Gas Sensor with Different Morphology of PANI Layer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensor Platform
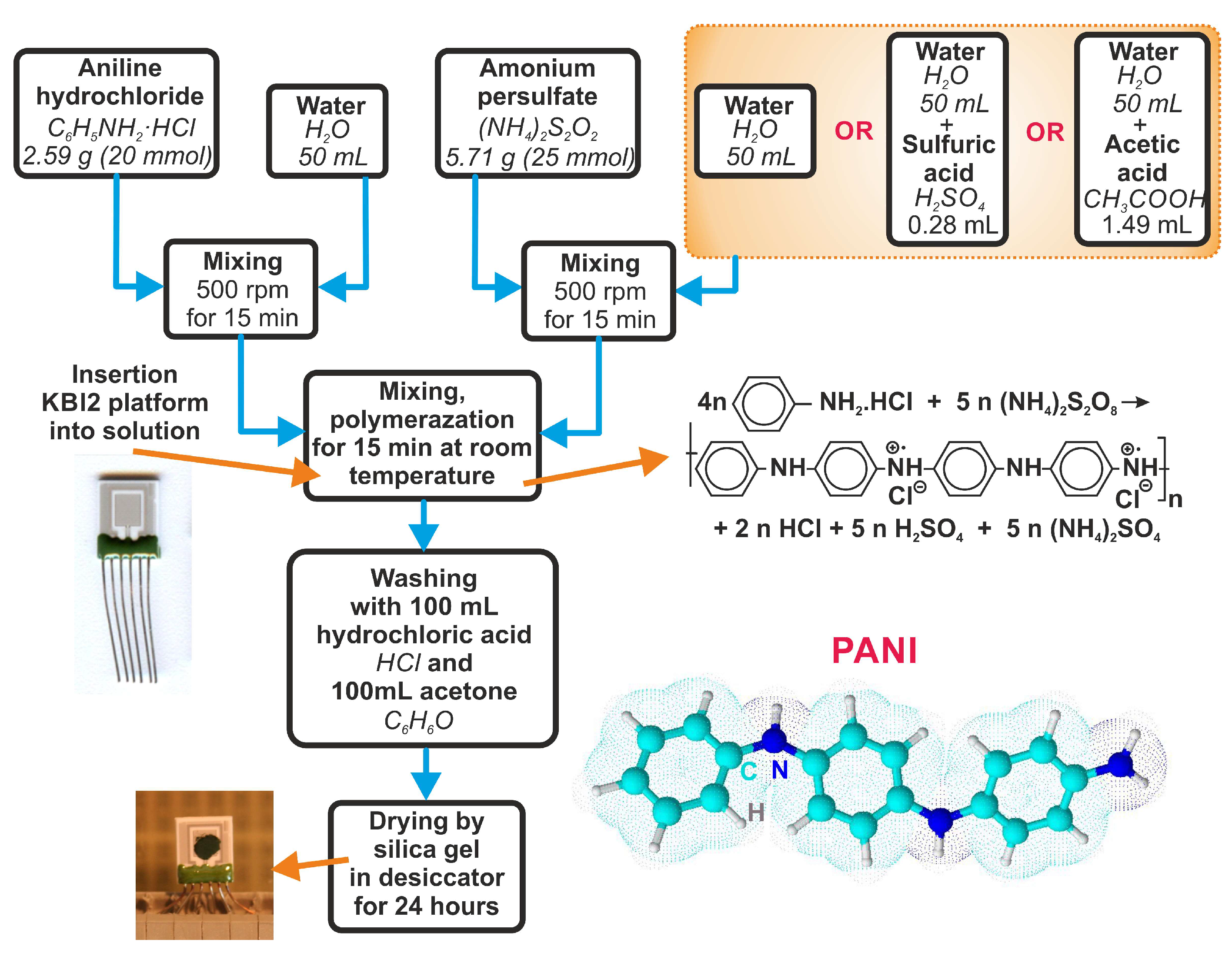
2.3. Material Synthesis and Sensor Fabrication
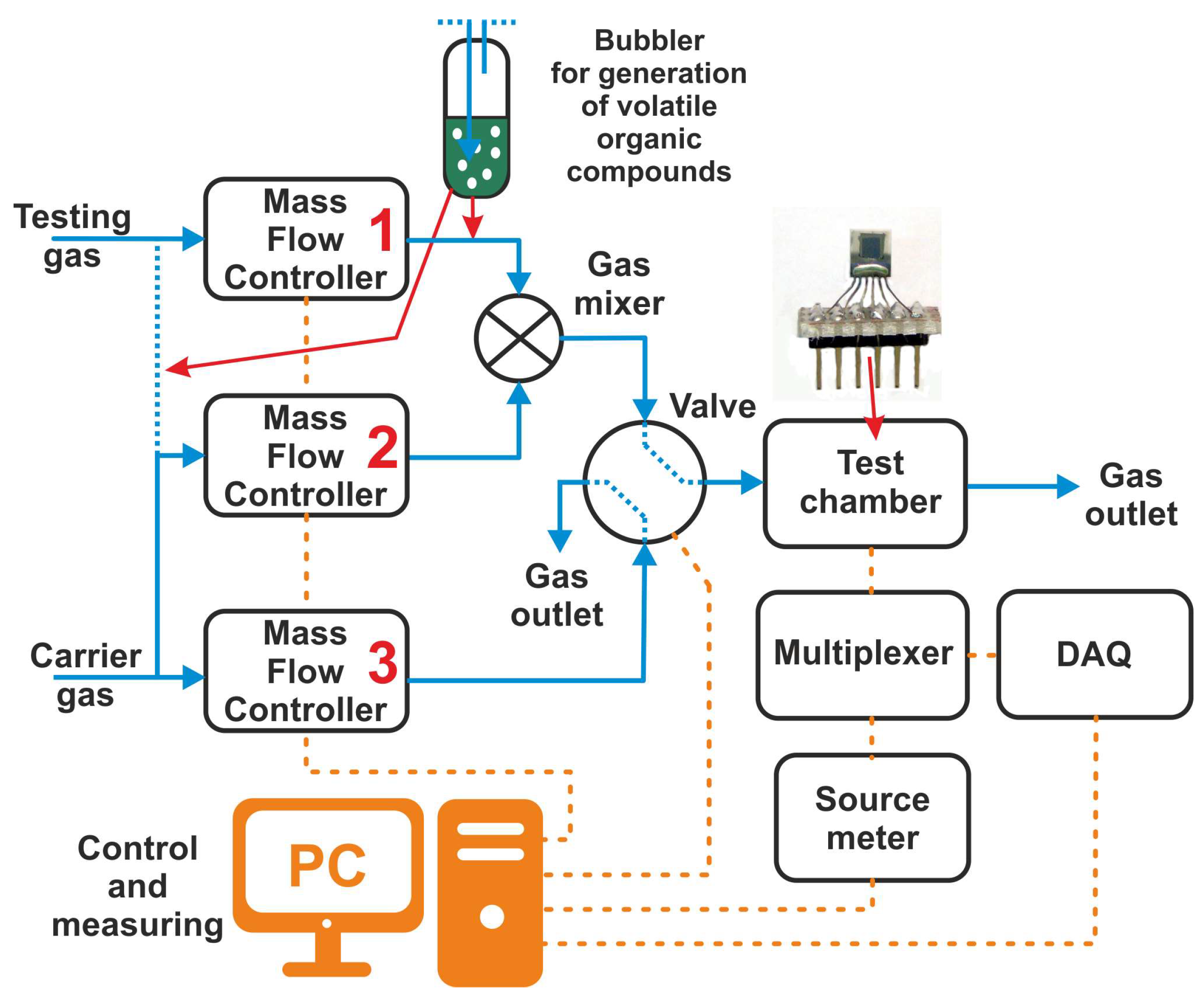
2.4. Preparation of the Gas Testing System
3. Results and Discussion
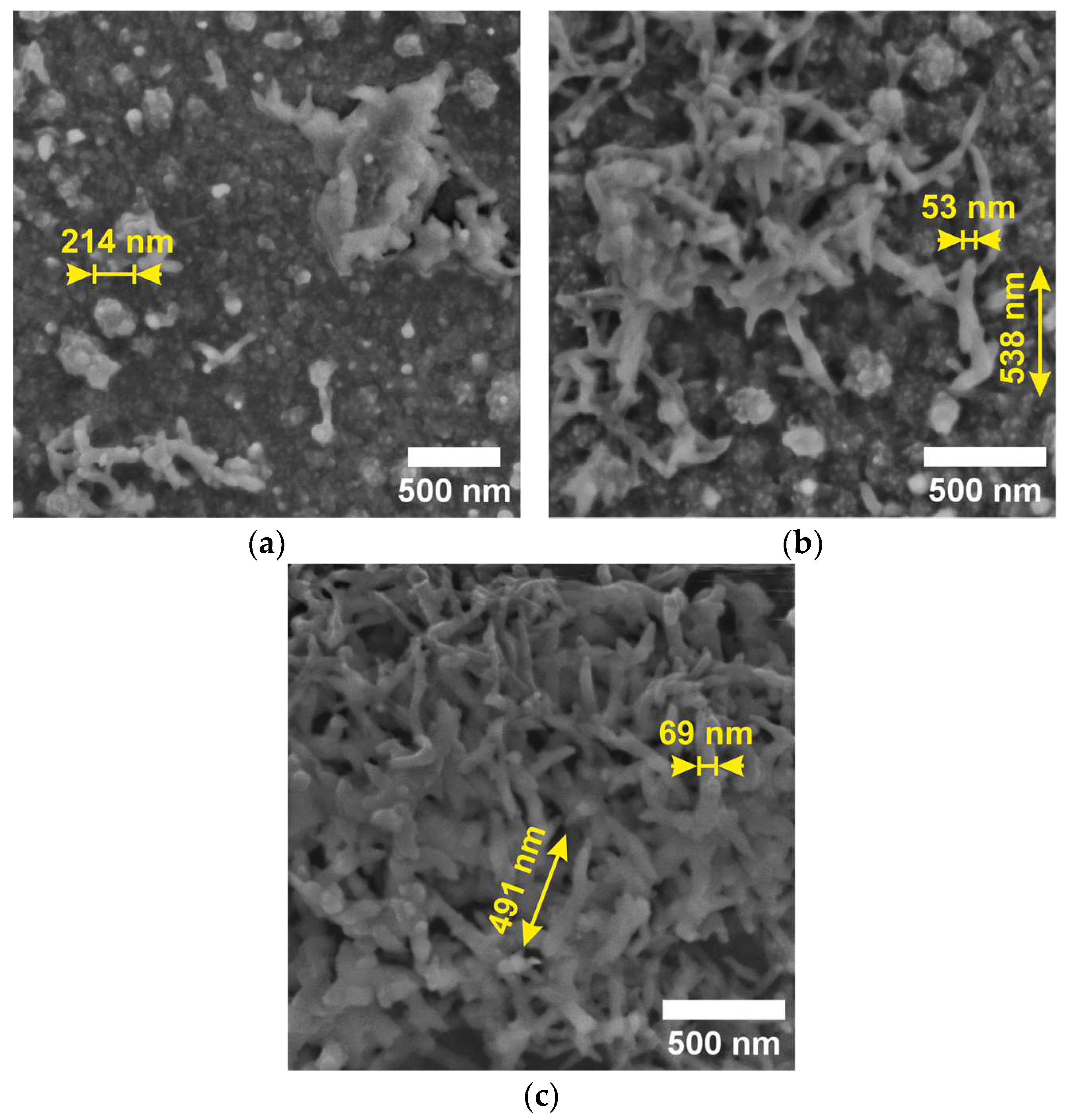
3.1. Scanning Electron Microscopy (SEM) and Raman Spectroscopy
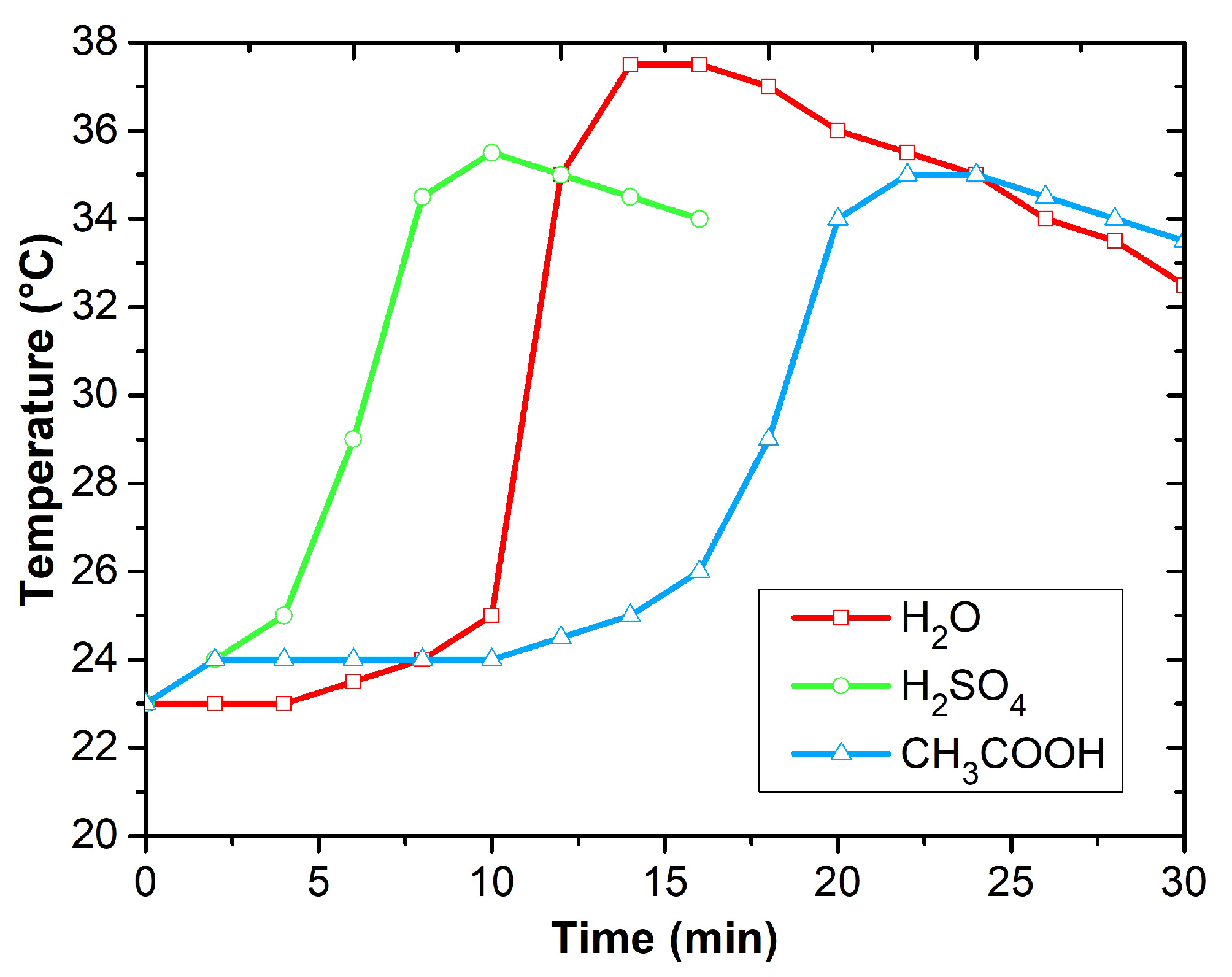
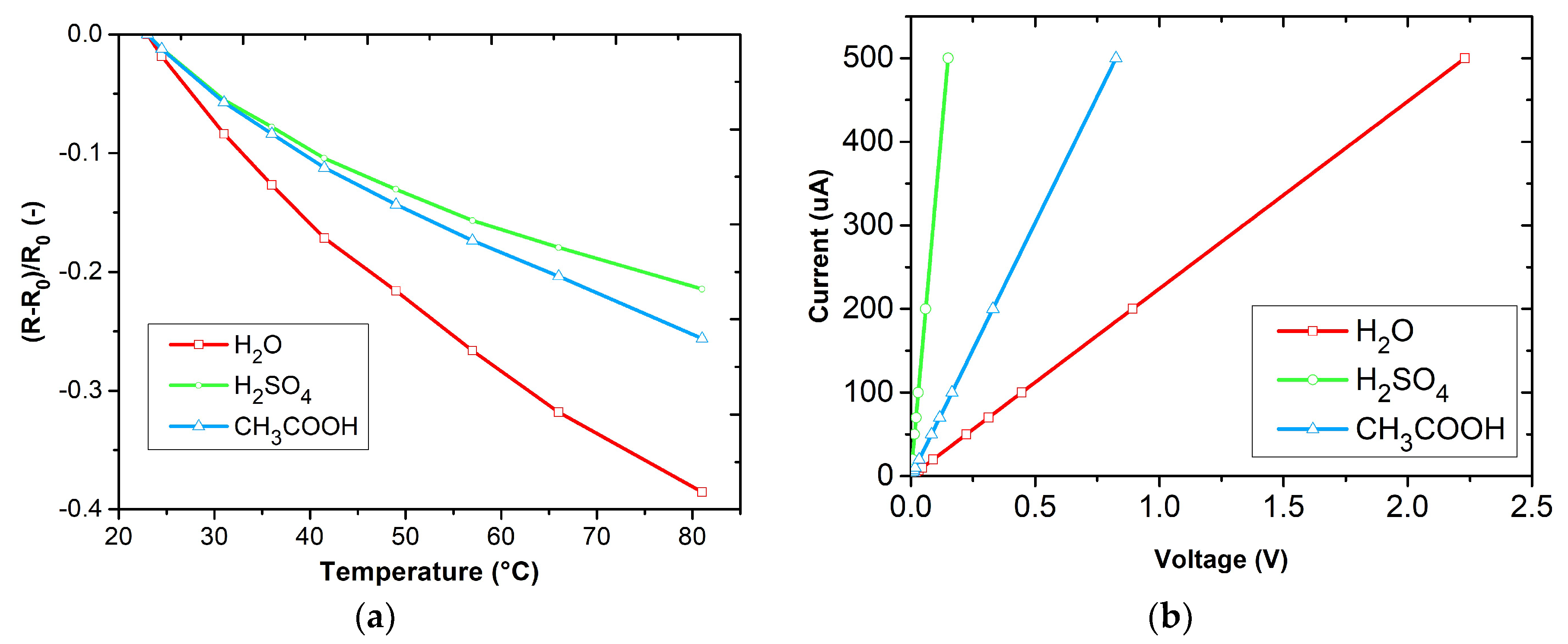
3.2. Temperature Analysis and Current-Voltage Characteristics of Polyaniline Layers
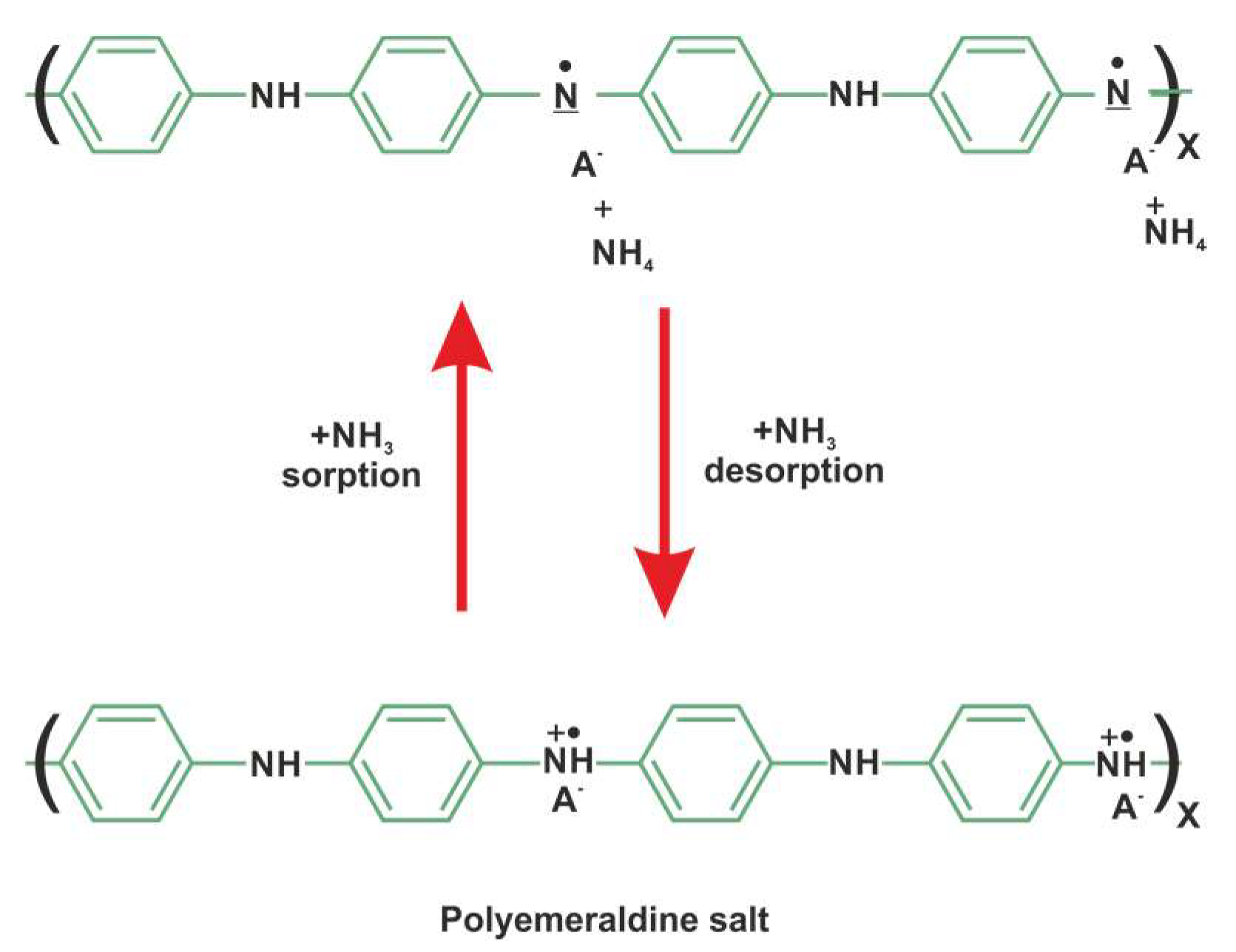
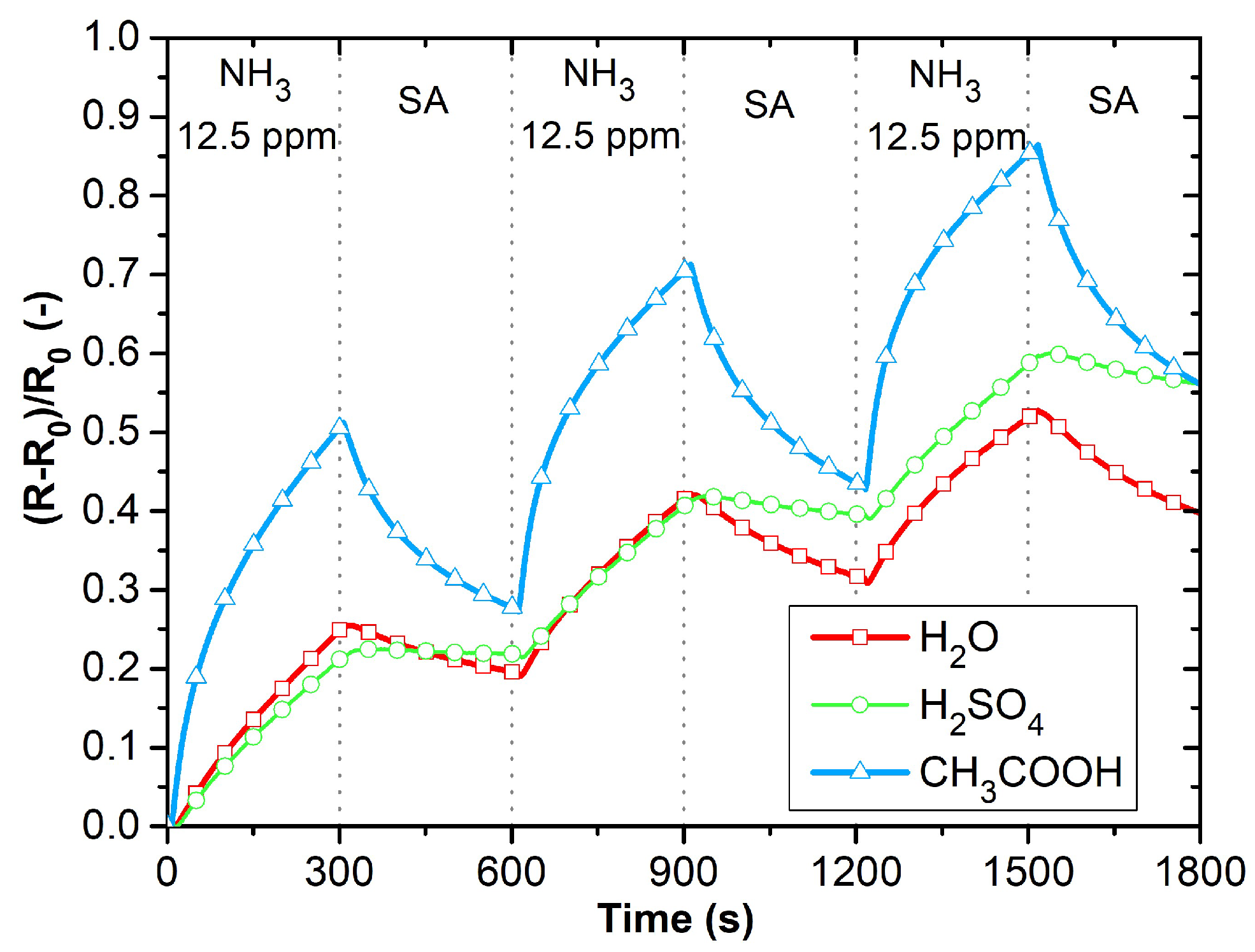
3.3. Gas Sensing Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAQ | Data Acquisition |
| DC | Direct Current |
| ID | Interdigital |
| PANI | Polyaniline |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| SA | Synthetic air |
| SEM | Scanning electron microscopy |
| VOC | Volatile Organic Compounds |
References
- Hosseini, S.H.; Khalkhali, R.A.; Noor, P. Study of polyaniline conducting/electroactive polymer as sensor for some agricultural phosphorus pesticides. Mon. Fur Chem. 2010, 141, 1049–1053. [Google Scholar] [CrossRef]
