Recent Advances of Enzyme-Free Electrochemical Sensors for Flexible Electronics in the Detection of Organophosphorus Compounds: A Review
Abstract
:1. Introduction
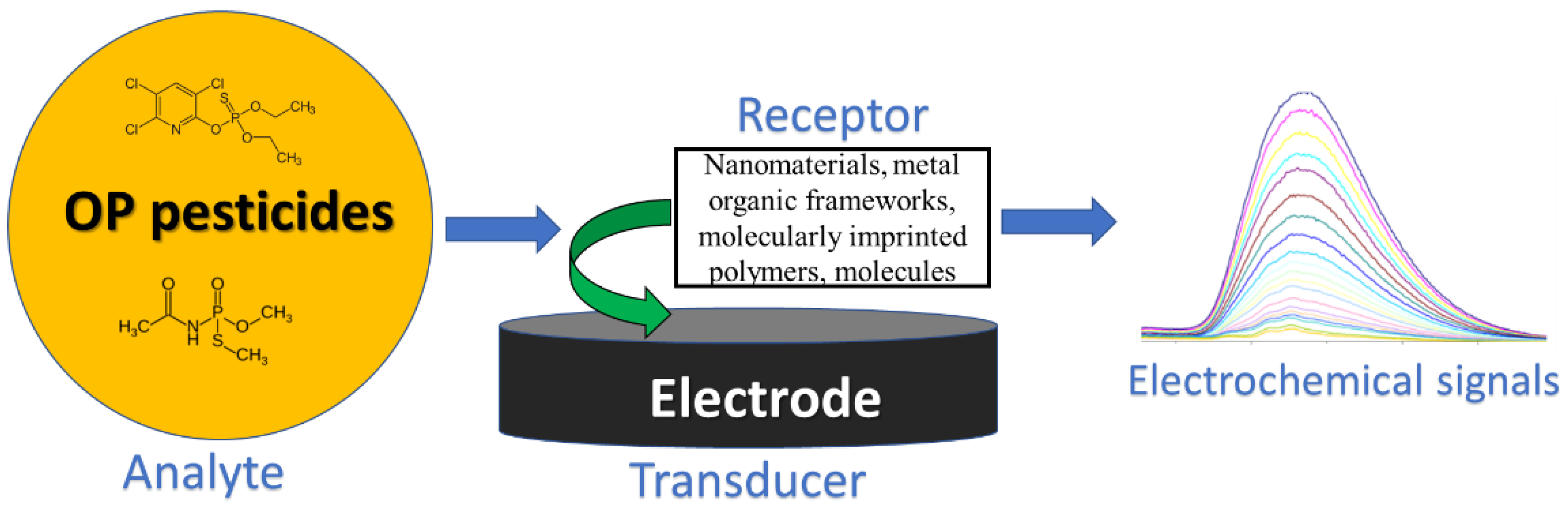
2. Electrochemical Sensors
2.1. Working Principle of an Electrochemical Sensor
2.2. Nanomaterial-Based Sensors
| Nanomaterials/ Electrode | OP Compound | Detection Technique | Concentration Range | LOD | Sensitivity | Published Year |
|---|---|---|---|---|---|---|
| ZrO2 NPs | Paraoxon, fenitrothion, Methyl parathion | Square wave voltammetry | Methyl parathion: 5–100 ng/mL | 3 ng/mL | - | 2005 [44] |
| ZrO2/Au nanocomposite | Parathion | Square wave voltammetry | 10–160 ng/ml | 3 ng/ml | - | 2008 [70] |
| MWCNTs | Methyl parathion | Square wave voltammetry | 0.05–2.0 µg/mL | 0.005 µg/mL | - | 2008 [87] |
| Bismuth-film/GCE | Methyl parathion | Square wave voltammetry | 3.0–100 ng/mL | 1.2 ng/ml | 0.0253 µA.mL.ng−1 | 2008 [83] |
| ZrO2 NPs | Methyl parathion | Square-wave voltammetry | 0.003–2.0 µg/mL | 0.001 µg/mL | - | 2008 [88] |
| Ionic liquid/ SWCNT/GCE | Methyl parathion | Linear sweep voltammetry | 2.0 × 10−9–4.0 × 10−6 M | 1.0 × 10−9 M | - | 2008 [81] |
| Electro deposited Au NPs on MWCNTs | Parathion | Linear scan voltammetry | 6.0 × 10−5–5.0 × 10−7 M | 1.0 × 10−7 M | - | 2009 [89] |
| ZrO2 NPs modified carbon paste electrode | Methyl parathion | Square wave voltammetry | 5.0–3000.0 ng/mL | 2.0 ng/mL | - | 2010 [90] |
| Pd NPs/MWCNTs nanocomposite | Methyl parathion | Differential pulse voltammetry | 0.10 μg/mL– 14 μg/mL | 0.05 μg/mL | - | 2010 [71] |
| Au NPs/Nafion/GCE | Methyl parathion | Square wave voltammetry | 5.0 × 10−7 to 1.2 × 10−4 M | 1.0 × 10−7 M | - | 2010 [91] |
