Role of Defects of Carbon Nanomaterials in the Detection of Ovarian Cancer Cells in Label-Free Electrochemical Immunosensors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Oxidation of MWCNTs, VGCFs, KS, and SP
2.4. Preparation of AuNPs-Carbon-Nafion (AuNPs@carbon)
2.5. Electrodeposition of AuNPs@Carbon Composites on GCE Electrode
2.6. Fabrication of the Immunosensor
3. Results and Discussion
3.1. Scanning Electron Microscopy Characterization of the Carbon Nanomaterial-Modified Electrodes
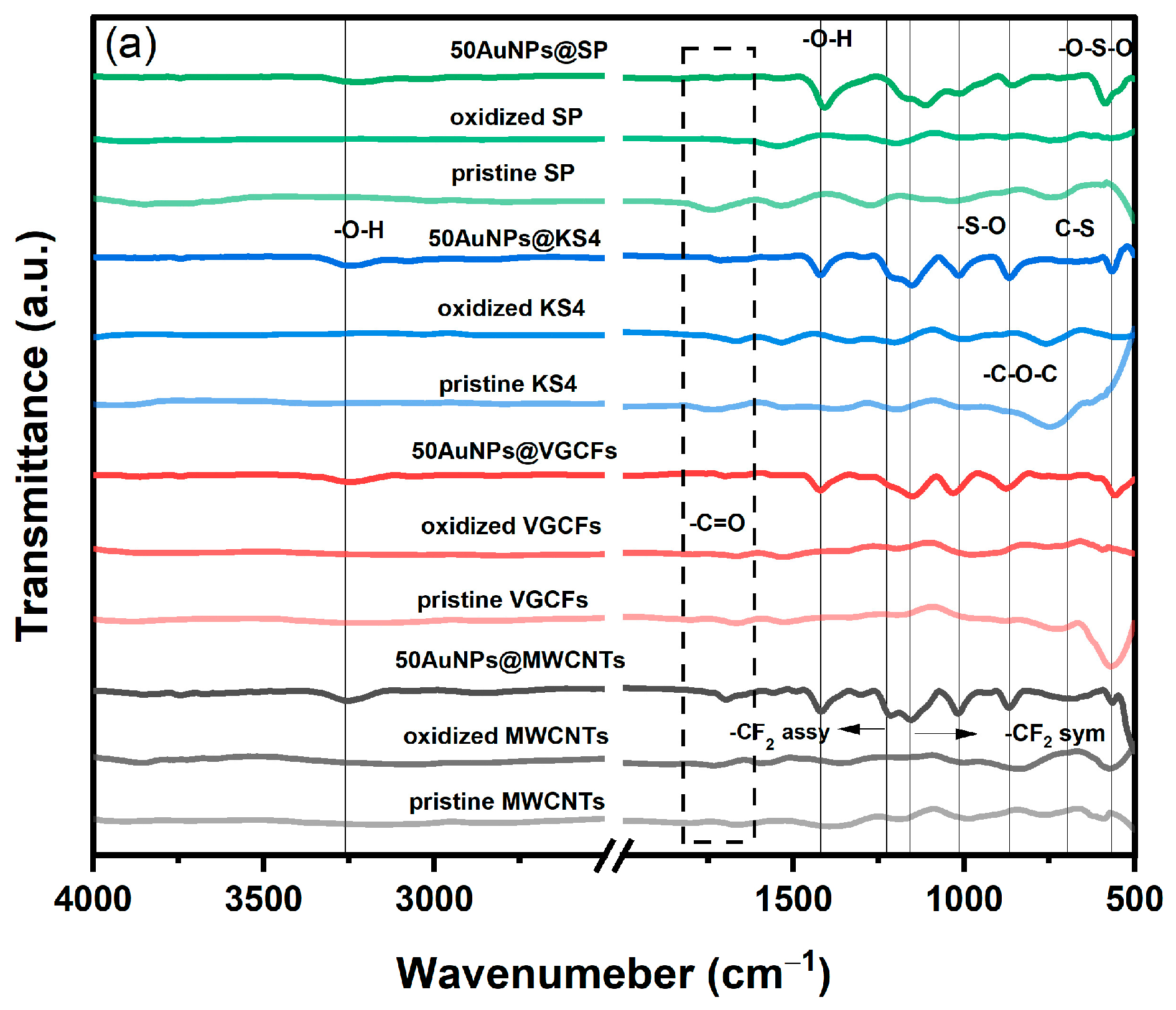
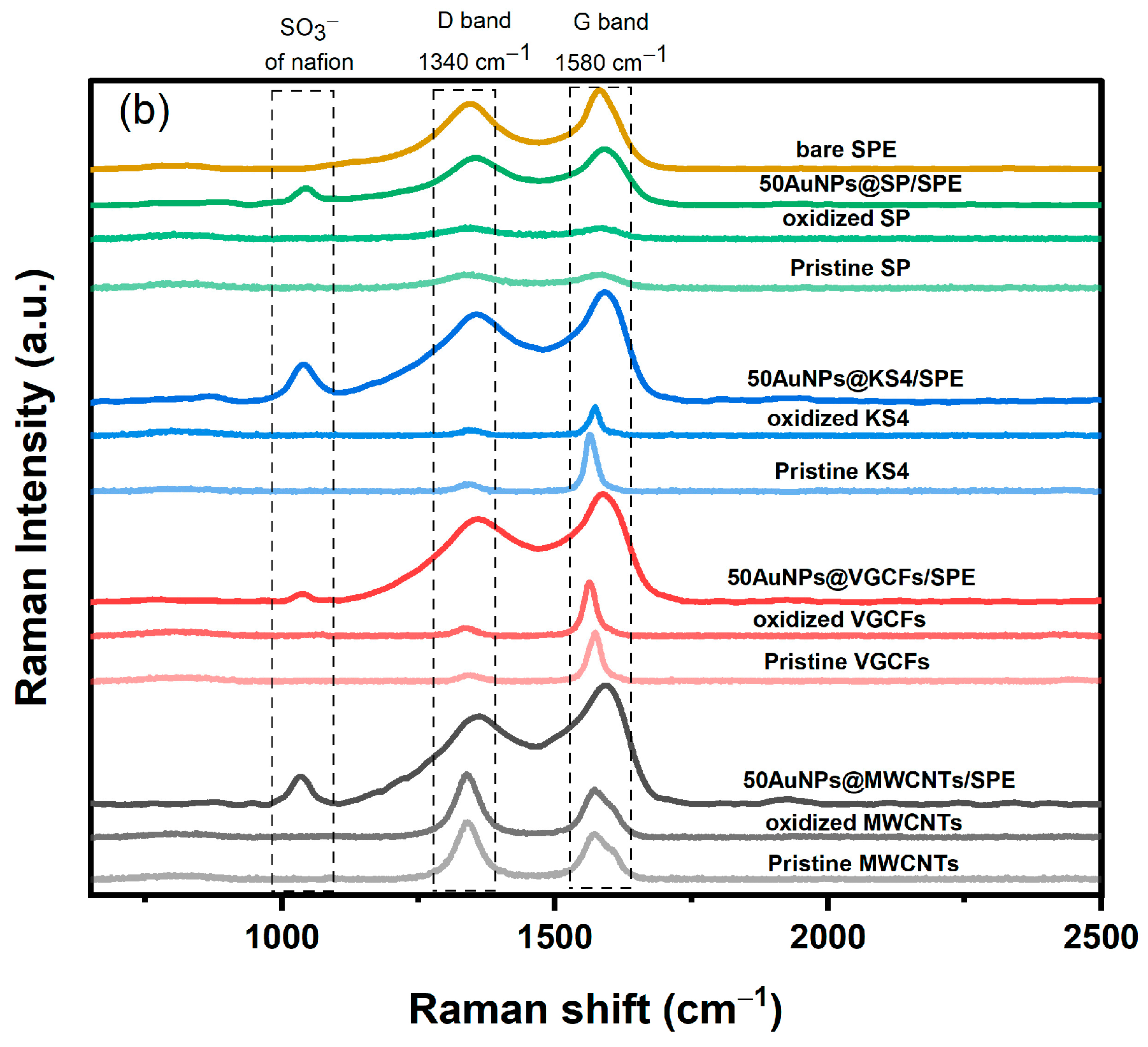
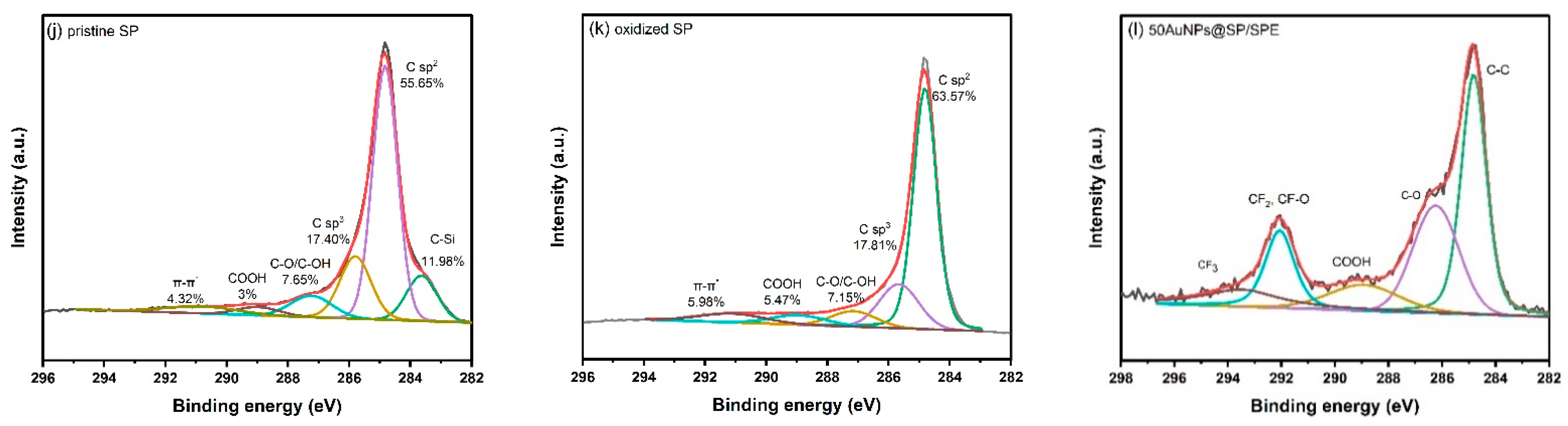
3.2. Characterization of the Electrode Using FTIR, Raman Spectroscopy, XRD, and XPS
3.3. Electrochemical Characterization of the Fabricated Immunosensor
3.4. Electrochemical Characterization of the Fabricated Immunosensor
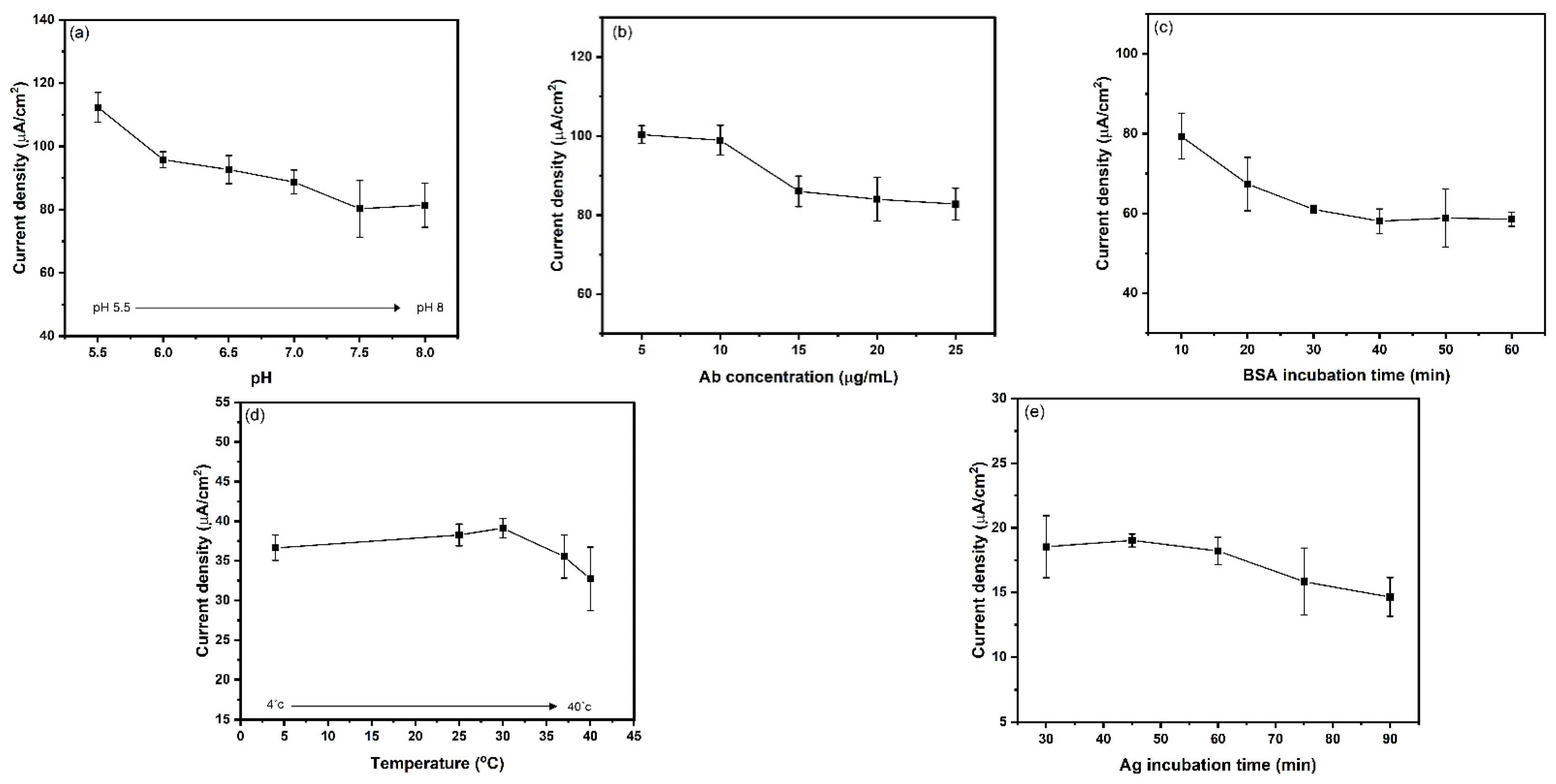
3.5. Optimization of Operational Parameters for Immunosensor
3.6. Electrochemical Immunosensing of the CA125 Antigen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- National Cancer Institute; National Institutes of Health. Ovarian Cancer. Available online: https://medlineplus.gov/ovariancancer.html (accessed on 22 August 2022).
- Staff, M.C. Ovarian Cancer. Available online: www.mayoclinic.org/diseases-conditions/ovarian-cancer/symptoms-causes/syc-20375941 (accessed on 22 August 2022).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tworoger, S.S.; Shafrir, A.L.; Hankinson, S.E. Ovarian cancer. In Cancer Epidemiology and Prevention; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Wu, M.; Liu, S.; Qi, F.; Qiu, R.; Feng, J.; Ren, X.; Rong, S.; Ma, H.; Chang, D.; Pan, H. A label-free electrochemical immunosensor for CA125 detection based on CMK-3(Au/Fc@MgAl-LDH)(n) multilayer nanocomposites modification. Talanta 2022, 241, 123254. [Google Scholar] [CrossRef] [PubMed]