- Akbar, S.; Dutta, P.; Lee, C. High-temperature ceramic gas sensors: A review. Int. J. Appl. Ceram. Technol. 2006, 3, 302–311. [Google Scholar] [CrossRef]
- Tatara, T.; Tsuzaki, K. An apnea monitor using a rapid-response hygrometer. J. Clin. Monit. 1997, 13, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, S.; Wang, T.; Fang, W. Air-flow sensor and humidity sensor application to neonatal infant respiration monitoring. Sens. Actuators A Phys. 1995, 49, 47–50. [Google Scholar] [CrossRef]
- Sun, Y.; Ong, K.Y. Detection Technologies for Chemical Warfare Agents and Toxic Vapors; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780203485705 020348570X. [Google Scholar]
- Kroutil, J.; Laposa, A.; Voves, J.; Davydova, M.; Nahlik, J.; Kulha, P.; Husak, M. Performance Evaluation of Low-Cost Flexible Gas Sensor Array with Nanocomposite Polyaniline Films. IEEE Sens. J. 2018, 18, 3759–3766. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, G. Nanostructured Gas Sensors; Jenny Stanford Publishing: New York, NY, USA, 2022; ISBN 9781003331230. [Google Scholar]
- Nagaraju, S.C.; Roy, A.S.; Kumar, J.B.P.; Anilkumar, K.R.; Ramagopal, G. Humidity Sensing Properties of Surface Modified Polyaniline Metal Oxide Composites. J. Eng. 2014, 2014, 925020. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857867. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Feast, W.J.; Tsibouklis, J.; Pouwer, K.L.; Groenendaal, L.; Meijer, E.W. Synthesis, processing and material properties of conjugated polymers. Polymer 1996, 37, 5017–5047. [Google Scholar] [CrossRef]
- Crowley, K.; Smyth, M.; Killard, A.; Morrin, A. Printing polyaniline for sensor applications. Chem. Pap. 2013, 67, 771–780. [Google Scholar] [CrossRef]
- Kang, H.; Park, H.; Park, Y.; Jung, M.; Kim, B.C.; Wallace, G.; Cho, G. Fully Roll-to-Roll Gravure Printable Wireless (13.56 MHz) Sensor-Signage Tags for Smart Packaging. Sci. Rep. 2015, 4, 5387. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Kumar, R.; Kumar, M.; Tyagi, S.; Kumar, D. Polyaniline nanofibers based gas sensor for detection of volatile organic compounds at room temperature. Mater. Res. Express 2019, 6, 1150d3. [Google Scholar] [CrossRef]
