Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables
Abstract
:1. Introduction
Bioimpedance
2. Electrical Impedance Tomography
- Choose the imaging modality (absolute or differential) and define the reference dataset for the differential approach.
- Select suitable electrodes (number, position and material).
- Define the measurement strategy (voltage-mode or current-mode).
- Design the electronic hardware, including both the current generation and the voltage readout blocks.
- Choose the numerical algorithm to solve the inverse problem.
2.1. EIT Imaging Modality
2.2. Measurement Strategies
- Adjacent method: Current is injected between two adjacent electrodes, and voltage is acquired between all the remaining pairs of adjacent electrodes. This is the most common technique [25].
- Opposite or Polar method: Current is injected between two electrodes that are 180° apart, and voltage is measured between the reference electrode and the remaining electrodes in pairs. This technique is used in the brain-imaging field [26].
- Cross or Diagonal method: One electrode is kept as a reference for the voltage measure, and current is injected between all the possible pairs of electrodes in turn; the measure is repeated by taking one electrode as a reference each time until all are used. This method is not usually used in EIT [26].
- Trigonometric method: Current is injected through all the electrodes at the same time instant, and the voltage is measured with respect to a reference electrode. This is the technique that guarantees the highest number of independent measures [25].
2.3. Electrodes
2.3.1. Number
2.3.2. Positioning
2.3.3. Contact Impedance
2.3.4. Interference Sources
2.3.5. Contact
2.3.6. Requirements for Wearable EIT
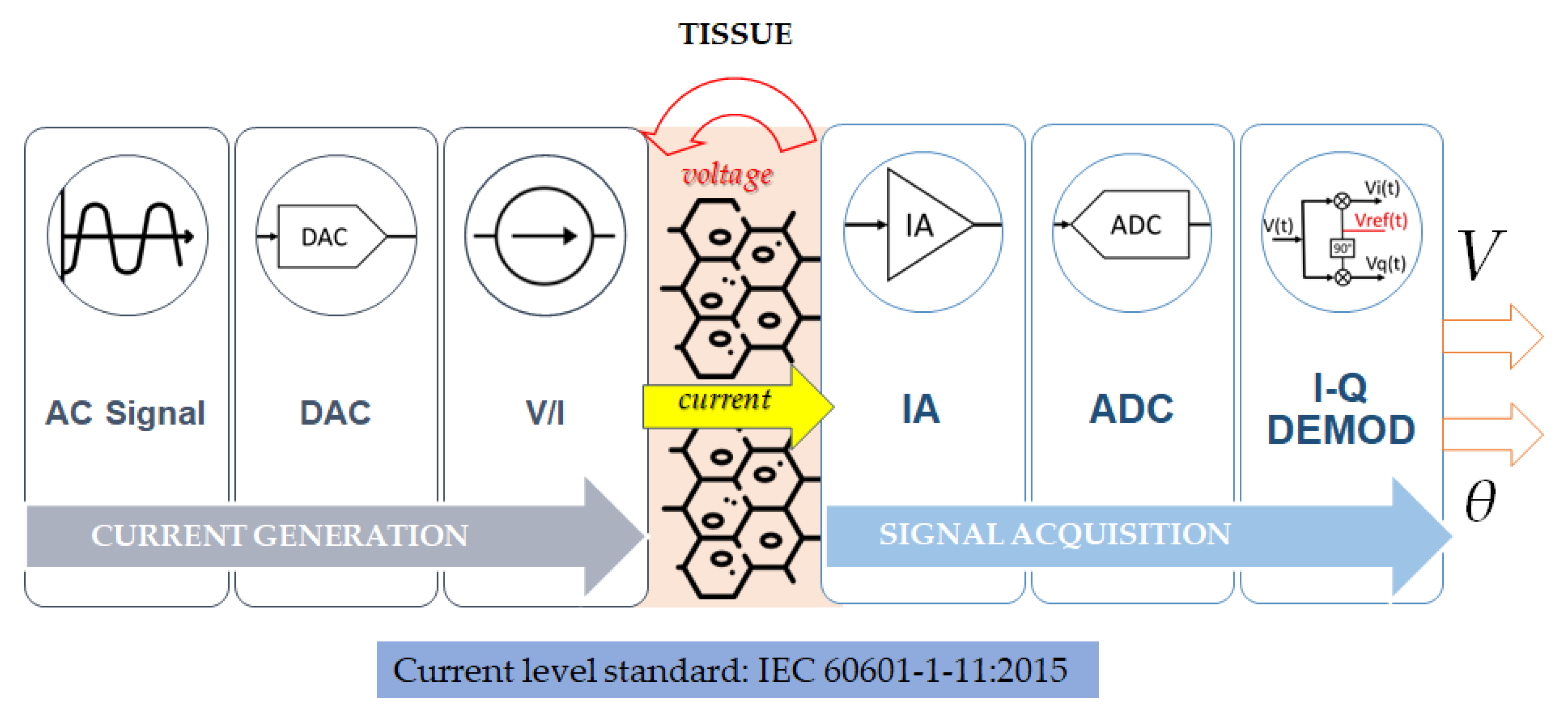
2.4. Hardware Design
2.4.1. Current Generation and Injection
2.4.2. Voltage Signal Acquisition
3. Applications
3.1. Pulmonary Imaging
3.2. Brain Imaging
3.3. Thermal Monitoring
3.4. Tumor Detection
3.5. Assessment of Muscle Health
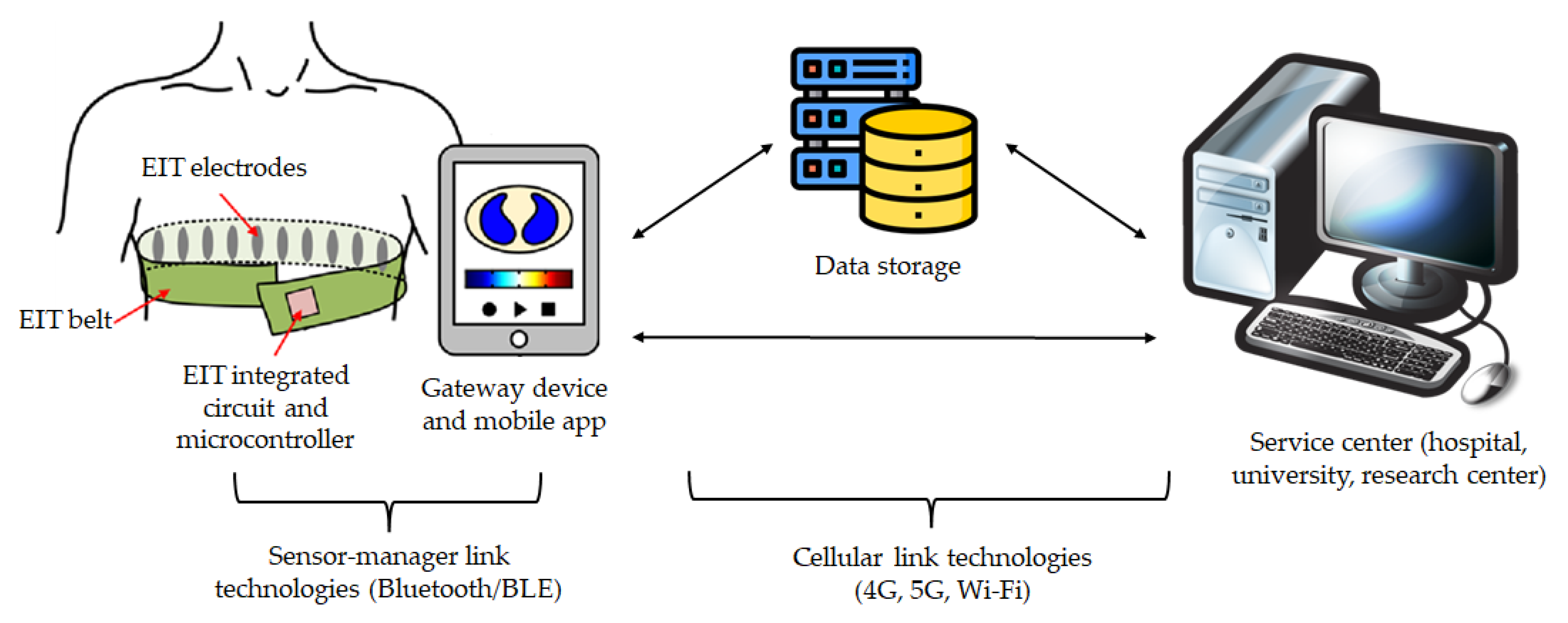
4. Wearable Solutions
4.1. Hardware Characteristics
4.1.1. Low Power Consumption
4.1.2. Electrode Configuration
4.1.3. Examples of Wearable EIT Implementations
4.2. Applications of Wearable EIT
4.2.1. Pulmonary Imaging
4.2.2. Cancer Detection
4.2.3. Gesture Recognition
4.2.4. Multi-Parameter EIT
4.3. Integration in Telemedicine Platforms
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayford, R.; Tizzard, A. Bioimpedance Imaging: An Overview of Potential Clinical Applications. Analyst 2012, 137, 4635–4643. [Google Scholar] [CrossRef] [PubMed]
- Rafiei-Naeini, M.; McCann, H. Low-Noise Current Excitation Sub-System for Medical EIT. Physiol. Meas. 2008, 29, S173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadleir, R.J.; Fox, R.A.; Turner, V.F. Inflatable belt for the application of electrode arrays. Rev. Sci. Instrum. 2000, 71, 530–535. [Google Scholar] [CrossRef]
- Dong, G.; Bayford, R.; Liu, H.; Zhou, Y.; Yan, W. EIT Images with Improved Spatial Resolution Using a Realistic Head Model. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 2006, pp. 1134–1137. [Google Scholar]
