A Portable Measurement Device Based on Phenanthroline Complex for Iron Determination in Water
Abstract
:1. Introduction
- Operate the device in the field in standalone operation, as opposed to instruments commonly used exclusively in the laboratory, at lower cost.
- Quantify iron concentrations in accordance with the value proposed by the regulations for human consumption.
- Use of a paired diode–photodiode with three radiation channels (red, green, and blue) allows the device to determine multiple water parameters without the necessity to modify the hardware configuration.
2. Materials and Methods
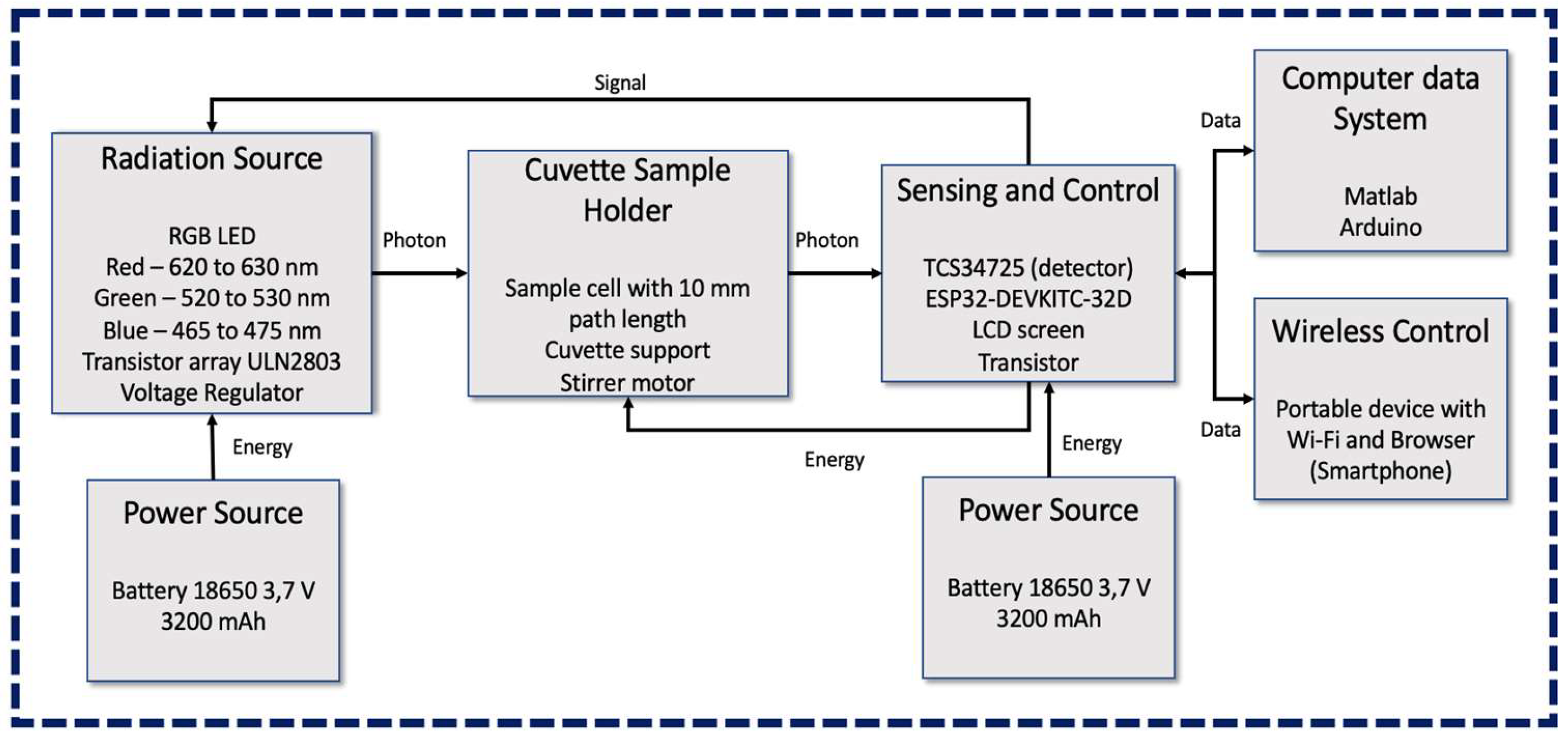
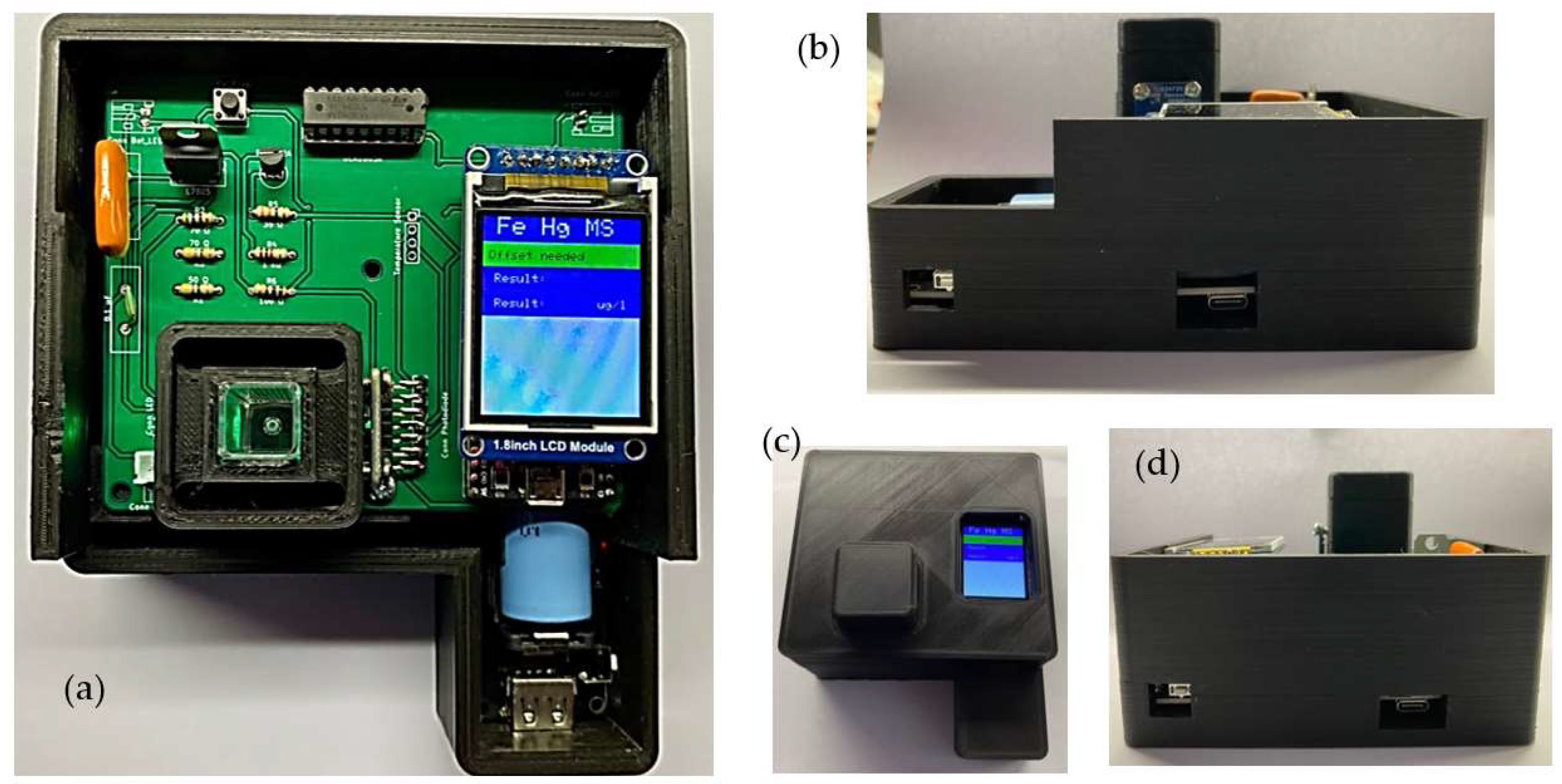
2.1. Instrument Description
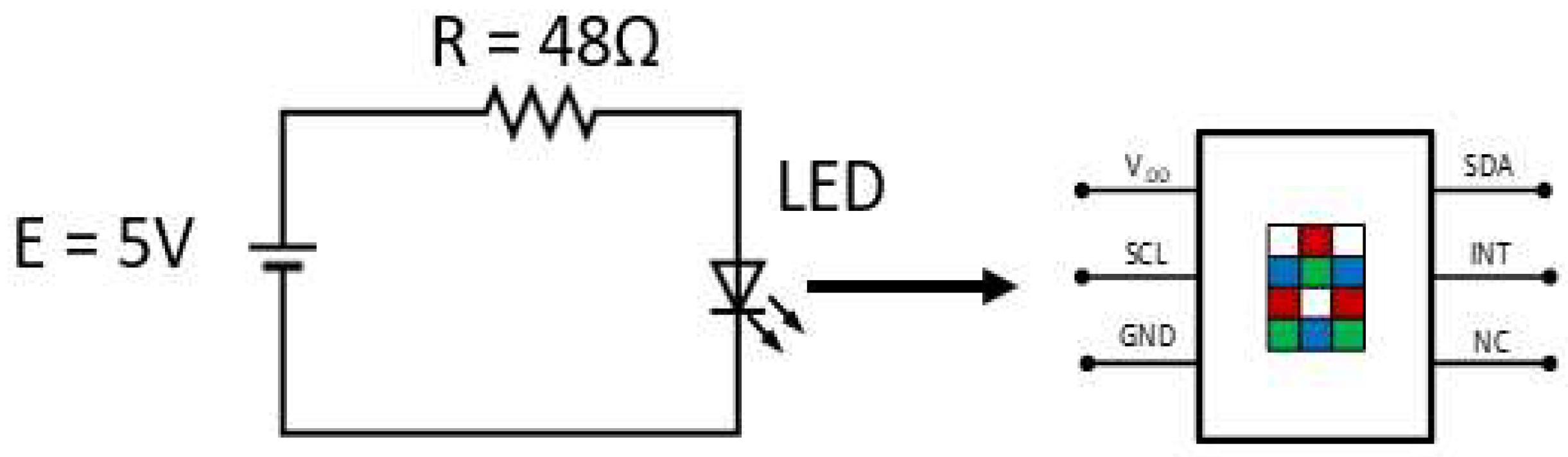
2.2. Sensing System Design
2.3. Iron Measurement System Design and Setup
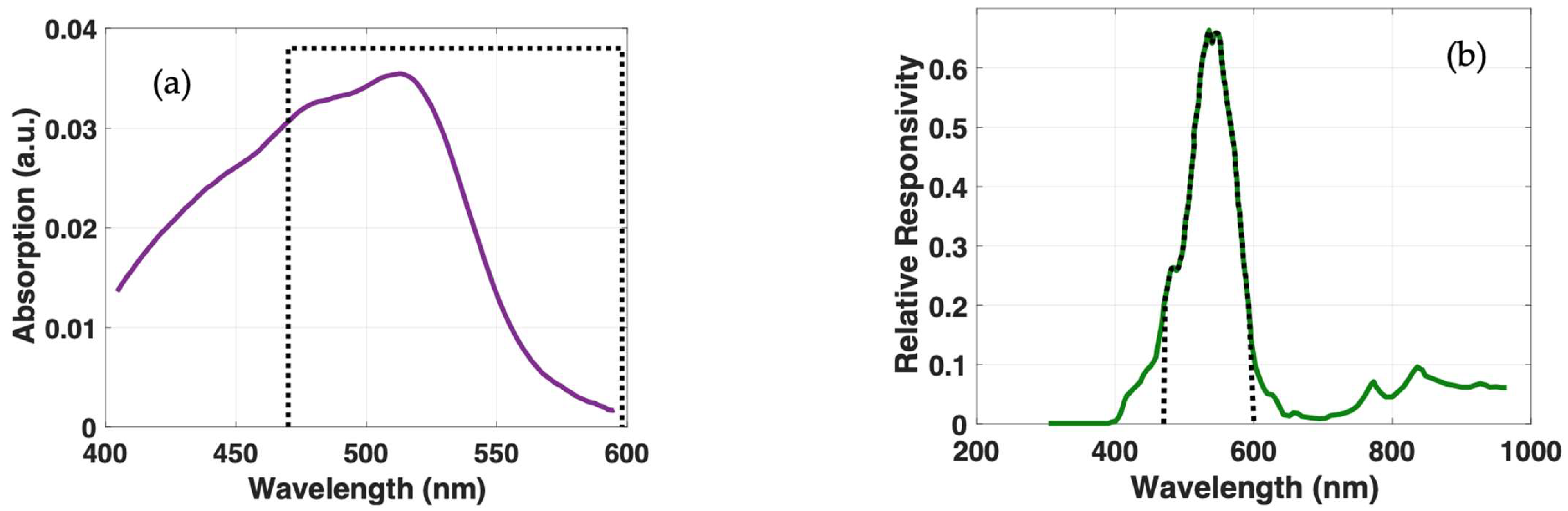
2.4. Photon Emission Characterization
2.5. Chemical Reagents and Fe2+ Samples
- (1)
- Stock solution of Fe2+ of 25 mg Fe2+/L obtained by diluting 0.0176 g of Fe(NH4)2(SO4)2•6H2O (Panreac) and one drop of concentrated hydrochloric acid to 100 mL.
- (2)
- Solution of hydroxylamine (0.1 M) prepared by dissolving 10 g of NH2OH•HCl (Panreac) to 100 mL.
- (3)
- Acetate buffer solution with pH 4.5, obtained by mixing 65 mL of acetic acid 0.1 M with 35 mL of sodium acetate (Panreac) 0.1 M.