- Safe, A.M.; Ahmadi Tabar, F.; Nikfarjam, A.; Sharif, F.; Hajghassem, H.; Mazinani, S. Hollow Polyaniline Nanofibers for Highly Sensitive Ammonia Detection Applications. IEEE Sens. J. 2019, 19, 9616–9623. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Reiner-Rozman, C.; Pichler, B.; Madi, V.; Weißenböck, P.; Hegedüs, T.; Aspermair, P.; Bintinger, J. Optimization of Printed Polyaniline Composites for Gas Sensing Applications. Sensors 2022, 22, 5379. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K.; Redon, N.; Wojkiewicz, J.-L.; Duc, C. Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems. Sensors 2020, 21, 169. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Baghaei Nejad, M.; Shahrokh Abadi, M.H.; Zou, Z.; Zheng, L.-R. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-Performance PANI-Based Ammonia Gas Sensor Promoted by Surface Nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Zhang, D.; Fan, X.; Jin, Y.; Guo, L. Room temperature ammonia gas sensor based on polyaniline/copper ferrite binary nanocomposites. Sens. Actuators B Chem. 2020, 322, 128615. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-Performance Gas Sensor of Polyaniline/Carbon Nanotube Composites Promoted by Interface Engineering. Sensors 2019, 20, 149. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Moura, L.G.; Oliveira, M.M.; Pimenta, M.A.; Zarbin, A.J.G. Resonant Raman spectroscopy and spectroelectrochemistry characterization of carbon nanotubes/polyaniline thin film obtained through interfacial polymerization. J. Raman Spectrosc. 2012, 43, 1094–1100. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting Polymers: A New Era in Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Akber, H.J.; Ibrahim, I.M.; Razeg, K.H. Hydrothermal Synthesis of Polyaniline Nano-fibers as H2S Gas Sensor. J. Phys. Conf. Ser. 2020, 1664, 012017. [Google Scholar] [CrossRef]
- Chabukswar, V.V.; Pethkar, S.; Athawale, A.A. Acrylic acid doped polyaniline as an ammonia sensor. Sens. Actuators B Chem. 2001, 77, 657–663. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Hernandez, A.; O’Malley, E.; Whitten, P.G.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of an ammonia gas sensor using inkjet-printed polyaniline nanoparticles. Talanta 2008, 77, 710–717. [Google Scholar] [CrossRef]