- Liu, S.; Jia, J.; Zhang, Y.D.; Yang, Y. Image Reconstruction in Electrical Impedance Tomography Based on Structure-Aware Sparse Bayesian Learning. IEEE Trans. Med. Imaging 2018, 37, 2090–2102. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.H. Electrical Impedance Tomography (EIT): A Review. J. Med. Eng. Technol. 2003, 27, 97–108. [Google Scholar] [CrossRef]
- Lionheart, W.R.B. EIT Reconstruction Algorithms: Pitfalls, Challenges and Recent Developments. Physiol. Meas. 2004, 25, 125–142. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, A.M.; Brown, B.H.; Leathard, A.D.; Harris, N.D.; Barber, D.C.; Edbrooke, D.L. Review Clinical Applications of Electrical Impedance Tomography. J. Med. Eng. Technol. 1993, 17, 89–98. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: III. Parametric Models for the Dielectric Spectrum of Tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [Green Version]
- Nopp, P.; Rapp, E.; Pfutzner, H.; Nakesch, H.; Rusham, C. Dielectric Properties of Lung Tissue as a Function of Air Content. Phys. Med. Biol. 1993, 38, 699–716. [Google Scholar] [CrossRef]
- Schwan, H.P. Electrical Properties of Tissue and Cell Suspensions. Adv. Biol. Med. Phys. 1957, 5, 147–209. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, E. The Dielectric Properties of Biological Tissues: I. Literature Survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Packham, B.; Koo, H.; Romsauerova, A.; Ahn, S.; McEwan, A.; Jun, S.C.; Holder, D.S. Comparison of Frequency Difference Reconstruction Algorithms for the Detection of Acute Stroke Using EIT in a Realistic Head-Shaped Tank. Physiol. Meas. 2012, 33, 767–786. [Google Scholar] [CrossRef]
- Lee, J.M.; Uhlmann, G. Determining Anisotropic Real-analytic Conductivities by Boundary Measurements. Commun. Pure Appl. Math. 1989, 42, 1097–1112. [Google Scholar] [CrossRef]
- Khan, T.A.; Ling, S.H. Review on Electrical Impedance Tomography: Artificial Intelligence Methods and Its Applications. Algorithms 2019, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Brazey, B.; Haddab, Y.; Zemiti, N. Robust Imaging Using Electrical Impedance Tomography: Review of Current Tools. Proc. R. Soc. A Math. Phys. Eng. Sci. 2022, 478, 20210713. [Google Scholar] [CrossRef]
- Riera, J.; Riu, P.J.; Casan, P.; Masclans, J.R. Tomografía de Impedancia Eléctrica En La Lesión Pulmonar Aguda. Med. Intensiv. 2011, 35, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Nissinen, A.; Kolehmainen, V.P.; Kaipio, J.P. Compensation of Modelling Errors Due to Unknown Domain Boundary in Electrical Impedance Tomography. IEEE Trans. Med. Imaging 2011, 30, 231–242. [Google Scholar] [CrossRef]
- Adler, A.; Holder, D. Electrical Impedance Tomography: Methods, History and Applications; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780429399886. [Google Scholar]
- Seo, J.K.; Harrach, B.; Woo, E.J. Recent Progress on Frequency Difference Electrical Impedance Tomography. ESAIM Proc. 2009, 26, 150–161. [Google Scholar] [CrossRef]
- Wu, C.; Soleimani, M. Frequency Difference EIT with Localization: A Potential Medical Imaging Tool during Cancer Treatment. IEEE Access 2019, 7, 21870–21878. [Google Scholar] [CrossRef]
