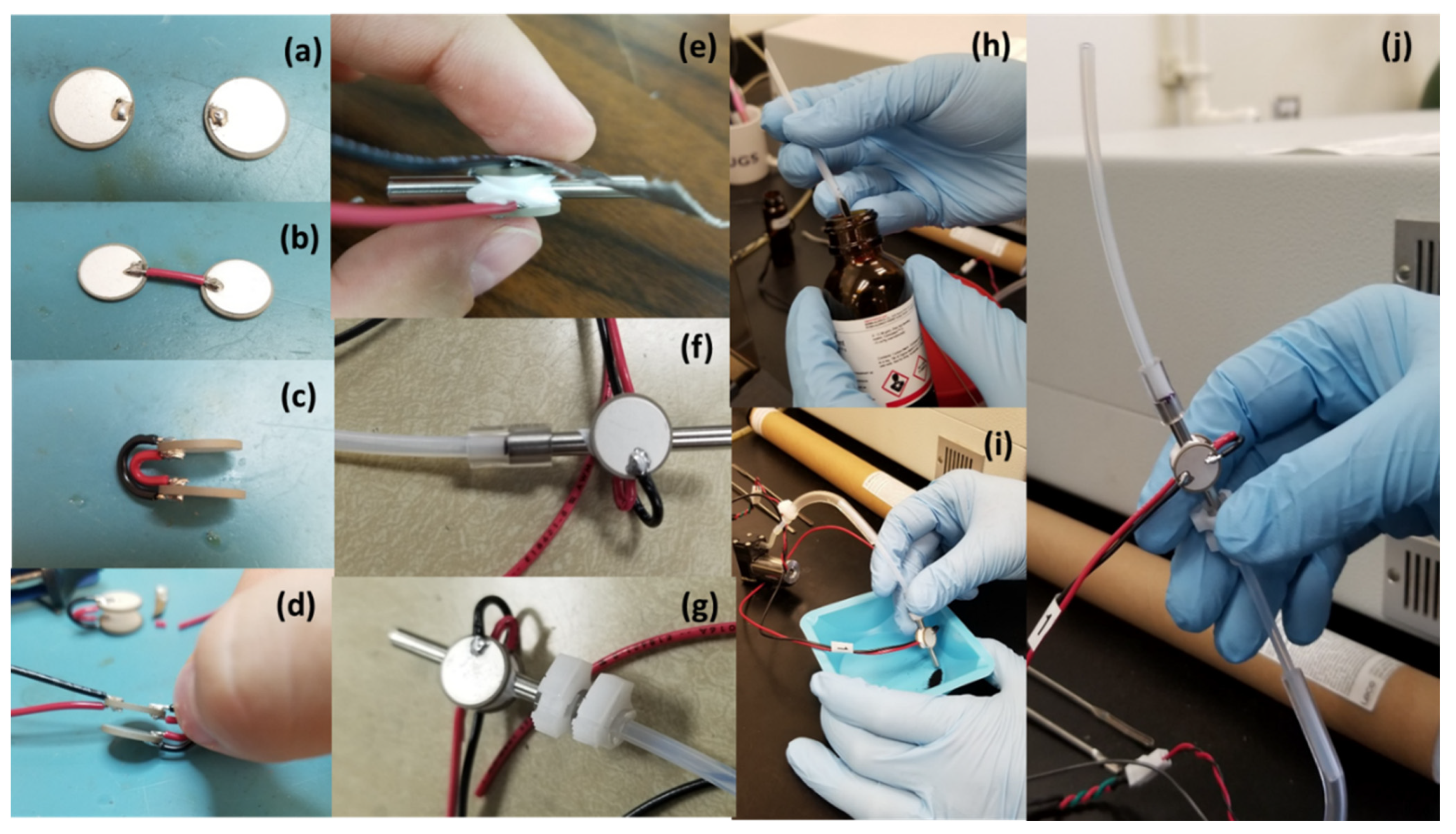
2. Materials and Methods
- Capable of extracting ethylene from the most complex background mix.