- Pakchin, P.S.; Ghanbari, H.; Saber, R.; Omidi, Y. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Scholler, N.; Crawford, M.; Sato, A.; Drescher, C.W.; O’Briant, K.C.; Kiviat, N.; Anderson, G.L.; Urban, N. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin. Cancer Res. 2006, 12, 2117–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Chen, Y.; Liu, W.; Zhao, M.; Li, H. Paper-based chemiluminescence immunodevice with temporal controls of reagent transport technique. Sens. Actuators B Chem. 2017, 250, 324–332. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017, 96, 308–316. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.; Li, S.; Pan, H. A highly sensitive electrochemiluminescence immunosensor based on magnetic nanoparticles and its application in CA125 determination. J. Solid State Electrochem. 2012, 16, 2891–2898. [Google Scholar] [CrossRef]
- Klug, T.L.; Bast, R.C.; Niloff, J.M.; Knapp, R.C.; Zurawski, V.R. Monoclonal antibody immunoradiometric assay for an antigenic determinant (CA 125) associated with human epithelial ovarian carcinomas. Cancer Res 1984, 44, 1048–1053. [Google Scholar]
- Sangili, A.; Kalyani, T.; Chen, S.-M.; Nanda, A.; Jana, S.K. Label-Free Electrochemical Immunosensor Based on One-Step Electrochemical Deposition of AuNP-RGO Nanocomposites for Detection of Endometriosis Marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620–7630. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Anusha, J.R.; Kim, B.C.; Yu, K.-H.; Raj, C.J. Electrochemical biosensing of mosquito-borne viral disease, dengue: A review. Biosens. Bioelectron. 2019, 142, 111511. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2. [Google Scholar]
- Gomathi, P.; Kim, M.K.; Park, J.J.; Ragupathy, D.; Rajendran, A.; Lee, S.C.; Kim, J.C.; Lee, S.H.; Ghim, H.D. Multiwalled carbon nanotubes grafted chitosan nanobiocomposite: A prosperous functional nanomaterials for glucose biosensor application. Sens. Actuators B Chem. 2011, 155, 897–902. [Google Scholar] [CrossRef]