- Takhti, M.; Odame, K. Structured Design Methodology to Achieve a High SNR Electrical Impedance Tomography. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Jandial, P.; Nersisson, R. A Technical Survey on Hardware Configurations for Electrical Impedance Tomography Systems. In Proceedings of the 3rd IEEE International Virtual Conference on Innovations in Power and Advanced Computing Technologies, i-PACT 2021, Kuala Lumpur, Malaysia, 27–29 November 2021. [Google Scholar]
- Harikumar, R.; Prabu, R.; Raghavan, S. Electrical Impedance Tomography (EIT) and Its Medical Applications: A Review. Int. J. Soft Comput. Eng. 2013, 3, 193–198. [Google Scholar]
- Rosa, B.M.G.; Yang, G.Z. Bladder Volume Monitoring Using Electrical Impedance Tomography with Simultaneous Multi-Tone Tissue Stimulation and DFT-Based Impedance Calculation Inside an FPGA. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 775–786. [Google Scholar] [CrossRef]
- Halter, R.J.; Hartov, A.; Paulsen, K.D. A Broadband High-Frequency Electrical Impedance Tomography System for Breast Imaging. IEEE Trans. Biomed. Eng. 2008, 55, 650–659. [Google Scholar] [CrossRef]
- Hong, S.; Lee, K.; Ha, U.; Kim, H.; Lee, Y.; Kim, Y.; Yoo, H.J. A 4.9 MΩ-Sensitivity Mobile Electrical Impedance Tomography IC for Early Breast-Cancer Detection System. IEEE J. Solid-State Circuits 2015, 50, 245–257. [Google Scholar] [CrossRef]
- Kim, M.; Jang, J.; Kim, H.; Lee, J.; Lee, J.; Lee, J.; Lee, K.R.; Kim, K.; Lee, Y.; Lee, K.J.; et al. A 1.4-m Ω-Sensitivity 94-DB Dynamic-Range Electrical Impedance Tomography SoC and 48-Channel Hub-SoC for 3-D Lung Ventilation Monitoring System. IEEE J. Solid-State Circuits 2017, 52, 2829–2842. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Zhang, S.; Yang, S.; Justin, G.A.; Sun, M.; Yan, W. A 128-Electrode Three Dimensional Electrical Impedance Tomography System. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Lyon, France, 22–26 August 2007; Volume 2007, pp. 4386–4389. [Google Scholar]
- Huang, C.N.; Yu, F.M.; Chung, H.Y. Rotational Electrical Impedance Tomography. Meas. Sci. Technol. 2007, 18, 2958–2966. [Google Scholar] [CrossRef]
- Lehti-Polojarvi, M.; Koskela, O.; Seppanen, A.; Figueiras, E.; Hyttinen, J. Rotational Electrical Impedance Tomography Using Electrodes with Limited Surface Coverage Provides Window for Multimodal Sensing. Meas. Sci. Technol. 2018, 29, 025401. [Google Scholar] [CrossRef]
- Murphy, E.K.; Mahara, A.; Halter, R.J. Absolute Reconstructions Using Rotational Electrical Impedance Tomography for Breast Cancer Imaging. IEEE Trans. Med. Imaging 2017, 36, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Gaggero, P.O.; Adler, A.; Brunner, J.; Seitz, P. Electrical Impedance Tomography System Based on Active Electrodes. Physiol. Meas. 2012, 33, 831–847. [Google Scholar] [CrossRef]