- (4)
- Phenanthroline solution (1,10−phenantroline 99+%; Acros Organics) prepared by diluting 100 mg of a homemade solid reagent (whose composition is described in the next paragraphs) and two drops of concentrated hydrochloric acid to 100 mL.
2.6. Calibration of the IMS Instrument
3. Sensitivity Analysis
4. Results and Discussion
| Instrument | Cost | Radiation Source | Measured Parameter | Sensitivity | Measuring Range | Precision | Accuracy | Recalibration | Power Source | Reagent | Wireless Communication | Remote Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMS | EUR 75 | LED | Iron (possibility to extend to more parameters) | 2.57–7.06 mg Fe2+/L | 0–1 mg Fe2+/L | ±1.0 – ±13.0 μg Fe2+/L | ±1.0–±3.7 μg Fe2+/L | Yes | Battery | Yes | Yes | Yes |
| HACH Iron DR300 [50] | EUR 952 | LED | Iron | - | 0–5 mg Fe2+/L | ±1.0 ± 0.2 mg/L Fe | - | Yes | Battery | Yes | Yes | Yes |
| HANNA HI-721 [51] | EUR 65 | LED | Iron | - | 0–5 mg Fe2+/L | ±0.01 mg Fe2+/L | ±0.04 mg Fe2+/L | Yes | Battery | Yes | No | No |
| Phytoplankton Fluorescence [52] | USD 150 | LED | Phytoplankton | - | 0.029 to 32.6 μg cla/L | - | - | Yes | Battery | No | No | No |
| Smart Turbidimeter [49] | EUR 8.30 | LED | Turbidimetry | - | 0–200 NTU | - | - | Yes | Battery | No | No | No |
| Water Turbidity Monitoring System [53] | - | LED | Turbidimetry | - | 0–40 NTU | - | - | Yes | - | No | No | No |