- Eissa, S.; Alshehri, N.; Rahman, A.M.A.; Dasouki, M.; Abu-Salah, K.M.; Zourob, M. Electrochemical immunosensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens. Bioelectron. 2018, 101, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Venkatesh, K.; Hsu, L.-F.; Arunachalam, P.; Yang, C.-C.; Ramaraj, S.K.; Ramalingam, R.J.; Arokiyaraj, S.; Al-Lohedan, H.A. An improving aqueous dispersion of polydopamine functionalized vapor grown carbon fiber for the effective sensing electrode fabrication to chloramphenicol drug detection in food samples. Microchem. J. 2021, 170, 106675. [Google Scholar] [CrossRef]
- Scotto, J.; Piccinini, E.; von Bilderling, C.; Coria-Oriundo, L.L.; Battaglini, F.; Knoll, W.; Marmisolle, W.A.; Azzaroni, O. Flexible conducting platforms based on PEDOT and graphite nanosheets for electrochemical biosensing applications. Appl. Surf. Sci. 2020, 525, 146440. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; García-Carmona, L.; Gil-Agustí, M.; García-Pellicer, M.; Quijano-López, A. Flexible and Conductive Bioelectrodes Based on Chitosan-Carbon Black Membranes: Towards the Development of Wearable Bioelectrodes. Nanomaterials 2021, 11, 2052. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Kita, J.; Suemasu, H.; Davies, I.; Koda, S.; Itatani, K. Fabrication of silicon carbide composites with carbon nanofiber addition and their fracture toughness. J. Mater. Sci. 2010, 45, 6052–6058. [Google Scholar] [CrossRef]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A novel and highly sensitive sandwich-type immunosensor for prostate-specific antigen detection based on MWCNTs-Fe3O4 nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Sukor, R.; Yusof, N.; Azri, F.; Hajian, R. Modification Strategy of Screen-Printed Carbon Electrode with Functionalized Multi-Walled Carbon Nanotube and Chitosan Matrix for Biosensor Development. Asian J. Chem. 2017, 29, 31–36. [Google Scholar]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, L.