- Rizzo, G.; Arena, A.; Donato, N.; Latino, M.; Saitta, G.; Bonavita, A.; Neri, G. Flexible, all-organic ammonia sensor based on dodecylbenzene sulfonic acid-doped polyaniline films. Thin Solid Film. 2010, 518, 7133–7137. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, F.; Li, F.; Zhu, Y. Ammonia sensitivity of polyaniline films via emulsion polymerization. Eur. Polym. J. 2000, 36, 679–683. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Asahi, T. Properties and stability of polyaniline nanofiber ammonia sensors fabricated by novel on-substrate method. Sens. Actuators B Chem. 2011, 160, 999–1004. [Google Scholar] [CrossRef]









| Raman Shift (cm−1) | Description of Band |
|---|---|
| 748 | Q ring bending, C–C ring deformation |
| 810 | out-of-plane C–H vibrations in the aromatic rings |
| 1169 | C–H bending of the quinoid rings |
| 1221 | C–N in benzene diamine units |
| 1260 | C–N in benzene diamine units |
| 1336 | C–N+, a characteristic band of the polaron radical cation |
| 1412 | phenazine structures |
| 1498 | C=N of the quinoid nonprotonated diimine units |
| 1590 | C=C stretching vibration of the quinonoid ring |
| Sensitive Layer | α (K−1) |
|---|---|
| PANI polymerized in H2O | −0.0066 |
| PANI polymerized in H2SO4 | −0.0038 |
| PANI polymerized in CH3COOH | −0.0044 |
| Sensitive Layer | R (Ω) |
|---|---|
| PANI polymerized in H2O | 4470 |
| PANI polymerized in H2SO4 | 306 |
| PANI polymerized in CH3COOH | 1660 |
| PANI Preparation Technology | Deposition Method of PANI | Substrate | Electrode Material | NH3 Concentration (ppm) | Operating Temperature (°C) | Relative Humidity (%) | Response ΔR/R0 (-) | Time Responses/Recovery (s) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PANI acrylic -acid-doped | - | - | - | 58 | 25 | - | 0.99 | 60/240 | [29] |
| Ink-jet printing | Ink-jet | PET | Ag | 50 | 70 | - | 0.15 | 100/200 | [30] |
| PANI doped with dodecylbenzenesulfonic acid | Spin coating | Polyester (Tartan 950) | MWCNT ink | 20 | 28 | 45 | 0.15 | 300/900 | [31] |
| PANI doped with dodecylbenzenesulfonic acid | data | Al2O3 | Au | 50 | 25 | Dry air | 0.8 | 90/180 | [32] |