- Lin, B.S.; Yu, H.R.; Kuo, Y.T.; Liu, Y.W.; Chen, H.Y.; Lin, B.S. Wearable Electrical Impedance Tomography Belt With Dry Electrodes. IEEE Trans. Biomed. Eng. 2022, 69, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Boone, K.G.; Holder, D.S. Effect of Skin Impedance on Image Quality and Variability in Electrical Impedance Tomography: A Model Study. Med. Biol. Eng. Comput. 1996, 34, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Woo, E.J.; Webster, J.G.; Tompkins, W.J. Using Compound Electrodes In Electrical Impedance Tomography. IEEE Trans. Biomed. Eng. 1993, 40, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, J.P.; Kolehmainen, V.; Lassas, M.J.; Ola, P.; Siltanen, S. Simultaneous Reconstruction of Conductivity, Boundary Shape, and Contact Impedances in Electrical Impedance Tomography. SIAM J. Imaging Sci. 2021, 14, 1407–1438. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Soleimani, M. Capacitively Coupled Electrical Impedance Tomography for Brain Imaging. IEEE Trans. Med. Imaging 2019, 38, 2104–2113. [Google Scholar] [CrossRef]
- Wu, Y.; Hanzaee, F.F.; Jiang, D.; Bayford, R.H.; Demosthenous, A. Electrical Impedance Tomography for Biomedical Applications: Circuits and Systems Review. IEEE Open J. Circuits Syst. 2021, 2, 380–397. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Bardill, A.; De Gelidi, S.; Bayford, R.; Demosthenous, A. A High Frame Rate Wearable EIT System Using Active Electrode ASICs for Lung Respiration and Heart Rate Monitoring. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 3810–3820. [Google Scholar] [CrossRef]
- Wu, Y.; Langlois, P.; Bayford, R.; Demosthenous, A. Design of a CMOS Active Electrode IC for Wearable Electrical Impedance Tomography Systems. In Proceedings of the IEEE International Symposium on Circuits and Systems, Montreal, QC, Canada, 22–25 May 2016; Volume 2016, pp. 846–849. [Google Scholar]
- Wu, Y.; Jiang, D.; Bardill, A.; Bayford, R.; Demosthenous, A. A 122 Fps, 1 MHz Bandwidth Multi-Frequency Wearable EIT Belt Featuring Novel Active Electrode Architecture for Neonatal Thorax Vital Sign Monitoring. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Asfaw, Y.; Adler, A. Automatic Detection of Detached and Erroneous Electrodes in Electrical Impedance Tomography. Physiol. Meas. 2005, 26, S175. [Google Scholar] [CrossRef] [Green Version]
- Rymarczyk, T.; Nita, P.; Vejar, A.; Wos, M.; Oleszek, M.; Adamkiewicz, P. Architecture of a Mobile System for the Analysis of Biomedical Signals Based on Electrical Tomography. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine, PTZE 2018, Raclawice, Poland, 9–12 September 2018; pp. 289–292. [Google Scholar]
- Hu, C.L.; Cheng, I.C.; Huang, C.H.; Liao, Y.T.; Lin, W.C.; Tsai, K.J.; Chi, C.H.; Chen, C.W.; Wu, C.H.; Lin, I.T.; et al. Dry Wearable Textile Electrodes for Portable Electrical Impedance Tomography. Sensors 2021, 21, 6789. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Kim, T.E.; Yoon, S.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Flexible Electrode Belt for EIT Using Nanofiber Web Dry Electrodes. Physiol. Meas. 2012, 33, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Van Helleputte, N.; Konijnenburg, M.; Pettine, J.; Jee, D.W.; Kim, H.; Morgado, A.; Van Wegberg, R.; Torfs, T.; Mohan, R.; Breeschoten, A.; et al. A 345 Μw Multi-Sensor Biomedical SoC with Bio-Impedance, 3-Channel ECG, Motion Artifact Reduction, and Integrated DSP. IEEE J. Solid-State Circuits 2015, 50, 230–244. [Google Scholar] [CrossRef]
- IEC 60601-1:2015; Medical Electrical Equipment Part 1: General Requirements for Basic Safety and Essential Performance. ISO: Geneva, Switzerland, 2015.