| Photoelectrochemical detection of L-Dopa detector [54] | - | LED | L-Dopa | 31.8 μA/L mmol | 20 up to 190 μmol/L | - | - | Yes | - | Yes | No | No |
| FOS-SI-LOV [55] | - | LED | Free chlorine | - | 10 to 400 μg/L | - | - | Yes | - | Yes | No | No |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, A.; Parveen, T.; Umar, K.; Ibrahim, M. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Albert, J.; Destouni, G.; Duke-Sylvester, S.; Magurran, A.; Oberdorff, T.; Reis, R.; Winemiller, K.; Ripple, W. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1988, 32. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31998L0083&from=EN (accessed on 1 November 2022).
- Ji, Y.; Wu, J.; Wang, Y.; Elumalai, V.; Subramani, T. Seasonal Variation of Drinking Water Quality and Human Health Risk Assessment in Hancheng City of Guanzhong Plain, China. Expo. Health 2020, 12, 469–485. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha; Shah, A.; Saeed, F.; Ali, M.; Qaisrani, S.; Dumat, C. Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 39852–39864. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Lone, F.; Bhat, R.; Mir, S.; Dar, Z.; Dar, S. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; pp. 1–26. Available online: https://link.springer.com/chapter/10.1007/978-3-030-35691-0_1 (accessed on 1 November 2022).