-Y.; Tseng, F.-Y.; Tan, Y.-C.; Li, J.; Feng, L.; Song, L.; Lai, C.-F.; Li, X.; He, J.-H.; et al. Label-free electrochemical immunosensor based on gold nanoparticle/polyethyleneimine/reduced graphene oxide nanocomposites for the ultrasensitive detection of cancer biomarker matrix metalloproteinase-1. Analyst 2021, 146, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Ma, L. Gold nanoparticle probes. Coord. Chem. Rev. 2009, 253, 1607–1618. [Google Scholar] [CrossRef]
- Mandal, S. Synthesis of radioactive gold nanoparticle in surfactant medium. J. Radioanal. Nucl. Chem. 2014, 299, 1209–1212. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y. Magnetoelectrochemistry of Gold Nanoparticle Quantized Capacitance Charging. J. Am. Chem. Soc. 2002, 124, 5280–5281. [Google Scholar] [CrossRef]
- Haruta, M.M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Gao, N.; He, H.; Zou, K.; Ma, M.; Zhou, Y.; Cai, Z.; Chang, G.; He, Y. A gold electrode modified with a gold-graphene oxide nanocomposite for non-enzymatic sensing of glucose at near-neutral pH values. Mikrochim. Acta 2019, 186, 722. [Google Scholar] [CrossRef]
- Kamal, N.A.; Anuar, N.S.; Noordin, R.; Rahumatullah, A.; Hamzah, H.H. A Study on the Electrodeposited Gold Nanoparticle (AuNP)-Film as a Nanoplatform for a Label-Free Electrochemical Strongyloidiasis Immunosensor. J. Electrochem. Soc. 2022, 169, 106514. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Gondran, C.; Holzinger, M.; Cosnier, S.; Zhang, Z. Voltammetric sensing of recombinant viral dengue virus 2 NS1 based on Au nanoparticle–decorated multiwalled carbon nanotube composites. Microchim. Acta 2020, 187, 363. [Google Scholar] [CrossRef]
- Benvidi, A.; Dehghani-Firouzabadi, A.; Mazloum-Ardakani, M.; Mirjalili, B.-B.F.; Zare, R. Electrochemical deposition of gold nanoparticles on reduced graphene oxide modified glassy carbon electrode for simultaneous determination of levodopa, uric acid and folic acid. J. Electroanal. Chem. 2015, 736, 22–29. [Google Scholar] [CrossRef]