- Zeng, L.; Heng, C.H. An 8-Channel 1.76-MW 4.84-Mm2Electrical Impedance Tomography SoC with Direct If Frequency Division Multiplexing. IEEE Trans. Circuits Syst. II Express Briefs 2021, 68, 3401–3405. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Langlois, P.; Bayford, R.; Demosthenous, A. A CMOS Current Driver with Built-in Common-Mode Signal Reduction Capability for EIT. In Proceedings of the ESSCIRC 201743rd IEEE European Solid State Circuits Conference, Leuven, Belgium, 11–14 September 2017; pp. 227–230. [Google Scholar]
- Langlois, P.J.; Wu, Y.; Bayford, R.H.; Demosthenous, A. On the Application of Frequency Selective Common Mode Feedback for Multifrequency EIT. Physiol. Meas. 2015, 36, 1337–1350. [Google Scholar] [CrossRef] [Green Version]
- Wicaksono, R.; Baidillah, M.R.; Darma, P.N.; Inoue, A.; Tsuji, H.; Takei, M. Pocket Electrical Impedance Tomography (p-EIT) System with Wide Impedance Range Buffer- Mirrored Current Source (BMCS) with Assist of Filter-Trained Quasi-3-D Method for Functional Gastric-Shape Imaging. IEEE Trans. Instrum. Meas. 2021, 70, 4507317. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, Z.; Han, B.; Dong, F. An FPGA-Based Multifrequency EIT System with Reference Signal Measurement. IEEE Trans. Instrum. Meas. 2021, 70, 4500710. [Google Scholar] [CrossRef]
- Li, W.; Xia, J.; Zhang, G.; Ma, H.; Liu, B.; Yang, L.; Zhou, Y.; Dong, X.; Fu, F.; Shi, X. Fast High-Precision Electrical Impedance Tomography System for Real-Time Perfusion Imaging. IEEE Access 2019, 7, 61570–61580. [Google Scholar] [CrossRef]
- Shahghasemi, M.; Odame, K.M. A Wide-Band, Wide-Swing Current Driver for Electrical Impedance Tomography Applications. In Proceedings of the Midwest Symposium on Circuits and Systems, Springfield, MA, USA, 9–12 August 2020; Volume 2020, pp. 659–662. [Google Scholar]
- Shi, X.; Li, W.; You, F.; Huo, X.; Xu, C.; Ji, Z.; Liu, R.; Liu, B.; Li, Y.; Fu, F.; et al. High-Precision Electrical Impedance Tomography Data Acquisition System for Brain Imaging. IEEE Sens. J. 2018, 18, 5974–5984. [Google Scholar] [CrossRef]
- Shi, X.; You, F.; Xu, C.; Ji, Z.; Liu, R.; Dong, X.; Fu, F.; Huo, X. Design and Implementation of a High-Precision Electrical Impedance Tomography Data Acquisition System for Brain Imaging. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference, BioCAS 2016, Shanghai, China, 17–19 October 2016; pp. 9–13. [Google Scholar]
- Yang, D.; Huang, G.; Xu, B.; Wang, X.; Wang, Z.; Wei, Z. A DSP-Based EIT System with Adaptive Boundary Voltage Acquisition. IEEE Sens. J. 2022, 22, 5743–5754. [Google Scholar] [CrossRef]
- Takhti, M.; Teng, Y.C.; Odame, K. A 10 MHz Read-Out Chain for Electrical Impedance Tomography. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, V.; Cornejo, R. Lung Monitoring with Electrical Impedance Tomography: Technical Considerations and Clinical Applications. J. Thorac. Dis. 2019, 11, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.R. Electric Properties of Flowing Blood and Impedance Cardiography. Ann. Biomed. Eng. 1989, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wrigge, H.; Zinserling, J.; Muders, T.; Varelmann, D.; Günther, U.; Von Der Groeben, C.; Magnusson, A.; Hedenstierna, G.; Putensen, C. Electrical Impedance Tomography Compared with Thoracic Computed Tomography during a Slow Inflation Maneuver in Experimental Models of Lung Injury. Crit. Care Med. 2008, 36, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Dudykevych, T.; Quintel, M.; Hellige, G. Detection of Local Lung Air Content by Electrical Impedance Tomography Compared with Electron Beam CT. J. Appl. Physiol. 2002, 93, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.C.; Pouzot, C.; Gros, A.; Tourevieille, C.; Lebars, D.; Lavenne, F.; Frerichs, I.; Guérin, C. Electrical Impedance Tomography Compared to Positron Emission Tomography for the Measurement of Regional Lung Ventilation: An Experimental Study. Crit. Care 2009, 13, R82. [Google Scholar] [CrossRef] [Green Version]
- Hinz, J.; Moerer, O.; Neumann, P.; Dudykevych, T.; Hellige, G.; Quintel, M. Effect of Positive End-Expiratory-Pressure on Regional Ventilation in Patients with Acute Lung Injury Evaluated by Electrical Impedance Tomography. Eur. J. Anaesthesiol. 2005, 22, 817–825. [Google Scholar] [CrossRef]
- Coulombe, N.; Gagnon, H.; Marquis, F.; Skrobik, Y.; Guardo, R. A Parametric Model of the Relationship between EIT and Total Lung Volume. Physiol. Meas. 2005, 26, 401–411. [Google Scholar] [CrossRef]
- Meier, T.; Luepschen, H.; Karsten, J.; Leibecke, T.; Großherr, M.; Gehring, H.; Leonhardt, S. Assessment of Regional Lung Recruitment and Derecruitment during a PEEP Trial Based on Electrical Impedance Tomography. Intensive Care Med. 2008, 34, 543–550. [Google Scholar] [CrossRef]