- Adesakin, T.; Oyewale, A.; Bayero, U.; Mohammed, A.; Aduwo, I.; Ahmed, P.; Abubakar, N.; Barje, I. Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon 2020, 6, e04773. [Google Scholar] [CrossRef]
- Yaqoob, A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.; Qari, H.; Umar, K.; Ibrahim, M.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Kotalik, C.; Cadmus, P.; Clements, W. Indirect Effects of Iron Oxide on Stream Benthic Communities: Capturing Ecological Complexity with Controlled Mesocosm Experiments. Environ. Sci. Technol. 2019, 53, 11532–11540. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Yang, Z.; Wang, Z.; Guo, C. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.; Ayton, S.; Bush, A. Iron and Alzheimer’s Disease: An Update on Emerging Mechanisms. J. Alzheimers Dis. 2018, 64, S379–S395. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, B.; Somarakis, A.; de Haan, L.; Dzyubachyk, O.; IJsselsteijn, M.; de Miranda, N.; Lelieveldt, B.; Dijkstra, J.; van Roon-Mom, W.; Höllt, T.; et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol. Commun. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Ndayisaba, A.; Kaindlstorfer, C.; Wenning, G. Iron in neurodegeneration—Cause or consequence? Front. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Reichmann, H. Role of iron in neurodegenerative diseases. J. Neural Transm. 2016, 123, 389–399. [Google Scholar] [CrossRef]
- Kempf, T.; Wollert, K. Iron and atherosclerosis: Too much of a good thing can be bad. Eur. Heart J. 2020, 41, 2696–2698. [Google Scholar] [CrossRef]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef]
- Sanjeevi, N.; Freeland-Graves, J.; Beretvas, N.; Sachdev, P. Trace element status in type 2 diabetes: A meta-analysis. J. Clin. Diagn. Res. 2018, 12, OE01. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, K.; Cen, C.; Wu, J.; Wu, X. Roles of metal ions in regulating the formation of a drinking water odorant (2,3,6-trichloroanisole) by Sphingomonas ursincola in drinking water. Sci. Total Environ. 2021, 776, 146054. [Google Scholar] [CrossRef]
- Liew, C.; Li, X.; Zhang, H.; Lee, H. A fully automated analytical platform integrating water sampling-miniscale-liquid-liquid extraction-full evaporation dynamic headspace concentration-gas chromatography-mass spectrometry for the analysis of ultraviolet filters. Anal. Chim. Acta 2018, 1006, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Mota, F.; Albuquerque, V.; Alexandria, A. Development of a Robotic Airboat for Online Water Quality Monitoring in Lakes. Robot 2019, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Cassivi, A.; Tilley, E.; Waygood, E.; Dorea, C. Evaluating self-reported measures and alternatives to monitor access to drinking water: A case study in Malawi. Sci. Total Environ. 2021, 750, 141516. [Google Scholar] [CrossRef] [PubMed]
- Didukh-Shadrina, S.; Losev, V.; Samoilo, A.; Trofimchuk, A.; Nesterenko, P. Determination of metals in natural waters by inductively coupled plasma optical emission spectroscopy after preconcentration on silica sequentially coated with layers of polyhexamethylene guanidinium and sulphonated nitrosonaphthols. Int. J. Anal. Chem. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesko, M.; Balbinot, F.; Scaglioni, P.; Nascimento, M.; Picoloto, R.; da Costa, V. Determination of halogens and sulfur in honey: A green analytical method using a single analysis. Anal. Bioanal. Chem. 2020, 412, 6475–6484. [Google Scholar] [CrossRef]
- Rocha, D.; Maringolo, V.; Araújo, A.; Amorim, C.; Montenegro, M. An overview of Structured Biosensors for Metal Ions Determination. Chemosensors 2021, 9, 324. [Google Scholar] [CrossRef]
- Hopwood, M.; Birchill, A.; Gledhill, M.; Achterberg, E.; Klar, J.; Milne, A. A Comparison between four analytical methods for the measurement of Fe(II) at nanomolar concentrations in coastal seawater. Front. Mar. Sci. 2017, 4, 192. [Google Scholar] [CrossRef] [Green Version]
- DR300 Pocket Colorimeter, Iron, Ferrover, with Box. Hach USA. Available online: https://www.hach.com/dr300-pocket-colorimeter-iron-ferrover-with-box/product?id=55321383871 (accessed on 24 April 2022).
- Model DC1500 Iron Colorimeter Lab. Available online: https://lamotte.com/model-dc1500-iron-colorimeter-lab (accessed on 24 April 2022).