| ZrO2/carbon paste electrode | Methyl parathion | Square wave voltammetry | 1.0 × 10−8– 1.0 × 10−5 mol/L | 4.6 × 10−9 mol/L | - | 2010 [92] |
| Au NPs decorated graphene hybrid nanosheets | Methyl parathion | Square wave voltammetry | 0.001–0.1 & 0.2–1.0 µg/mL | 0.6 ng/mL | - | 2011 [69] |
| Graphene-ZrO2 nanocomposite | Methyl parathion | Square wave voltammetry | 0.5 ng/mL–100 ng/mL | 0.1 ng/mL | - | 2011 [93] |
| Au NP-MWCNTs composite | Methyl parathion | Differential pulse voltammetry | 0.5–16.0 mg/mL | 50 µg/mL | 1.91 μAμg−1mL | 2011 [94] |
| ZrO2 NPs-graphene nanosheet nanocomposite | Methyl parathion | Square wave voltammetry | 0.002–0.9 µg/mL | 0.6 ng/mL | - | 2012 [73] |
| Au–ZrO2–SiO2 nanocomposite | Paraoxon-ethyl | Square wave voltammetry | 1.0–500 ng/mL | 0.5 ng/mL | - | 2012 [95] |
| MWCNTs/poly(acrylamide) nanocomposite/GCE | Methyl parathion | Differential pulse voltammetry | 5.0 × 10−9– 1.0 × 10−5 mol/L | 2.0 × 10−9 mol/L | 0.882 μA.μM−1 | 2012 [96] |
| Graphene-Nafion/GCE | Methyl parathion | Square wave voltammetry | 0.02–20 µg/mL | 1.6 ng/mL | - | 2013 [97] |
| MWCNTs-CeO2-Au nanocomposite | Methyl parathion | Electrochemical stripping voltammetry | 10−10 × 10−7 M | 3.02 × 10−11 M | - | 2013 [98] |
| CuO NWs-SWCNTs nanocomposite | Malathion | Differential pulse voltammetry | 0-0.4 ppb | 0.1 ppb | 628.71 μA.cm−2.ppb−1 | 2014 [37] |
| ZrO2 NPs | Omethoate | Square wave voltammetry | 98.5 pmol/L–985 nmol/L | 52.5 pmol/L | - | 2015 [99] |
| mono-6-thio-b-cyclodextrin/ Au NPs/SWCNT/ GCE | Methyl parathion | Square wave voltammetry | 2.0–80.0 nM | 0.1 nM | 0.035 μA/nM | 2015 [100] |
| Reduced graphene oxide/AuNPs nanocomposite | Fenitrothion | Differential pulse voltammetry | 0.1–6.25 ng/mL | 0.036 ng/mL | - | 2016 [101] |