- Zick, G.; Elke, G.; Becher, T.; Schädler, D.; Pulletz, S.; Freitag-Wolf, S.; Weiler, N.; Frerichs, I. Effect of PEEP and Tidal Volume on Ventilation Distribution and End-Expiratory Lung Volume: A Prospective Experimental Animal and Pilot Clinical Study. PLoS ONE 2013, 8, e72675. [Google Scholar] [CrossRef] [Green Version]
- Odenstedt, H.; Lindgren, S.; Olegård, C.; Erlandsson, K.; Lethvall, S.; Åneman, A.; Stenqvist, O.; Lundin, S. Slow Moderate Pressure Recruitment Maneuver Minimizes Negative Circulatory and Lung Mechanic Side Effects: Evaluation of Recruitment Maneuvers Using Electric Impedance Tomography. Intensive Care Med. 2005, 31, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Van Heerde, M.; Roubik, K.; Kopelent, V.; Kneyber, M.C.J.; Markhorst, D.G. Spontaneous Breathing during High-Frequency Oscillatory Ventilation Improves Regional Lung Characteristics in Experimental Lung Injury. Acta Anaesthesiol. Scand. 2010, 54, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.L.V.; Chaves, C.N.; Gomes, S.; Beraldo, M.A.; Volpe, M.S.; Tucci, M.R.; Schettino, I.A.L.; Bohm, S.H.; Carvalho, C.R.R.; Tanaka, H.; et al. Real-Time Detection of Pneumothorax Using Electrical Impedance Tomography. Crit. Care Med. 2008, 36, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Schiffmann, H.; Hahn, G.; Hellige, G. Non-Invasive Radiation-Free Monitoring of Regional Lung Ventilation in Critically Ill Infants. Intensive Care Med. 2001, 27, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Miedema, M.; De Jongh, F.H.; Frerichs, I.; Van Veenendaal, M.B.; Van Kaam, A.H. Changes in Lung Volume and Ventilation during Surfactant Treatment in Ventilated Preterm Infants. Am. J. Respir. Crit. Care Med. 2011, 184, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Veenendaal, M.B.; Miedema, M.; De Jongh, F.H.C.; Van Der Lee, J.H.; Frerichs, I.; Van Kaam, A.H. Effect of Closed Endotracheal Suction in High-Frequency Ventilated Premature Infants Measured with Electrical Impedance Tomography. Intensive Care Med. 2009, 35, 2130–2134. [Google Scholar] [CrossRef] [Green Version]
- Deibele, J.M.; Luepschen, H.; Leonhardt, S. Dynamic Separation of Pulmonary and Cardiac Changes in Electrical Impedance Tomography. Physiol. Meas. 2008, 29, S1–S14. [Google Scholar] [CrossRef]
- Frerichs, I.; Hinz, J.; Herrmann, P.; Weisser, G.; Hahn, G.; Quintel, M.; Hellige, G. Regional Lung Perfusion as Determined by Electrical Impedance Tomography in Comparison with Electron Beam CT Imaging. IEEE Trans. Med. Imaging 2002, 21, 646–652. [Google Scholar] [CrossRef]
- Shono, A.; Kotani, T.; Frerichs, I. Personalisation of Therapies in COVID-19 Associated Acute Respiratory Distress Syndrome, Using Electrical Impedance Tomography. J. Crit. Care Med. 2021, 7, 62–66. [Google Scholar] [CrossRef]
- Mauri, T.; Spinelli, E.; Scotti, E.; Colussi, G.; Basile, M.C.; Crotti, S.; Tubiolo, D.; Tagliabue, P.; Zanella, A.; Grasselli, G.; et al. Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients with the Acute Respiratory Distress Syndrome from Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1129–1134. [Google Scholar] [CrossRef]
- Pulletz, S.; Van Genderingen, H.R.; Schmitz, G.; Zick, G.; Schädler, D.; Scholz, J.; Weiler, N.; Frerichs, I. Comparison of Different Methods to Define Regions of Interest for Evaluation of Regional Lung Ventilation by EIT. Physiol. Meas. 2006, 27, S115–S127. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, S.; Linz, K.; Braun, C.; Dzierzawski, P.; Spassov, S.; Wenzel, C.; Schumann, S. Lung Area Estimation Using Functional Tidal Electrical Impedance Variation Images and Active Contouring. Physiol. Meas. 2022, 43, 075010. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.Y.; Hou, W.; Huang, Q.; Hou, X.; Bao, X.Y.; Kong, W.X.; Li, C.X.; Qiu, Y.Q.; Hu, S.Y.; Dong, L.H. Advances in Electrical Impedance Tomography-Based Brain Imaging. Mil. Med. Res. 2022, 9, 10. [Google Scholar] [CrossRef]
- Hannan, S.; Faulkner, M.; Aristovich, K.; Avery, J.; Walker, M.; Holder, D. Imaging Fast Electrical Activity in the Brain during Ictal Epileptiform Discharges with Electrical Impedance Tomography. NeuroImage Clin. 2018, 20, 674–684. [Google Scholar] [CrossRef]
- Hannan, S.; Faulkner, M.; Aristovich, K.; Avery, J.; Walker, M.C.; Holder, D.S. In Vivo Imaging of Deep Neural Activity from the Cortical Surface during Hippocampal Epileptiform Events in the Rat Brain Using Electrical Impedance Tomography. Neuroimage 2020, 209, 116525. [Google Scholar] [CrossRef] [PubMed]
- Holder, D.S. Detection of Cortical Spreading Depression in the Anaesthetised Rat by Impedance Measurement with Scalp Electrodes: Implications for Non-Invasive Imaging of the Brain with Electrical Impedance Tomography. Clin. Phys. Physiol. Meas. 1992, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Boverman, G.; Kao, T.J.; Wang, X.; Ashe, J.M.; Davenport, D.M.; Amm, B.C. Detection of Small Bleeds in the Brain with Electrical Impedance Tomography. Physiol. Meas. 2016, 37, 727–750. [Google Scholar] [CrossRef]