- Kareem, H.; Dunaev, D. The Working Principles of ESP32 and Analytical Comparison of using Low-Cost Microcontroller Modules in Embedded Systems Design. In Proceedings of the 2021 4th International Conference on Circuits, Systems and Simulation (ICCSS), Kuala Lumpur, Malaysia, 28 May 2021; pp. 130–135. [Google Scholar] [CrossRef]
- Maier, A.; Sharp, A.; Vagapov, Y. Comparative analysis and practical implementation of the ESP32 microcontroller module for the internet of things. In Proceedings of the 2017 Internet Technologies and Applications (ITA), Wrexham, UK, 12–15 September 2017; pp. 143–148. [Google Scholar] [CrossRef]
- Dasgupta, P.; Bellamy, H.; Liu, H.; Lopez, J.; Loree, E.; Morris, K.; Petersen, K.; Mir, K. Light emitting diode based flow-through optical absorption detectors. Talanta 1993, 40, 53–74. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Pan, Z.; Li, D. A High-Performance Optoelectronic Sensor Device for Nitrate Nitrogen in Recirculating Aquaculture Systems. Sensors 2018, 18, 3382. [Google Scholar] [CrossRef]
- Fitzhenry, C.; Jowett, L.; Roche, P.; Harrington, K.; Moore, B.; Paull, B.; Murray, E. Portable analyser using two-dimensional ion chromatography with ultra-violet light-emitting diode-based absorbance detection for nitrate monitoring within both saline and freshwaters. J. Chromatogr. A 2021, 1652, 462368. [Google Scholar] [CrossRef]
- Fay, C.; Nattestad, A. Advances in Optical Based Turbidity Sensing Using LED Photometry (PEDD). Sensors 2021, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.; Maguire, I.; Heery, B.; Nwankire, C.; Ducrée, J.; Regan, F. PhosphaSense: A fully integrated, portable lab-on-a-disc device for phosphate determination in water. Sens. Actuators B Chem. 2017, 246, 1085–1091. [Google Scholar] [CrossRef]
- Pal, A.; Kulkarni, M.; Gupta, H.; Ponnalagu, R.; Dubey, S.; Goel, S. Portable and Autonomous Device for Real-time Colorimetric Detection: Validation for Phosphorous and Nitrite Detection. Sens. Actuators A Phys. 2021, 330, 112896. [Google Scholar] [CrossRef]
- Seetasang, S.; Kaneta, T. Portable two-color photometer based on paired light emitter detector diodes and its application to the determination of paraquat and diquat. Microchem. J. 2021, 171, 106777. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Ulusoy, H.; Morgul, U.; Ulusoy, S.; Tartaglia, A.; Yılmaz, E.; Soylak, M.; Locatelli, M.; Kabir, A. Sensitive determination of Fluoxetine and Citalopram antidepressants in urine and wastewater samples by liquid chromatography coupled with photodiode array detector. J. Chromatogr. A 2021, 1648, 462215. [Google Scholar] [CrossRef]
- Duffy, G.; Maguire, I.; Heery, B.; Gers, P.; Ducrée, J.; Regan, F. ChromiSense: A colourimetric lab-on-a-disc sensor for chromium speciation in water. Talanta 2018, 178, 392–399. [Google Scholar] [CrossRef]
- Yan, J.; Ren, J.; Ren, L.; Yang, Y.; Yang, S.; Ren, T. A novel structure design and fabrication method for low liquid consumption and high precision device of colorimeter in water quality detection. Sens. Actuators A Phys. 2019, 289, 1–10. [Google Scholar] [CrossRef]
- Oliveira, G.C.; Machado, C.; Inácio, D.; da Silveira Petruci, J.; Silva, S. RGB color sensor for colorimetric determinations: Evaluation and quantitative analysis of colored liquid samples. Talanta 2022, 241, 123244. [Google Scholar] [CrossRef]
- De Berg, K.; Maeder, M.; Clifford, S. A new approach to the equilibrium study of iron(III) thiocyanates which accounts for the kinetic instability of the complexes particularly observable under high thiocyanate concentrations. Inorg. Chim. Acta 2016, 445, 155–159. [Google Scholar] [CrossRef]
- Amendola, C.; Lacerenza, M.; Pirovano, I.; Contini, D.; Spinelli, L.; Cubeddu, R.; Torricelli, A.; Re, R. Optical characterization of 3D printed PLA and ABS filaments for diffuse optics applications. PLoS ONE 2021, 16, e0253181. [Google Scholar] [CrossRef]
- Hossain, M.; Biswas, P.; Rani, S.; Eskender, S.; Islam, M.; Chakma, A.; Canning, J. Low-Cost 3D Printer Drawn Optical Microfibers for Smartphone Colorimetric Detection. Biosensors 2022, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement—100:2008; International Organization for Standardization: Geneva, Switzerland, 2008.
- Parra, L.; Rocher, J.; Escrivá, J.; Lloret, J. Design and development of low cost smart turbidity sensor for water quality monitoring in fish farms. Aquac. Eng. 2018, 81, 10–18. [Google Scholar] [CrossRef]
- DR300 Pocket Colorimeter, Chlorine, Free + Total, LR/HR, with Box. Available online: https://www.hach.com/p-dr300-colorimeters-parameter/LPV445.97.00110 (accessed on 8 January 2023).
- HI-721 High Range Iron Handheld Colorimeter—Checker. Available online: https://www.hannainstruments.co.uk/photometers/808-pocket-checker-for-iron-testing (accessed on 8 January 2023).