| Ag/graphene nanoribbons nanocomposite | Methyl parathion | Amperometry | 0.005–2780 µM | 0.5 nM | 0.5940 μA.μM−1 cm−2 | 2017 [72] |
| MoS2/graphene nanocomposite | Methyl parathion | Amperometry | 0.01–1905 µM | 3.23 µM | 0.457 (±0.008) μA.μM−1.cm−2 | 2017 [102] |
| Graphene oxide/PEDOT: PSS & polypyrrole/AuNPs | Malathion, cadusafos | Impedance spectroscopy | N/A | 0.1 nmol/L | - | 2017 [84] |
| 3 D graphene/AuNPs nanocomposite | Diethylcyanophosphone | Differential pulse voltammetry | 1 × 10−11–7 × 10−8 M | 3.45 × 10−12 M | - | 2017 [103] |
| MWCNTs/TiO2 NP/ GCE | Diazinon | Square wave voltammetry | 11–8360 nM | 3 nM | - | 2017 [82] |
| NiO nanoplatelets | Parathion | Differential pulse voltammetry | 0.1–30 µM | 0.024 µM | - | 2018 [25] |
| CuO NPs/ 3 D graphene nanocomposite | Malathion | Differential pulse voltammetry | 0.03–1.5 nM | 0.01 nM | - | 2018 [77] |
| CuO/TiO2 nanocomposite | Methyl parathion | Differential pulse voltammetry | 0–2000 ppb | 1.21 ppb | - | 2018 [76] |
| Pralidoxime chlorideimmobilized CuO nanostructure | Chlorpyrifos, fenthion, methyl parathion | Differential pulse voltammetry | 0.01–0.16 µM | Chlorpyrifos-1.6 × 10−9 M Fenthion-2.5 × 10−9 M Methyl parathion-6.7 × 10−9 M | - | 2018 [104] |
| NbC/Mo NPs nanocomposite | Fenitrothion | Differential pulse voltammetry | 0.01–1889 µM | 0.15 nM | 0.355 μA.μM−1 cm−2 | 2018 [105] |
| Peptide nanotube/ pencil graphite electrode | Fenitrothion | Square wave voltammetry | 0.114 μM–1.712 μM | 0.0196 μM | - | 2018 [106] |
| Ionic liquid/chitosan/AuNPs | Malathion | Square wave voltammetry | 0.89–5.94 nM & 5.94–44.6 nM | 0.68 nM | - | 2018 [38] |
| AuNPs/neutral red-protein functionalized graphene/GCE | Methyl Parathion | Differential pulse voltammetry | 0.02–0.153 μM & 0.153–1.36 μM | 6 nM | - | 2019 [107] |