- Capable of separating ethylene from the light compounds, especially plant-produced compounds, such as methane, hydrogen sulfide and ammonia.
- Capable of separating and quantifying the greatest number of plant-produced VOCs.
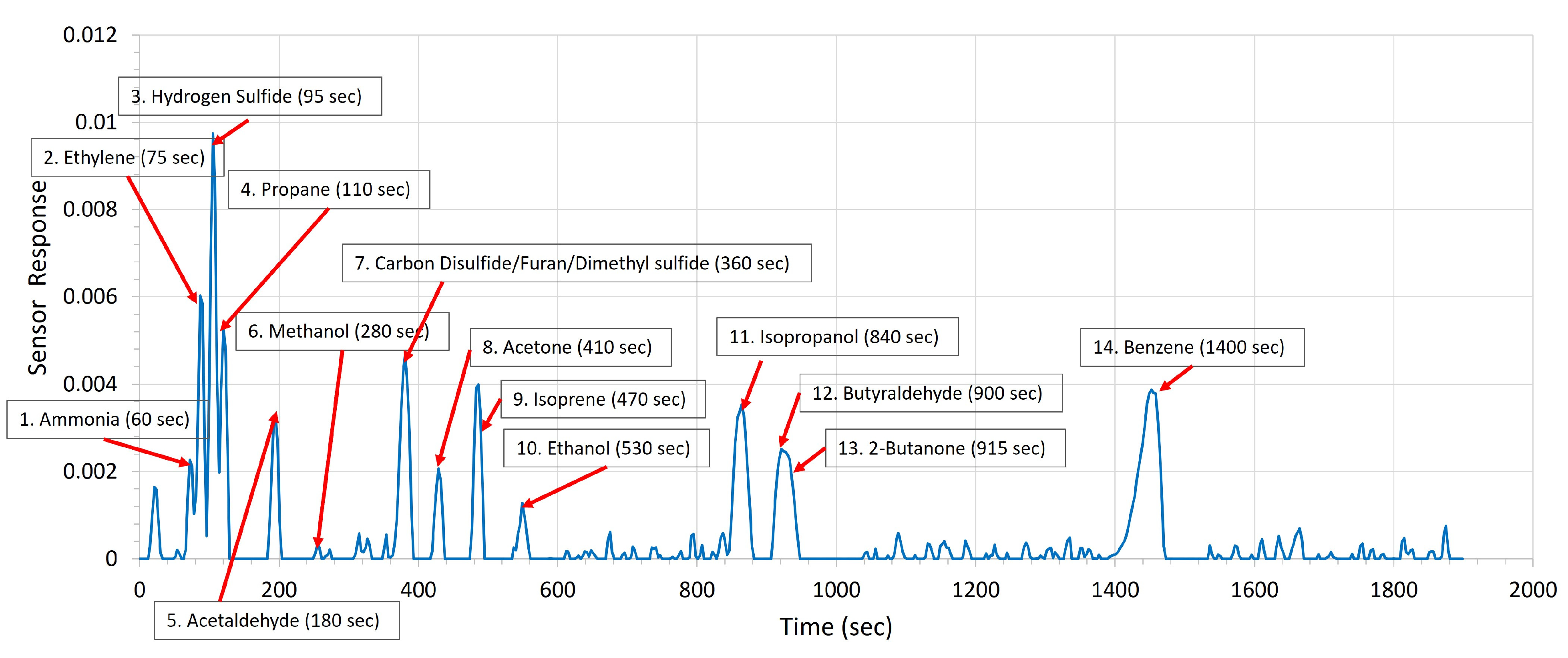
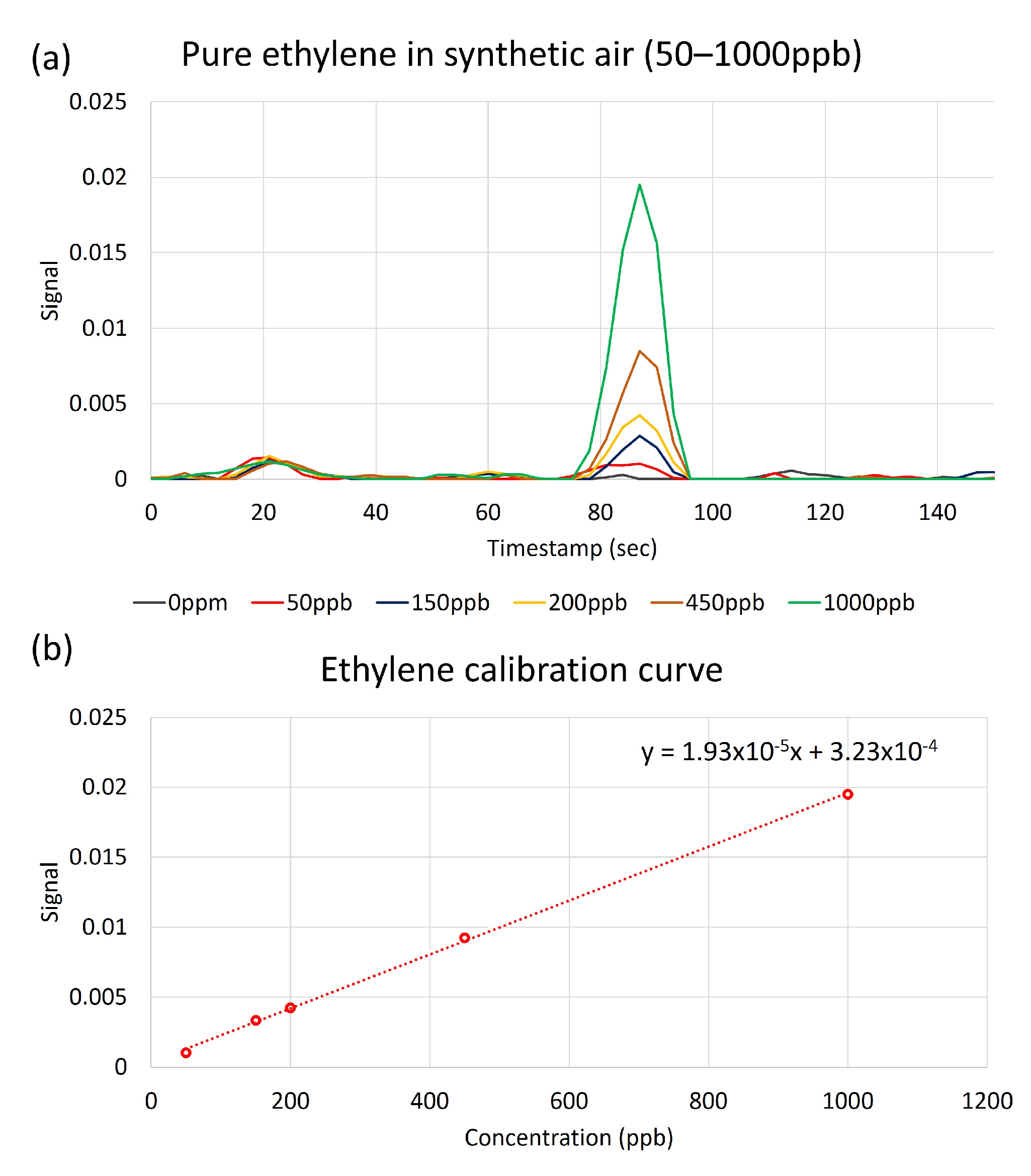
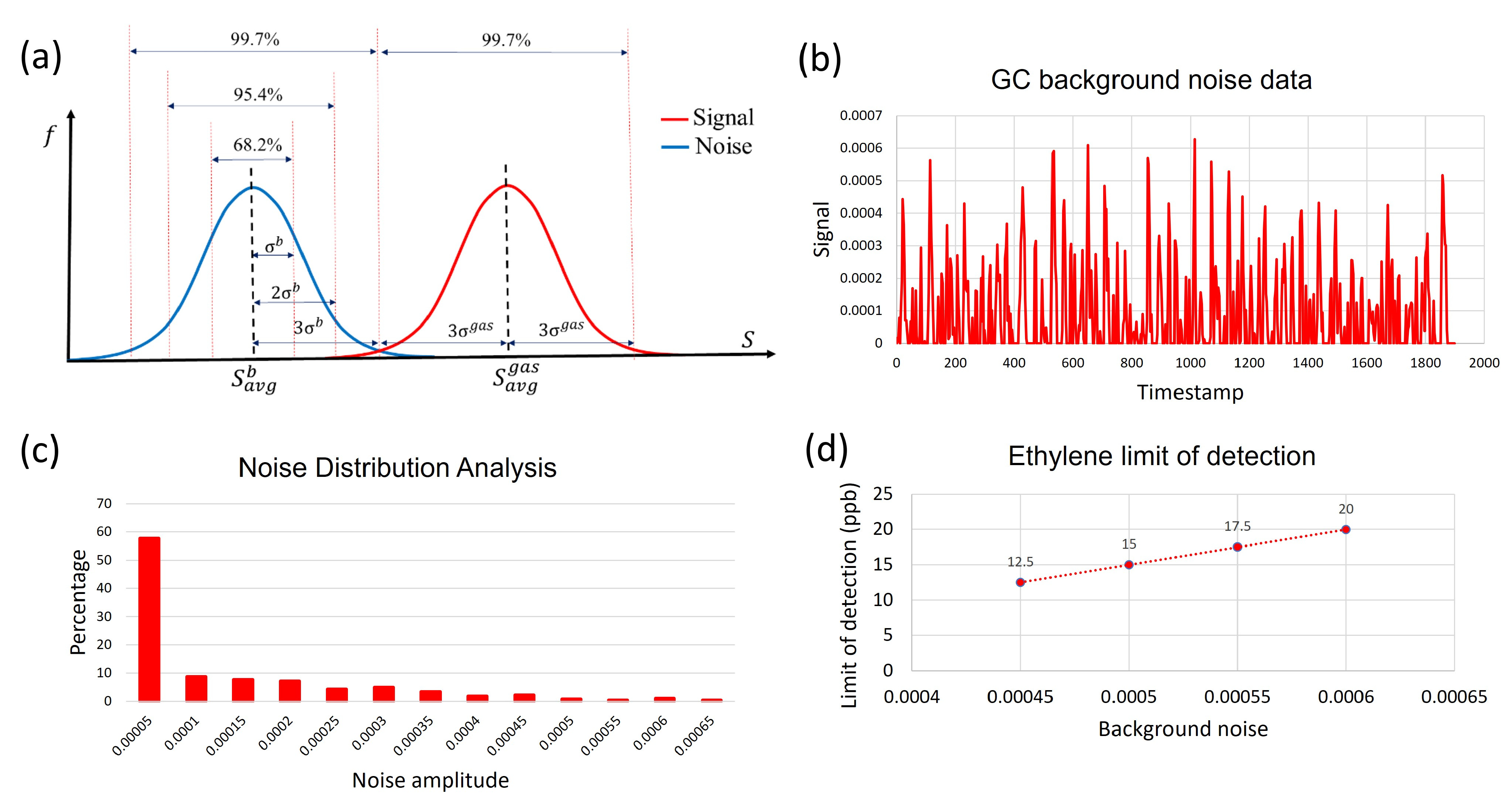
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salisbury, F.B.; Campbell, W.F.; Carman, J.G.; Bingham, G.E.; Bubenheim, D.L.; Yendler, B.; Sytchev, V.; Levinskikh, M.A.; Ivanova, I.; Chernova, L.; et al. Plant growth during the greenhouse II experiment on the Mir orbital station. Adv. Space Res. 2003, 31, 221–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckart, P. Life Support and Biospherics; Herbert Utz Publishers: Munchen, Germany, 1994. [Google Scholar]
- Perino, M.A.; Lobascio, C.; Pastor, S.; Maffei, M. Greenhouse: A strategic element to support humans in space. In Proceedings of the Seventh ISU Annual International Symposium, Strasbourg, France, 4–7 June 2002. [Google Scholar]
- Wang, Z.; Fingas, M.; Yang, C.; Christensen, J.H. Crude Oil and Refined Product Fingerprinting: Principles. In Environmental Forensics; Morrison, R.D., Murphy, B.L., Eds.; Academic Press: Burlington, MA, USA, 2005; pp. 339–407. [Google Scholar]
- Aguado, S.; Bergeret, G.; Daniel, C.; Farrusseng, D. Absolute Molecular Sieve Separation of Ethylene/Ethane Mixtures with Silver Zeolite A. J. Am. Chem. Soc. 2012, 134, 14635–14637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Schmitt, K.; Blanke, M.; Bauersfeld, M.L.; Wöllenstein, J.; Lang, W. Ethylene detection in fruit supply chains. Philos. Trans. R. Soc. 2014, 372, 20130311. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Ethylene in root growth and development. In The Plant Hormone Ethylene; Jackson, M.B., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 159–181. [Google Scholar]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Fu, W.; van Dijkman, T.F.; Lima, L.M.C.; Jiang, F.; Schneider, G.F.; Bouwman, E. Ultrasensitive Ethene Detector Based on a Graphene–Copper(I) Hybrid Material. Nano Lett. 2017, 17, 7980–7988. [Google Scholar] [CrossRef] [PubMed]