- Singh, R.; Premkumar, T.; Shin, J.-Y.; Geckeler, K.E. Carbon Nanotube and Gold-Based Materials: A Symbiosis. Chem. A Eur. J. 2010, 16, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, V.I.; Imae, T.; Ujihara, M.; Murthy, C.N. Surface immobilization of carbon nanotubes by beta-cyclodextrins and their inclusion ability. J Nanosci. Nanotechnol. 2013, 13, 2604–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.-C.; Wang, Y.; Zhang, Q.; Levon, K. Optimization of the Electrodeposition of Gold Nanoparticles for the Application of Highly Sensitive, Label-Free Biosensor. Biosensors 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, N.D.; Omar, M.H.; Ahmad Kamal, N.N.; Abdul Razak, K.; Sönmez, T.; Balakrishnan, V.; Hamzah, H.H. Effect of Supporting Background Electrolytes on the Nanostructure Morphologies and Electrochemical Behaviors of Electrodeposited Gold Nanoparticles on Glassy Carbon Electrode Surfaces. ACS Omega 2021, 6, 24419–24431. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of 17β-estradiol. Sci. Rep. 2019, 9, 13859. [Google Scholar] [CrossRef] [Green Version]
- Asiaei, S.; Smith, B.; Nieva, P. Enhancing conjugation rate of antibodies to carboxylates: Numerical modeling of conjugation kinetics in microfluidic channels and characterization of chemical over-exposure in conventional protocols by quartz crystal microbalance. Biomicrofluidics 2015, 9, 064115. [Google Scholar] [CrossRef] [Green Version]
- Sigwadi, R.; Mokrani, T.; Msomi, P.; Nemavhola, F. The Effect of Sulfated Zirconia and Zirconium Phosphate Nanocomposite Membranes on Fuel-Cell Efficiency. Polymers 2022, 14, 263. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [Green Version]