| SiC NPs/MWCNTs/t chitosan | Parathion | Differential pulse voltammetry | 0–10,000 ng/mL | 20 ng/mL | - | 2019 [85] |
| CNT/carbon paste electrode | Diazinon | Differential pulse voltammetry | 1 × 10−10–6 × 10−8 M | 4.5 × 10−10 M | - | 2019 [108] |
| MWCNTs/ZrO2 nanocomposite | Methyl parathion | Differential pulse voltammetry | 19.9–176.8 × 10−6 mol/L | 9.0 × 10−9 mol/L | - | 2020 [74] |
| MWCNTs | Methyl parathion | Differential pulse voltammetry | 1.0 × 10−7−3.4 × 10−5 M | 3.52 × 10−8 M-acid treatment, 3.33 × 10−8 M-base treatment | 0.55 μA/μM-acid 0.52 μA/μM-base | 2020 [109] |
| Pt/Zr-based metal organic framework/carbon paste microelectrode | Phosalone | Square wave voltammetry | 0.50 nM–20 μM | 0.078 nM | - | 2020 [110] |
| Carbon nanoballs/Glove-Right finger pretreated | Fenitrothion | Square wave voltammetry | 1.0 × 10−6– 10 × 10−6 mol/L | 6.4 × 10−7 mol/L | - | 2021 [111] |
| Graphene nanoplatelet/ ZrO2/ Ag SPE | Methyl parathion | Square wave voltammetry | 1–20 µM | 1 µM | 2021 [112] | |
| Zinc(II) phthalocyanine/ SWCNT/GCE | Methyl parathion | Differential pulse voltammetry | 2.45 nM–4.0 × 10−8 M | 1.49 nM | 0.1847 μA.nM−1 | 2021 [113] |
| CuO Nanorods electrode | chlorpyrifos, parathion, paraoxon, pirimiphos | Cyclic Voltammetry | 0.29–0.61 μM | 10−7 M | 1.269 μA/ngmL−1 -chlorpyrifos, 1.425 μA/ngmL−1 -parathion, 1.657 μA/ngmL−1 -paraoxon, 2.833 μA/ngmL−1 -pirimiphos | 2021 [75] |
| Halloysite nanotubes/MWCNTs | Methyl parathion | Differential pulse voltammetry | 0.5–11 μM | 0.034 μM | - | 2021 [86] |
| Ag NPs/PGE functionalized CNT/graphite electrode | Diazinon, malathion, chlorpyrifos | Multiple pulse amperometry | Diazinon: 0.1–20 μM, malathion: 1–30 μM, chlorpyrifos: 0.25–50 μM | Diazinon-0.35 μmol/L, malathion-0.89 μmol/L, chlorpyrifos-0.53 μmol/L | Diazinon- 0.068 mALμmol−1, Malathion-0.030 mALμmol−1 Chlorpyrifos-0.043 mALμmol−1 | 2022 [114] |