- Mallick, M.; Hossain, S.M.; Das, J. Graphene Oxide Based Fruit Ripeness Sensing e-Nose. Mater. Today Proc. 2018, 5, 9866–9870. [Google Scholar] [CrossRef]
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective Detection of Ethylene Gas Using Carbon Nanotube-based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R. An infrared-based sensor system for the detection of ethylene for the discrimination of fruit ripening. Infrared Phys. Technol. 2017, 85, 403–409. [Google Scholar] [CrossRef]
- Popa, C. Ethylene Measurements from Sweet Fruits Flowers Using Photoacoustic Spectroscopy. Molecules 2019, 24, 1144. [Google Scholar] [CrossRef]
- Fong, D.; Luo, S.-X.; Andre, R.D.S.; Swager, T.M. Trace Ethylene Sensing via Wacker Oxidation. ACS Cent. Sci. 2020, 6, 507–512. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.; Morgan, P.; Saltveit, M. Ethylene in Plant Biology. Vol. 3; Academic Press, Inc.: San Diego, CA, USA, 1992. [Google Scholar]
- Chen, X.; Wreyford, R.; Nasiri, N. Recent Advances in Ethylene Gas Detection. Materials 2022, 15, 5813. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, S.; Mandon, J.; Arslanov, D.; DePessemier, J.; Hermans, C.; Harren, F. Current methods for detecting ethylene in plants. Ann. Bot. 2013, 111, 347–360. [Google Scholar] [CrossRef] [PubMed]
- De Blas, M.; Navazo, M.; Alonso, L.; Durana, N.; Iza, J. Automatic on-line monitoring of atmospheric volatile organic compounds: Gas chromatography-mass spectrometry and gas chromatography-flame ionization detection as complementary systems. Sci. Total Environ. 2011, 409, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Cui, D.F.; Chen, X.; Zhang, L.L.; Cai, H.Y.; Li, H. A micro gas chromatography column with a micro thermal conductivity detector for volatile organic compound analysis. Rev. Sci. Instrum. 2013, 84, 025001. [Google Scholar] [CrossRef]