- Aragaw, B.A.; Su, W.-N.; Rick, J.; Hwang, B.-J. Highly efficient synthesis of reduced graphene oxide—Nafion nanocomposites with strong coupling for enhanced proton and electron conduction. RSC Adv. 2013, 3, 23212–23221. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Ryan, J.J.; Spontak, R.J.; Deng, L. Nafion/IL hybrid membranes with tuned nanostructure for enhanced CO2 separation: Effects of ionic liquid and water vapor. Green Chem. 2018, 20, 1391–1404. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Ferlazzo, A.; Lanza, M.; Milone, C.; Iannazzo, D.; Piperno, A.; Piperopoulos, E.; Galvagno, S. Morphological modification of MWCNT functionalized with HNO3/H2SO4 mixtures. J. Nanosci. Nanotechnol. 2012, 12, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Jean, D.I.; Ji, C.; Zou, S. In situ surface-enhanced Raman spectroscopic studies of nafion adsorption on Au and Pt electrodes. Langmuir 2012, 28, 957–964. [Google Scholar] [CrossRef]
- Rojas, J.; Toro Gonzalez, M.; Molina Higgins, M.; Castano, C. Facile radiolytic synthesis of ruthenium nanoparticles on graphene oxide and carbon nanotubes. Mater. Sci. Eng. B 2015, 205, 28–35. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Chang, C.-M.; Wang, D.-M.; Lai, J.-Y. Preparation and applications of Nafion-functionalized multiwalled carbon nanotubes for proton exchange membrane fuel cells. J. Mater. Chem. 2010, 20, 4409–4416. [Google Scholar] [CrossRef]
- Young, J.K.; Lewinski, N.A.; Langsner, R.J.; Kennedy, L.C.; Satyanarayan, A.; Nammalvar, V.; Lin, A.Y.; Drezek, R.A. Size-controlled synthesis of monodispersed gold nanoparticles via carbon monoxide gas reduction. Nanoscale Res. Lett. 2011, 6, 428. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Kumar, S.J. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an Immunosensing Platform for Noninvasive Electrochemical Detection of Oral Cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Mucin-16 Human Mucin-16 (MUC16) ELISA Kit. Available online: https://www.abbexa.com/human-carbohydrate-antigen-125-elisa-kit (accessed on 2 January 2023).