- Fu, F.; Li, B.; Dai, M.; Hu, S.J.; Li, X.; Xu, C.H.; Wang, B.; Yang, B.; Tang, M.X.; Dong, X.Z.; et al. Use of Electrical Impedance Tomography to Monitor Regional Cerebral Edema during Clinical Dehydration Treatment. PLoS ONE 2014, 9, e113202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Tian, Z.; Wang, M. Simultaneous Imaging of Intracerebral Hemorrhage and Secondary Ischemia with Electrical Impedance Tomography. In Proceedings of the 2021 IEEE International Conference on Consumer Electronics and Computer Engineering, ICCECE 2021, Guangzhou, China, 15–17 January 2021; pp. 723–726. [Google Scholar]
- Ferraioli, F.; Formisano, A.; Martone, R. Effective Exploitation of Prior Information in Electrical Impedance Tomography for Thermal Monitoring of Hyperthermia Treatments. IEEE Trans. Magn. 2009, 45, 1554–1557. [Google Scholar] [CrossRef]
- Bottiglieri, A.; Dunne, E.; McDermott, B.; Cavagnaro, M.; Porter, E.; Farina, L. Monitoring Microwave Thermal Ablation Using Electrical Impedance Tomography: An Experimental Feasibility Study. In Proceedings of the 14th European Conference on Antennas and Propagation, EuCAP 2020, Copenhagen, Denmark, 15–20 March 2020. [Google Scholar]
- Kao, T.J.; Boverman, G.; Kim, B.S.; Isaacson, D.; Saulnier, G.J.; Newell, J.C.; Choi, M.H.; Moore, R.H.; Kopans, D.B. Regional Admittivity Spectra with Tomosynthesis Images for Breast Cancer Detection: Preliminary Patient Study. IEEE Trans. Med. Imaging 2008, 27, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Akhtari-Zavare, M.; Latiff, L.A. Electrical Impedance Tomography as a Primary Screening Technique for Breast Cancer Detection. Asian Pacific J. Cancer Prev. 2015, 16, 5595–5597. [Google Scholar] [CrossRef]
- Mansouri, S.; Chabchoub, S.; Alharbi, Y.; Alshrouf, A. EIT 40-Electrodes Breast Cancer Detection and Screening. IEEJ Trans. Electr. Electron. Eng. 2022, 17, 1141–1147. [Google Scholar] [CrossRef]
- Ma, G.; Soleimani, M. Spectral Capacitively Coupled Electrical Resistivity Tomography for Breast Cancer Detection. IEEE Access 2020, 8, 50900–50910. [Google Scholar] [CrossRef]
- Braun, R.P.; Mangana, J.; Goldinger, S.; French, L.; Dummer, R.; Marghoob, A.A. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 489–493. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Fields, S.I.; Shriver, C.D.; Lenington, S.; Ginor, R.; Peoples, G.E.; Burch, H.B.; Peretz, T.; Freund, H.R.; Nissan, A. Electrical Impedance Scanning of Thyroid Nodules before Thyroid Surgery: A Prospective Study. Ann. Surg. Oncol. 2005, 12, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Ivorra, A.; Reuter, V.E.; Rubinsky, B.; Solomon, S.B. Electrical Impedance Characterization of Normal and Cancerous Human Hepatic Tissue. Physiol. Meas. 2010, 31, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Das, S.; Chatterjee, J. Electrical Bioimpedance Analysis: A New Method in Cervical Cancer Screening. J. Med. Eng. 2015, 2015, 636075. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga-Gomez, J.; Zerhouni, N.; Al Masry, Z.; Devalland, C.; Varnier, C. A Survey of Breast Cancer Screening Techniques: Thermography and Electrical Impedance Tomography. J. Med. Eng. Technol. 2019, 43, 305–322. [Google Scholar] [CrossRef] [Green Version]
- Pathiraja, A.A.; Pathiraja, A.A.; Weerakkody, R.A.; Weerakkody, R.A.; Von Roon, A.C.; Von Roon, A.C.; Ziprin, P.; Ziprin, P.; Bayford, R.; Bayford, R. The Clinical Application of Electrical Impedance Technology in the Detection of Malignant Neoplasms: A Systematic Review. J. Transl. Med. 2020, 18, 227. [Google Scholar] [CrossRef]
- Yang, D.; Gu, C.; Gu, Y.; Zhang, X.; Ge, D.; Zhang, Y.; Wang, N.; Zheng, X.; Wang, H.; Yang, L.; et al. Electrical Impedance Analysis for Lung Cancer: A Prospective, Multicenter, Blind Validation Study. Front. Oncol. 2022, 12, 3418. [Google Scholar] [CrossRef]
- Ye, G.; Lim, K.H.; George, R.T.; Ybarra, G.A.; Joines, W.T.; Liu, Q.H. 3D EIT for Breast Cancer Imaging: System, Measurements, and Reconstruction. Microw. Opt. Technol. Lett. 2008, 50, 3261–3271. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Bae, J.; Yoo, H.J. A 10.4 MW Electrical Impedance Tomography SoC for Portable Real-Time Lung Ventilation Monitoring System. IEEE J. Solid-State Circuits 2015, 50, 2501–2512. [Google Scholar] [CrossRef]
- Murphy, E.K.; Skinner, J.; Martucci, M.; Rutkove, S.B.; Halter, R.J. Toward Electrical Impedance Tomography Coupled Ultrasound Imaging for Assessing Muscle Health. IEEE Trans. Med. Imaging 2019, 38, 1409–1419. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in Healthcare Wearable Devices. npj Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Huang, J.J.; Hung, Y.H.; Wang, J.J.; Lin, B.S. Design of Wearable and Wireless Electrical Impedance Tomography System. Meas. J. Int. Meas. Confed. 2016, 78, 9–17. [Google Scholar] [CrossRef]