- Leeuw, T.; Boss, E.; Wright, D. In situ Measurements of Phytoplankton Fluorescence Using Low Cost Electronics. Sensors 2013, 13, 7872–7883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulyana, Y.; Hakim, D. Prototype of Water Turbidity Monitoring System. IOP Conf. Ser. Mater. Sci. Eng. 2018, 384, 012052. [Google Scholar] [CrossRef]
- Neto, S.Y.; De Cássia Silva Luz, R.; Damos, F. Visible LED light photoelectrochemical sensor for detection of L-Dopa based on oxygen reduction on TiO2 sensitized with iron phthalocyanine. Electrochem. Commun. 2016, 62, 1–4. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, J.; Fang, S.; Wang, C.; Wang, Q.; Wu, J.; Chen, J.; Duan, M. A LED-based fiber-optic sensor integrated with lab-on-valve manifold for colorimetric determination of free chlorine in water. Talanta 2017, 167, 103–110. [Google Scholar] [CrossRef]







| Parameter | Value |
|---|---|
| Holographic grating | 1200 lines/mm, blazed at 240 nm |
| Maximum resolution | 0.5 nm |
| Range | 190 to 1100 nm |
| Accuracy | ±0.20 nm (546.11 nm Hg emission line) ±30 nm (190 to 900 nm) |
| Repeatable peak separation of repetitive scanning of Hg line source | <0.10 nm |
| Standard deviation of 10 measurements | <0.05 nm |
| Accuracy of instrument | 1A: ±0.004 A 2A: ±0.004 A 3A: ±0.006 A |
| Repeatability of light intensity measurement | 1A: ±0.0025 A |
| Drift | <0.0005 Abs/h at 500 nm, 2.0 nm SBW, 2 h warmup |
| Baseline flatness | ±0.0015 A (200–800 nm), 2.0 nm SBW, smoothed |
| Standard Solution (µg Fe2+/L) | Standard Sample Uncertainty (µg Fe2+/L) | First Series | Second Series | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMS (µg Fe2+/L) | IMS Prec. (µg Fe2+/L) | IMS Uncert. (µg Fe2+/L) | Nicolet (µg Fe2+/L) | Nicolet Prec. (µg Fe2+/L) | Nicolet Uncert. (µg Fe2+/L) | IMS (µg Fe2+/L) | IMS Prec. (µg Fe2+/L) | IMS Uncert. (µg Fe2+/L) | Nicolet (µg Fe2+/L) | Nicolet Prec. (µg Fe2+/L) | Nicolet Uncert. (µg Fe2+/L) | ||
| 25 | ±0.25 | - | - | - | - | 28.7 | ±88.3 | ±9.4 | 28.2 | ±795.2 | ±28.2 | ||
| 50 | ±0.50 | 54.8 | ±27.0 | ±5.2 | 54.7 | ±275.5 | ±16.6 | 44.4 | ±22.0 | ±4.7 | 41.3 | ±795.2 | ±28.2 |