| PANI nanofibers doped with HCl | Spin coating | Al2O3 | Au | 200 | 50 | Dry N2 | 2.9 | 600/300 | [33] |
| PANI prepared by polymerization in solution on a substrate | Coating during polymerization | Al2O3 | Pt | 50 | 25 | Dry synthetic air | 0.85 | 300/- | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroutil, J.; Laposa, A.; Povolny, V.; Klimsa, L.; Husak, M. Gas Sensor with Different Morphology of PANI Layer. Sensors 2023, 23, 1106. https://doi.org/10.3390/s23031106
Kroutil J, Laposa A, Povolny V, Klimsa L, Husak M. Gas Sensor with Different Morphology of PANI Layer. Sensors. 2023; 23(3):1106. https://doi.org/10.3390/s23031106
Chicago/Turabian StyleKroutil, Jiri, Alexandr Laposa, Vojtech Povolny, Ladislav Klimsa, and Miroslav Husak. 2023. "Gas Sensor with Different Morphology of PANI Layer" Sensors 23, no. 3: 1106. https://doi.org/10.3390/s23031106
APA StyleKroutil, J., Laposa, A., Povolny, V., Klimsa, L., & Husak, M. (2023). Gas Sensor with Different Morphology of PANI Layer. Sensors, 23(3), 1106. https://doi.org/10.3390/s23031106