- Skov, H.; Lindskog, A.; Palmgren, F.; Christensen, C.S. An overview of commonly used methods for measuring benzene in ambient air. Atmos. Environ. 2001, 35, 141–148. [Google Scholar] [CrossRef]
- Liaud, C.; Nguyen, N.T.; Nasreddine, R.; Le Calvé, S. Experimental performances study of a transportable GC-PID and two thermo-desorption based methods coupled to FID and MS detection to assess BTEX exposure at sub-ppb level in air. Talanta 2014, 127, 33–42. [Google Scholar] [CrossRef]
- Gallego, E.; Roca, F.; Perales, J.; Sánchez, G.; Esplugas, P. Characterization and determination of the odorous charge in the indoor air of a waste treatment facility through the evaluation of volatile organic compounds (VOCs) using TD–GC/MS. Waste Manag. 2012, 32, 2469–2481. [Google Scholar] [CrossRef]
- de Blas, M.; Navazo, M.; Alonso, L.; Durana, N.; Gomez, M.C.; Iza, J. Simultaneous indoor and outdoor on-line hourly monitoring of atmospheric volatile organic compounds in an urban building. The role of inside and outside sources. Sci. Total. Environ. 2012, 426, 327–335. [Google Scholar] [CrossRef]
- Zimmermann, S.; Wischhusen, S.; Müller, J. Micro flame ionization detector and micro flame spectrometer. Sens. Actuators B Chem. 2000, 63, 159–166. [Google Scholar] [CrossRef]
- Nasreddine, R.; Person, V.; Serra, C.A.; Le Calvé, S. Development of a novel portable miniaturized GC for near real-time low level detection of BTEX. Sens. Actuators B Chem. 2016, 224, 159–169. [Google Scholar] [CrossRef]
- Garg, A.; Akbar, M.; Vejerano, E.; Narayanan, S.; Nazhandali, L.; Marrb, L.C.; Agaha, M. Zebra GC: A mini gas chromatography system for trace-level determination of hazardous air pollutants. Sens. Actuators B Chem. 2015, 212, 145–154. [Google Scholar] [CrossRef]