- Rapin, M.; Braun, F.; Adler, A.; Wacker, J.; Frerichs, I.; Vogt, B.; Chetelat, O. Wearable Sensors for Frequency-Multiplexed EIT and Multilead ECG Data Acquisition. IEEE Trans. Biomed. Eng. 2019, 66, 810–820. [Google Scholar] [CrossRef]
- Zlochiver, S.; Arad, M.; Radai, M.M.; Barak-Shinar, D.; Krief, H.; Engelman, T.; Ben-Yehuda, R.; Adunsky, A.; Abboud, S. A Portable Bio-Impedance System for Monitoring Lung Resistivity. Med. Eng. Phys. 2007, 29, 93–100. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Duan, J.; Liu, X.; Bayford, R.; Demosthenous, A. Towards a High Accuracy Wearable Hand Gesture Recognition System Using EIT. In Proceedings of the IEEE International Symposium on Circuits and Systems, Florence, Italy, 27–30 May 2018; Volume 2018. [Google Scholar]
- Lu, X.; Sun, S.; Liu, K.; Sun, J.; Xu, L. Development of a Wearable Gesture Recognition System Based on Two-Terminal Electrical Impedance Tomography. IEEE J. Biomed. Health Inform. 2022, 26, 2515–2523. [Google Scholar] [CrossRef]
- Lee, M.H.; Jang, G.Y.; Kim, Y.E.; Yoo, P.J.; Wi, H.; Oh, T.I.; Woo, E.J. Portable Multi-Parameter Electrical Impedance Tomography for Sleep Apnea and Hypoventilation Monitoring: Feasibility Study. Physiol. Meas. 2018, 39, 124004. [Google Scholar] [CrossRef]
- Aliverti, A. Wearable Technology: Role in Respiratory Health and Disease. Breathe 2017, 13, e27–e36. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, A.; Aliverti, A. Telemonitoring Systems for Respiratory Patients: Technological Aspects. Pulmonology 2020, 26, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart Textiles and Sensorized Garments for Physiological Monitoring: A Review of Available Solutions and Techniques. Sensors 2021, 21, 814. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, J.; Yoo, H.J. Wearable Lung-Health Monitoring System with Electrical Impedance Tomography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 1707–1710. [Google Scholar]
- Adler, A.; Grychtol, B.; Bayford, R. Why Is EIT so Hard, and What Are We Doing about It? Physiol. Meas. 2015, 36, 1067–1073. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Output | Application | Advantages (+) and Disadvantages (−) |
|---|---|---|---|
| Absolute | Absolute impedance | Computer simulations | Always applicable (+) Sensitive to instrumentation and modelling errors (−) Not reliable in a clinical setting (−) |
| Time- difference | Time-dependent impedance change | Time-varying phenomena | Robust to modelling errors (+) Time-referenced data required (−) |
| Frequency-difference | Frequency-dependent impedance change | Identification of different tissues | Robust to modelling errors (+) Time-referenced data not required (+) Sufficient contact between tissues’ electrical frequency properties required (−) |
| Issue | Challenge | Innovative Solutions |
|---|---|---|
| Optimal number | Trade-off between image resolution and computation time | Rotational EIT |
| Correct positioning | Lack of robustness of image reconstruction algorithms | Elastic or adjustable electrode belt |
| Contact impedance | Modelling errors | Multi-pole measurement strategy; simultaneous reconstruction of electrodes and electrical properties; capacitively coupled EIT |
| Noise at the interface | Degradation of image resolution | Active electrode-based EIT |
| Ensure good contact | Variable contact impedance and reconstruction errors | Conductive gel; faulty contact detection algorithm |
| Ensure good contact (wearable systems) | Variable contact impedance and reconstruction errors | Dry and flexible electrodes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennati, F.; Angelucci, A.; Morelli, L.; Bardini, S.; Barzanti, E.; Cavallini, F.; Conelli, A.; Di Federico, G.; Paganelli, C.; Aliverti, A. Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables. Sensors 2023, 23, 1182. https://doi.org/10.3390/s23031182
Pennati F, Angelucci A, Morelli L, Bardini S, Barzanti E, Cavallini F, Conelli A, Di Federico G, Paganelli C, Aliverti A. Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables. Sensors. 2023; 23(3):1182. https://doi.org/10.3390/s23031182
Chicago/Turabian StylePennati, Francesca, Alessandra Angelucci, Letizia Morelli, Susanna Bardini, Elena Barzanti, Federico Cavallini, Antonello Conelli, Gaia Di Federico, Chiara Paganelli, and Andrea Aliverti. 2023. "Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables" Sensors 23, no. 3: 1182. https://doi.org/10.3390/s23031182
APA StylePennati, F., Angelucci, A., Morelli, L., Bardini, S., Barzanti, E., Cavallini, F., Conelli, A., Di Federico, G., Paganelli, C., & Aliverti, A. (2023). Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables. Sensors, 23(3), 1182. https://doi.org/10.3390/s23031182