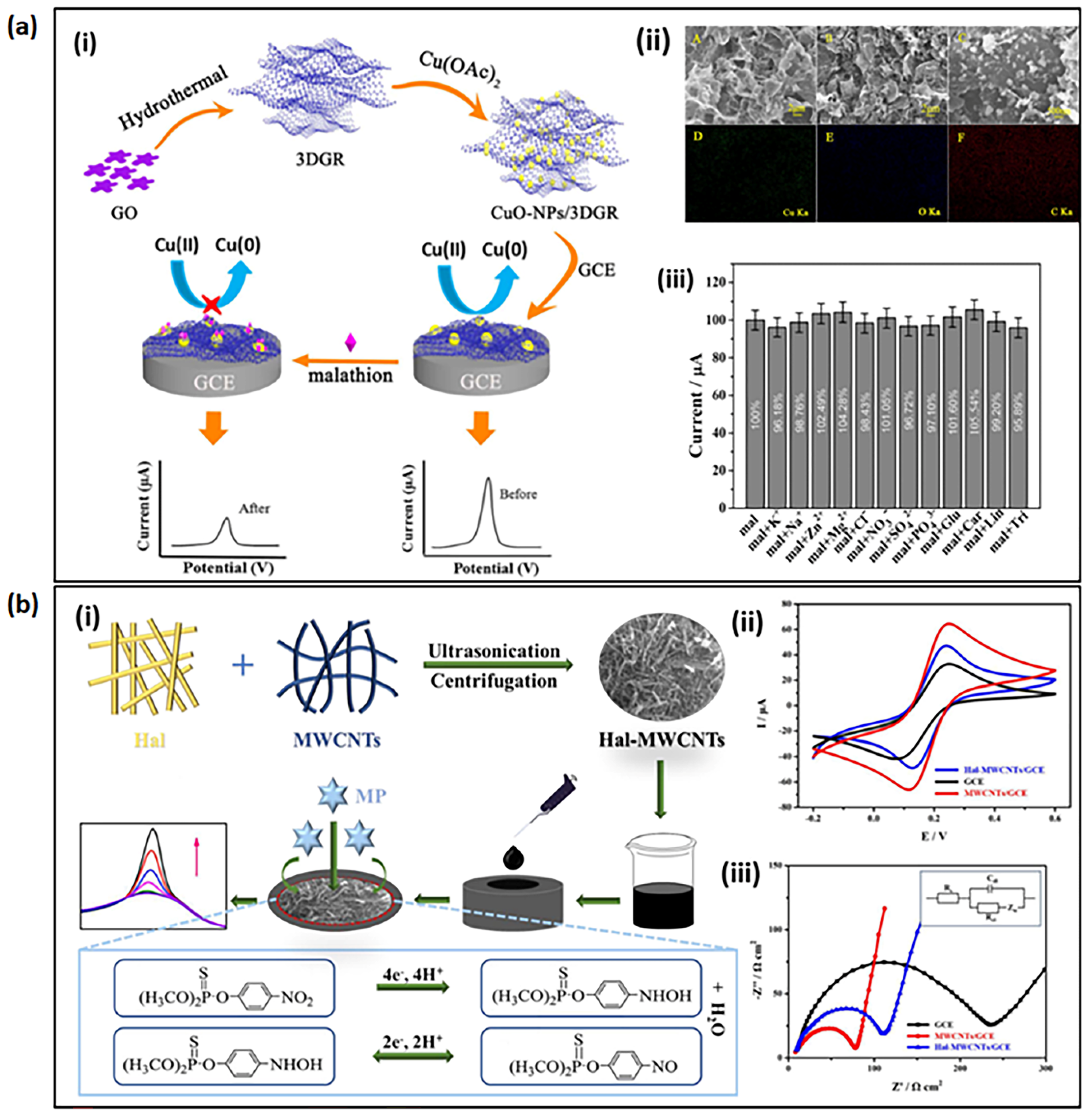
2.3. Molecular Electrochemical Sensors
| Molecule/ Electrode | OP Compound | Detection Technique | Concentration Range | LOD | Sensitivity | Published Year |
|---|---|---|---|---|---|---|
| Cobalt metallophthalocyanines/GCE | Fenitrothion | Square wave voltammetry | 1.20–42.0 μmoldm−3 | 0.460 μmoldm−3 | 0.26 Acm−2M−1 | 2017 [130] |
| CoPc(MOR-NAF)/GCE | Diazinon, Parathion | Square wave voltammetry | Diazinon-0.38–5.07 μmoldm−3, Parathion-0.07–5.75 μmoldm−3 | Diazinon-0.120 Μmoldm−3 Parathion-0.020 μmoldm−3 | 3.46 Acm−2M−1 | 2017 [131] |
| Manganese Phthalocyanine-4-azido polyaniline hybrid/ITO | Fenitrothion, eserine, diazinon | Square wave voltammetry | Fenitrothion-0.12–15.00 μmoldm−3 Eserine-0.10–5.00 μmoldm−3 Diazinon-0.20–7.50 μmoldm−3 | Fenitrothion-0.049 μmoldm−3 Eserine-0.088 μmol dm−3 Diazinon-0.062 μmol dm−3 | 4.67 Acm−2M−1 | 2019 [132] |
3. Flexible Electronic Sensors
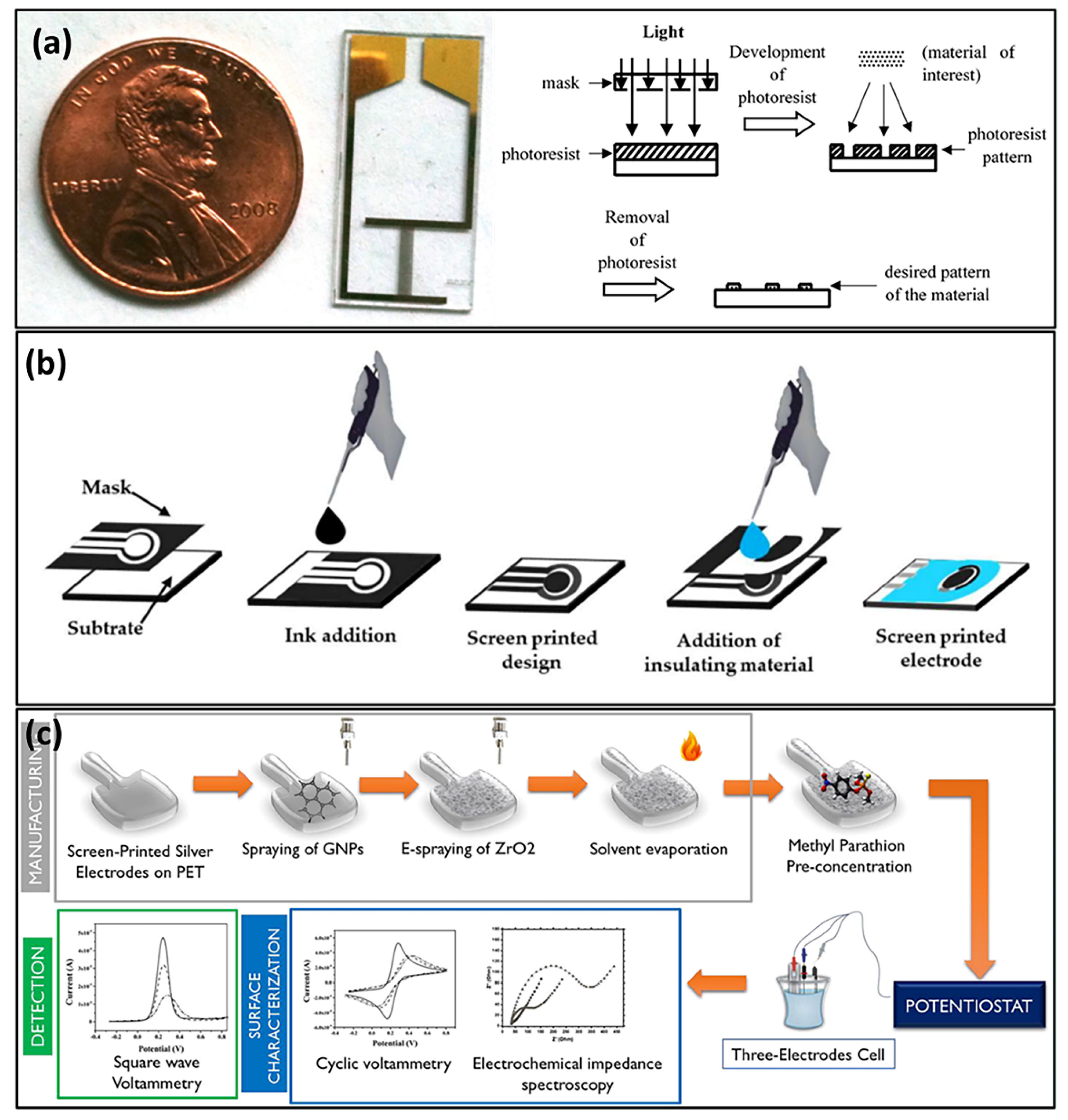
4. Summary, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; White, S. Tracking Phosphorus Security: Indicators of Phosphorus Vulnerability in the Global Food System. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Hashmi, Z.; Adriyani, R.; Yuniarto, A.; Mazari, S.A.; Akhter, F.; Mubarak, N.M. Recent Trends and Future Challenges of Pesticide Removal Techniques—A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 105571. [Google Scholar] [CrossRef]
- EPA, U.S. Report of Pesticide Industry Sales and Usage, 2008–2012 Market Estimates; United States Environmental Protection Agency: Washington, DC, USA, 2017.