| 75 | ±0.75 | - | - | - | - | - | - | 75.6 | ±36.0 | ±6.0 | 79.4 | ±795.2 | ±28.2 |
| 100 | ±1.00 | 98.4 | ±25.0 | ±5.0 | 98.1 | ±275.5 | ±16.6 | 97.0 | ±21.1 | ±4.6 | 98.6 | ±795.2 | ±28.2 |
| 125 | ±1.25 | - | - | - | - | - | - | 122.6 | ±49.0 | ±7.0 | 124.1 | ±795.2 | ±28.2 |
| 150 | ±1.50 | - | - | - | - | - | - | 152.2 | ±36.0 | ±6.0 | 149.7 | ±795.2 | ±28.2 |
| 200 | ±2.00 | 196.6 | ±18.4 | ±4.3 | 196,0 | ±275.5 | ±16.6 | 201.5 | ±28.0 | ±5.3 | 200.9 | ±795.2 | ±28.2 |
| 500 | ±5.00 | 513.8 | ±28.0 | ±5.3 | 511.2 | ±275.5 | ±16.6 | - | - | - | - | - | - |
| 1000 | ±10.00 | 993.3 | ±27.0 | ±5.2 | 994.9 | ±275.5 | ±16.6 | - | - | - | - | - | - |
| Standard Solution (µg Fe2+/L) | Standard Sample Uncertainty (µg Fe2+/L) | Third Series | Fourth Series | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMS µg (Fe2+/L) | IMS Prec. (µg Fe2+/L) | IMS Uncert. (µg Fe2+/L) | Nicolet (µg Fe2+/L) | Nicolet Prec. (µg Fe2+/L) | Nicolet Uncert. (µg Fe2+/L) | IMS (µg Fe2+/L) | IMS Prec. (µg Fe2+/L) | IMS Uncert. (µg Fe2+/L) | Nicolet (µg Fe2+/L) | Nicolet Prec. (µg Fe2+/L) | Nicolet Uncert. (µg Fe2+/L) | ||
| 25 | ±0.25 | 25.4 | ±13.6 | ±3.7 | 25.3 | ±470.8 | ±21.7 | 21.27 | ±46.2 | ±6.8 | 27.4 | ±750.6 | ±27.4 |
| 50 | ±0.50 | 50.3 | ±9.0 | ±3.0 | 48.6 | ±470.8 | ±21.7 | 48.58 | ±39.6 | ±6.3 | 43.3 | ±750.6 | ±27.4 |
| 75 | ±0.75 | 73.3 | ±4.0 | ±2.0 | 77.7 | ±470.8 | ±21.7 | - | - | - | - | - | - |
| 100 | ±1.00 | 101.0 | ±5.2 | ±2.3 | 95.2 | ±470.8 | ±21.7 | 101.19 | ±193.2 | ±13.9 | 91,0 | ±750.6 | ±27.4 |
| 125 | ±1.25 | 122.3 | ±3.6 | ±1.9 | 124.3 | ±470.8 | ±21.7 | - | - | - | - | - | - |
| 150 | ±1.50 | 154.0 | ±4.8 | ±2.2 | 159.3 | ±470.8 | ±21.7 | 158.1 | ±163.8 | ±12.8 | 159.9 | ±750.6 | ±27.4 |
| 200 | ±2.00 | 198.4 | ±1.0 | ±1.0 | 194.2 | ±470.8 | ±21.7 | 193.4 | ±46.2 | ±6.8 | 197.0 | ±750.6 | ±27.4 |
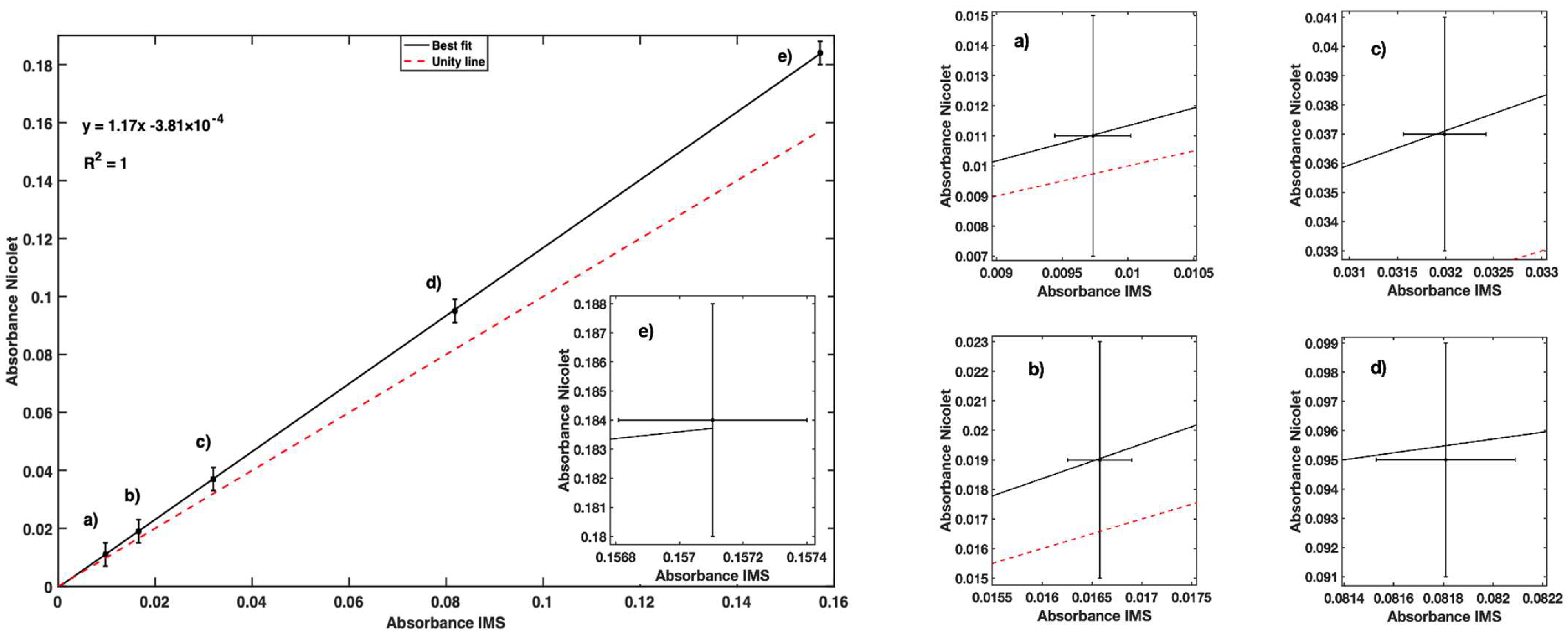
| Sample Conc. (Fe2+/L) | IMS Mean Abs. | Nicolet Abs. (525 nm) | IMS Variance |
|---|---|---|---|
| 50 | 0.010 | 0.011 | 2.06 × 10−8 |
| 100 | 0.016 | 0.019 | 2.59 × 10−8 |
| 200 | 0.032 | 0.037 | 4.62 × 10−8 |