- Alinoori, A.H.; Masoum, S. Multicapillary Gas Chromatography-Temperature Modulated Metal Oxide Semiconductor Sensors Array Detector for Monitoring of Volatile Organic Compounds in Closed Atmosphere Using Gaussian Apodization Factor Analysis. Anal. Chem. 2018, 90, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Zellers, E.T. Dual-Chemiresistor GC Detector Employing Monolayer-Protected Metal Nanocluster Interfaces. Anal. Chem. 2002, 74, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Tessmann, T.; Lang, W. High sensitive and selective ethylene measurement by using a large-capacity-on-chip pre-concentrator device. Sens. Actuators B Chem. 2014, 197, 405–413. [Google Scholar] [CrossRef]
- Zaidi, N.A.; Tahir, M.W.; Vellekoop, M.J.; Lang, W. A Gas Chromatographic System for the Detection of Ethylene Gas Using Ambient Air as a Carrier Gas. Sensors 2017, 17, 2283. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Larin, A. Multisensory Gas Chromatography for Field Analysis of Complex Gaseous Mixtures. ChemEngineering 2019, 3, 13–30. [Google Scholar] [CrossRef]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Novel highly-integrated MEMS based solid state detectors for analytical gas chromatography. Sens. Actuators B Chem. 2018, 256, 1057–1068. [Google Scholar] [CrossRef]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Hybrid SnO2/TiO2 Nanocomposites for Selective Detection of Ultra-Low Hydrogen Sulfide Concentrations in Complex Backgrounds. Sensors 2016, 16, 1373. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Larin, A. Doped, Metal Oxide-Based Chemical Sensors. U.S. Patent 11,009,475 B2, 18 May 2021. [Google Scholar]
- Steen, H.; Dobrokhotov, V.; Lineberry, Q.; Paschal, J. 3-D Glass Printable Hand-Held Gas Chromatograph for Biomedical and Environmental Applications. U.S. Patent 11,243,192 B2, 8 February 2022. [Google Scholar]