- Torati, S.R.; Kasturi, K.C.S.B.; Lim, B.; Kim, C. Hierarchical gold nanostructures modified electrode for electrochemical detection of cancer antigen CA125. Sens. Actuators B Chem. 2017, 243, 64–71. [Google Scholar] [CrossRef]
- Rong, Q.; Feng, F.; Ma, Z. Metal ions doped chitosan–poly(acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Nara, S. Dual gold nanostructure-based electrochemical immunosensor for CA125 detection. Appl. Nanosci. 2018, 8, 1843–1853. [Google Scholar] [CrossRef]
- Wu, L.; Yan, F.; Ju, H. An amperometric immunosensor for separation-free immunoassay of CA125 based on its covalent immobilization coupled with thionine on carbon nanofiber. J. Immunol. Methods 2007, 322, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; dos Santos, G.P.; Ferroni, M.; Faglia, G.; Yamanaka, H.; Marrazza, G. New label free CA125 detection based on gold nanostructured screen-printed electrode. Sens. Actuators B Chem. 2013, 179, 194–200. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Rashidi, M.R.; Barar, J.; Aghaie, M.; Mohammadnejad, D.; Ramazani, A.; Karami, P.; Coukos, G.; Omidi, Y. An ultra-sensitive impedimetric immunosensor for detection of the serum oncomarker CA-125 in ovarian cancer patients. Nanoscale 2015, 7, 3768–3779. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Mohammadzadeh, A.; Jafari, M.; Habibi, B. Ultrasensitive immunoassay of glycoprotein 125 (CA 125) in untreated human plasma samples using poly (CTAB-chitosan) doped with silver nanoparticles. Int. J. Biol. Macromol. 2018, 120, 2048–2064. [Google Scholar] [CrossRef]



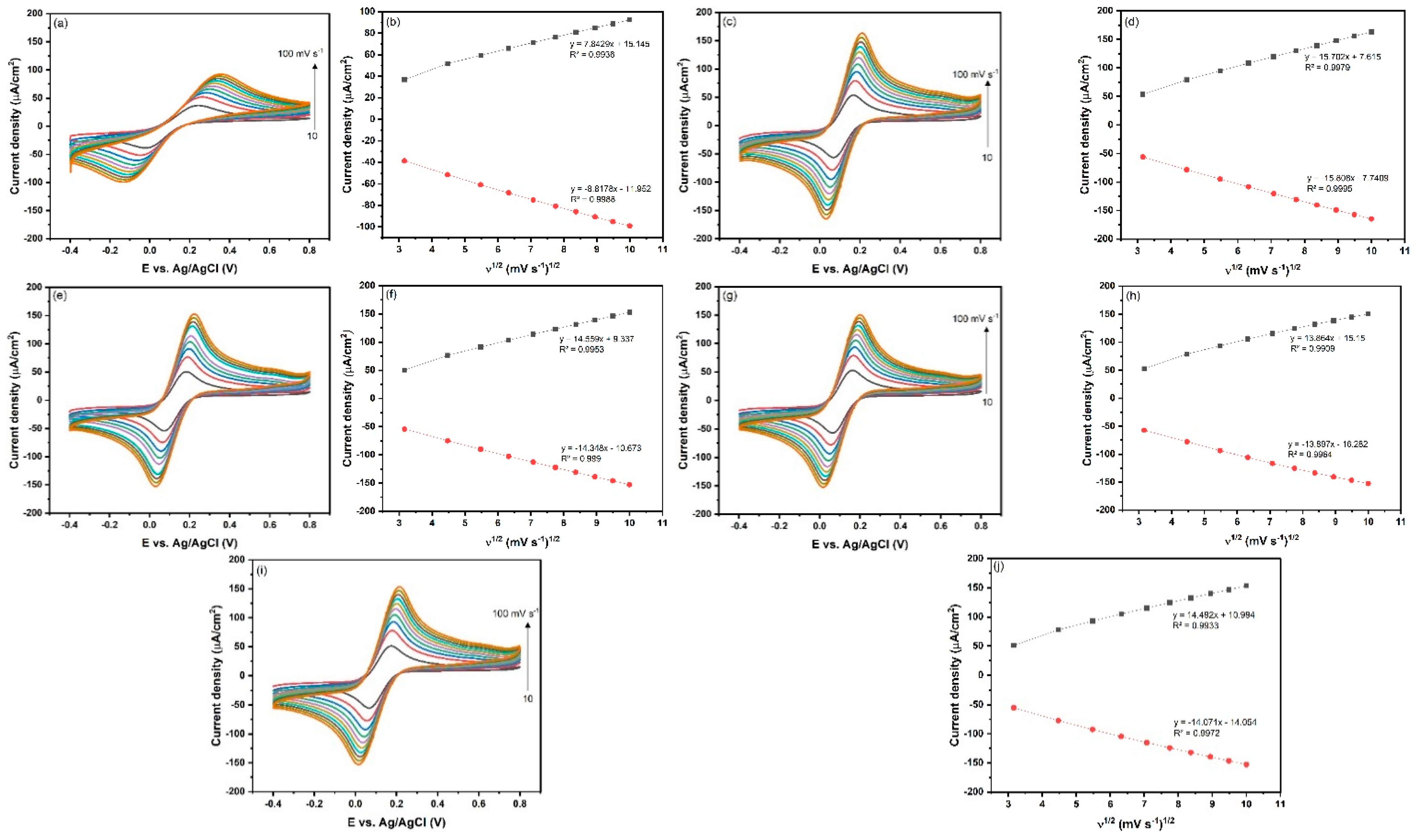


| Electrode | Ipa (μA) | Ipc (μA) | A (cm2) |
|---|---|---|---|
| Bare GCE | 7.8429 | −8.8178 | 0.532 |
| 50AuNPs@MWCNTs/GCE | 15.702 | −15.808 | 0.953 |
| 50AuNPs@VGCFs/GCE | 14.559 | −14.348 | 0.865 |