| 500 | 0.081 | 0.095 | 1.96 × 10−8 |
| 1000 | 0.157 | 0.184 | 2.18 × 10−8 |
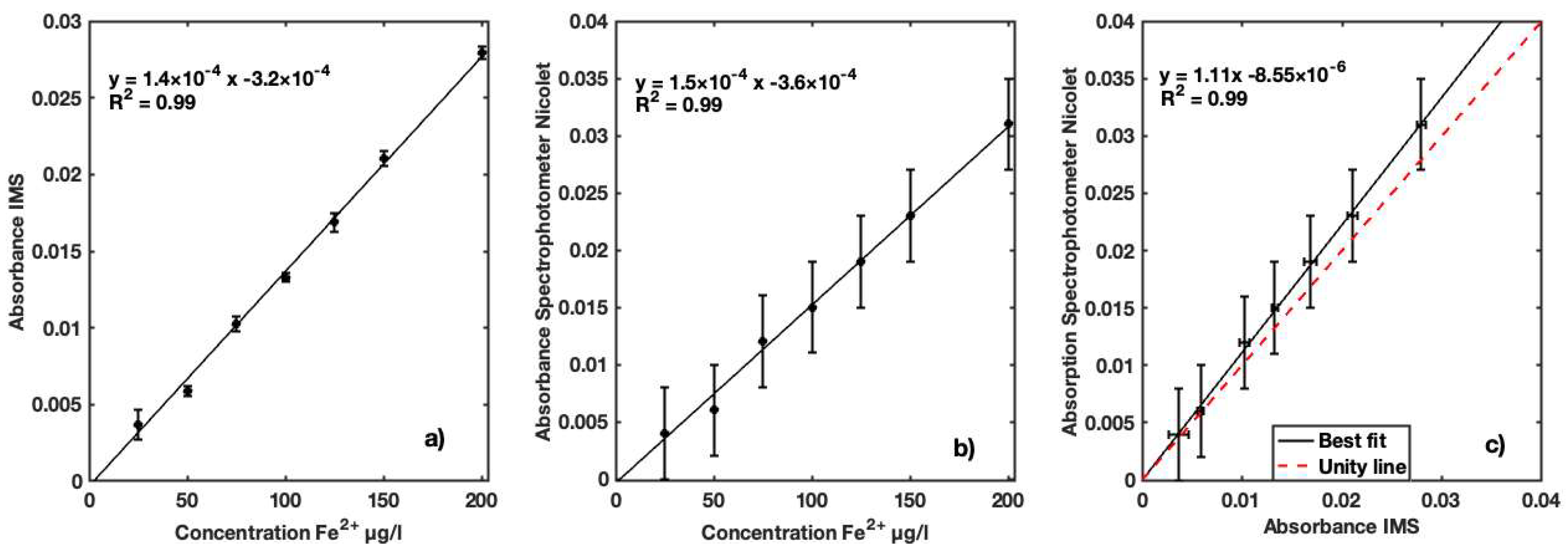
| Sample Conc. (µg Fe2+/L) | IMS Mean Abs. | Nicolet Abs. (525 nm) | IMS Variance | Retrieved (µg Fe2+/L) | Recovery (%) |
|---|---|---|---|---|---|
| 25 | 0.003 | 0.004 | 2.37 × 10−7 | 27.62 | 110.50 |
| 50 | 0.005 | 0.006 | 2.46 × 10−8 | 51.97 | 103.95 |
| 75 | 0.010 | 0.012 | 6.15 × 10−8 | 74.49 | 99.32 |
| 100 | 0.013 | 0.015 | 2.21 × 10−8 | 101.71 | 101.71 |
| 125 | 0.016 | 0.019 | 9.99 × 10−8 | 122.52 | 98.02 |
| 150 | 0.021 | 0.023 | 6.12 × 10−8 | 153.54 | 102.36 |
| 200 | 0.027 | 0.031 | 3.96 × 10−8 | 197.09 | 98.54 |
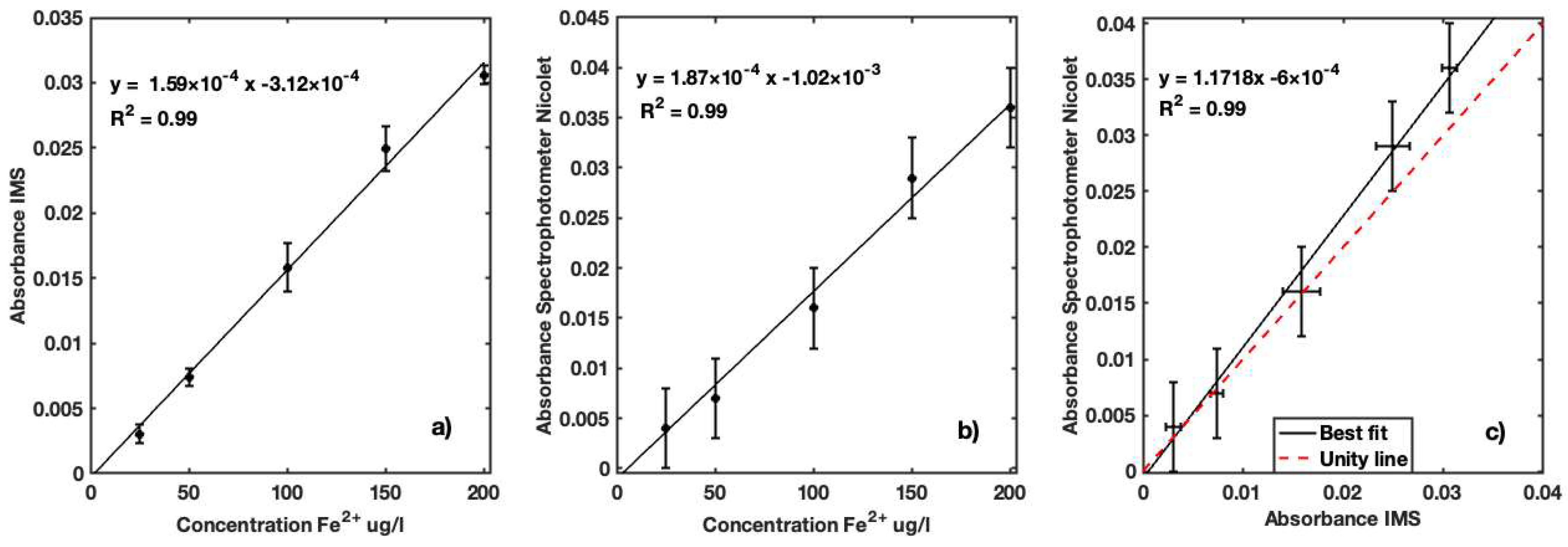
| Sample Conc. (µg Fe2+/L) | IMS Mean Abs. | Nicolet Abs. (525 nm) | IMS Variance |
|---|---|---|---|
| 25 | 0.004 | 0.007 | 5.47 × 10−8 |
| 50 | 0.008 | 0.011 | 8.30 × 10−8 |
| 75 | 0.012 | 0.016 | 1.32 × 10−7 |
| 100 | 0.016 | 0.019 | 1.17 × 10−7 |
| 125 | 0.019 | 0.024 | 1.35 × 10−7 |
| 150 | 0.024 | 0.030 | 1.19 × 10−7 |
| 200 | 0.031 | 0.036 | 3.58 × 10−7 |
| Sample Conc. (µg Fe2+/L) | IMS Mean Abs. | Nicolet Abs. (525 nm) | IMS Variance |
|---|---|---|---|
| 25 | 0.003 | 0.004 | 1.30 × 10−7 |
| 50 | 0.007 | 0.007 | 1.03 × 10−7 |
| 100 | 0.015 | 0.016 | 8.63 × 10−7 |
| 150 | 0.024 | 0.029 | 7.14 × 10−7 |
| 200 | 0.030 | 0.036 | 1.31 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, S.; Tlemçani, M.; Bortoli, D.; Feliciano, M.; Lopes, M.E. A Portable Measurement Device Based on Phenanthroline Complex for Iron Determination in Water. Sensors 2023, 23, 1058. https://doi.org/10.3390/s23031058
Fernandes S, Tlemçani M, Bortoli D, Feliciano M, Lopes ME. A Portable Measurement Device Based on Phenanthroline Complex for Iron Determination in Water. Sensors. 2023; 23(3):1058. https://doi.org/10.3390/s23031058
Chicago/Turabian StyleFernandes, Samuel, Mouhaydine Tlemçani, Daniele Bortoli, Manuel Feliciano, and Maria Elmina Lopes. 2023. "A Portable Measurement Device Based on Phenanthroline Complex for Iron Determination in Water" Sensors 23, no. 3: 1058. https://doi.org/10.3390/s23031058