- Dobrokhotov, V.; Larin, A. Bimetal Doped-Metal Oxide-Based Chemical Sensors. U.S. Patent 10,802,008 B2, 13 October 2020. [Google Scholar]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter. 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Gurlo, A.; Barsan, N.; Oprea, A.; Sahm, M.; Sahm, T.; Weimar, U. An n- to p-type conductivity transition induced by oxygen adsorption on a-Fe2O3. Appl. Phys. Lett. 2004, 85, 2280–2282. [Google Scholar] [CrossRef]
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Gas’kov, A.M.; Rumyantseva, M. N Nature of gas sensitivity in nanocrystalline metal oxides. J. Appl. Chem. 2001, 74, 440–444. [Google Scholar]
- Gas’kov, A.M.; Rumyantseva, M.N. Materials for solid-state gas sensors. Inorg. Mater. 2000, 36, 293–301. [Google Scholar] [CrossRef]
- Roy, S.; Basu, S. Improved zinc oxide film for gas sensor applications. Bull. Mater. Sci. 2002, 25, 513–515. [Google Scholar] [CrossRef]
- Chatterjee, A.P.; Mitra, P.; Mukhopadhyay, A.K. Chemically deposited zinc oxide thin film gas sensor. J. Mater. Sci. 1999, 34, 4225–4231. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Oakes, L.; Sowell, D.; Larin, A.; Hall, J.; Kengne, A.; Bakharev, P.; Corti, G.; Cantrell, T.; Prakash, T.; et al. Towards the Nanospring-Based Artificial Olfactory System for Trace-Detection of Flammable and Explosive Vapors. Sens. Actuators B Chem. 2012, 168, 138–148. [Google Scholar] [CrossRef]
- Mason, P. Challenges of gas monitoring and interpretation in underground coal mines following an emergency. In Proceedings of the 2012 Coal Operators’ Conference, The University of Wollongong, Wollongong, Australia, 14–15 February 2023. [Google Scholar]
- Batten, J.H.; Stutte, G.W.; Wheeler, R.M. Volatile organic compounds detected in the atmosphere of NASA’s biomass production chamber. Adv. Space Res. 1996, 18, 189–192. [Google Scholar] [CrossRef]