| 50AuNPs@KS4/GCE | 13.864 | −13.897 | 0.838 |
| 50AuNPs@SP/GCE | 14.492 | −14.071 | 0.849 |
| Modified Electrode Materials | Linear Range (μg/mL) | R2 |
|---|---|---|
| 50AuNPs@MWCNTs | 10–0.001 | 0.9624 |
| 50AuNPs@VGCFs | 10–0.0001 | 0.2216 |
| 50AuNPs@KS4 | 10–0.001 | 0.7449 |
| 50AuNPs@SP | 10–0.001 | 0.7039 |
| Modified Electrode Materials | Linear Range (U/mL) | LOD (U/mL) | Ref. |
|---|---|---|---|
| Gold nanostructures (GNs) modified electrode | 10–100 | 5.5 | [56] |
| Chitosan–poly(acrylic acid) nanosphere | 1−150 | 0.3 | [57] |
| Gold nanorods (GNRs)/ITO as capture anti-CA125. and GNPs@Cd ²⁺ | 20−100 | 3.4 | [58] |
| Thionine-CNF | 2−75 | 1.8 | [59] |
| electrodepositing of AuNPs on SPGE | 0−1000 | 6.7 | [60] |
| AuNP@SiO2-CdSe quantum dots | 0–0.1 | 0.0016 | [61] |
| PAMAM/AuNP-3DrGO-MWCNT @O-succinyl-chitosan-magnetic nanoparticles (Suc-CS@MNPs) | 0.0005–10,10–75 | 0.0006 | [62] |
| Poly(CTAB-CS)-Ag NPs | 0.01–400 | 0.001 | [63] |
| 50AuNPs@MWCNTs | 0.0004–4 | 0.0004 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runprapan, N.; Wang, F.-M.; Ramar, A.; Yuan, C.-C. Role of Defects of Carbon Nanomaterials in the Detection of Ovarian Cancer Cells in Label-Free Electrochemical Immunosensors. Sensors 2023, 23, 1131. https://doi.org/10.3390/s23031131
Runprapan N, Wang F-M, Ramar A, Yuan C-C. Role of Defects of Carbon Nanomaterials in the Detection of Ovarian Cancer Cells in Label-Free Electrochemical Immunosensors. Sensors. 2023; 23(3):1131. https://doi.org/10.3390/s23031131
Chicago/Turabian StyleRunprapan, Nattharika, Fu-Ming Wang, Alagar Ramar, and Chiou-Chung Yuan. 2023. "Role of Defects of Carbon Nanomaterials in the Detection of Ovarian Cancer Cells in Label-Free Electrochemical Immunosensors" Sensors 23, no. 3: 1131. https://doi.org/10.3390/s23031131