- Larrat, E.P.; Stutte, G.W.; Wheeler, R.M. Potential Effects of Biogenic Compound Production on Human Health in Closed Life Support Systems; SAE Technical Paper 2005-01-2772; SAE International: Warrendale, PA, USA, 2005. [Google Scholar]
- Batten, J.H.; Stutte, G.W.; Wheeler, R.M. Effect of crop development on biogenic emissions from plant populations grown in closed plant growth chambers. Phytochemistry 1995, 39, 1351–1357. [Google Scholar] [CrossRef]









| Column Name | Type | Coating Material | OD | ID | Column Length (m) | Mesh |
|---|---|---|---|---|---|---|
| CorboWax | packed/micropacked | CarboBlack B (S12) (Fisher Scientific, Hampton, NH, USA) | 1/8 in | 2.1 mm | 2 m | 80/120 |
| CarboXen | packed/micropacked | Carboxen (Sigma-Aldrich, St. Louis, MO, USA) | 1/8 in | 2.1 mm | 4.25 ft | 60/80 |
| MXT-BAC1 | capillary | MXT™-BAC1 (Fisher Scientific, Hampton, NH, USA) | 0.83 mm | 0.53 mm | 30 m | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrokhotov, V.; Larin, A.; Viugina, E.; Emberton, A.; Livchak, A.; Cremer, J.T., Jr.; Gary, C.K. A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions. Sensors 2023, 23, 9713. https://doi.org/10.3390/s23249713
Dobrokhotov V, Larin A, Viugina E, Emberton A, Livchak A, Cremer JT Jr., Gary CK. A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions. Sensors. 2023; 23(24):9713. https://doi.org/10.3390/s23249713
Chicago/Turabian StyleDobrokhotov, Vladimir, Alexander Larin, Elena Viugina, Adam Emberton, Andrey Livchak, Jay T. Cremer, Jr., and Charles K. Gary. 2023. "A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions" Sensors 23, no. 24: 9713. https://doi.org/10.3390/s23249713
APA StyleDobrokhotov, V., Larin, A., Viugina, E., Emberton, A., Livchak, A., Cremer, J. T., Jr., & Gary, C. K. (2023). A Compact Monitor for Ethylene and Other Plant-Produced Volatile Organic Compounds for NASA Space Missions. Sensors, 23(24), 9713. https://doi.org/10.3390/s23249713