- Wieben, C.M. Estimated Annual Agricultural Pesticide Use by Major Crop or Crop Group for States of the Conterminous United States. 1992–2017 (ver. 2.0, May 2020): U.S. Geological Survey data release 2013; U.S. Geological Survey: Reston, VA, USA, 20 May 2019. [Google Scholar]
- Earthjustice Council Organophosphate Pesticides in the United States. 2021. Available online: https://earthjustice.org/features/organophosphate-pesticides-united-states (accessed on 10 December 2022).
- Baker, N.T. Estimated Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 2008–12; U.S. Geological Survey: Reston, VA, USA, 2015.
- United States Department of Agriculture. Pesticide Data Program (PDP) Annual Summary, Calendar Year. 2021. Available online: https://www.ams.usda.gov/sites/default/files/media/2021PDPAnnualSummary.pdf (accessed on 10 December 2022).
- Hertz-Picciotto, I.; Sass, J.B.; Engel, S.; Bennett, D.H.; Bradman, A.; Eskenazi, B.; Lanphear, B.; Whyatt, R. Organophosphate Exposures during Pregnancy and Child Neurodevelopment: Recommendations for Essential Policy Reforms. PLoS Med. 2018, 15, e1002671. [Google Scholar] [CrossRef] [PubMed]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018–7043. [Google Scholar] [CrossRef] [PubMed]
- Samuels, A.T.; Obare, S.O. Advances in Analytical Methods for Organophosphorus Pesticide Detection. In Pesticides in the Modern World—Trends in Pesticides Analysis; Stoytcheva, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-437-5. [Google Scholar]
- Adhya, T.K.; Wahid, P.A.; Sethunathan, N. Persistence and Biodegradation of Selected Organophosphorus Insecticides in Flooded versus Non-Flooded Soils. Biol. Fertil. Soils 1987, 5, 36–40. [Google Scholar] [CrossRef]
- Tahara, M.; Kubota, R.; Nakazawa, H.; Tokunaga, H.; Nishimura, T. Use of Cholinesterase Activity as an Indicator for the Effects of Combinations of Organophosphorus Pesticides in Water from Environmental Sources. Water Res. 2005, 39, 5112–5118. [Google Scholar] [CrossRef]
- Steiner, W.E.; Klopsch, S.J.; English, W.A.; Clowers, B.H.; Hill, H.H. Detection of a Chemical Warfare Agent Simulant in Various Aerosol Matrixes by Ion Mobility Time-of-Flight Mass Spectrometry. Anal. Chem. 2005, 77, 4792–4799. [Google Scholar] [CrossRef]
- John, H.; Worek, F.; Thiermann, H. LC-MS-Based Procedures for Monitoring of Toxic Organophosphorus Compounds and Verification of Pesticide and Nerve Agent Poisoning. Anal. Bioanal. Chem. 2008, 391, 97–116. [Google Scholar] [CrossRef]
- Zeng, K.; Yang, T.; Zhong, P.; Zhou, S.; Qu, L.; He, J.; Jiang, Z. Development of an Indirect Competitive Immunoassay for Parathion in Vegetables. Food Chem. 2007, 102, 1076–1082. [Google Scholar] [CrossRef]
- Skládal, P.; Horáček, J.; Malina, M. Direct piezoelectric immunosensors for pesticides. In Biosensors for Direct Monitoring of Environmental Pollutants in Field; Nikolelis, D.P., Krull, U.J., Wang, J., Mascini, M., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 145–153. ISBN 978-90-481-4959-9. [Google Scholar]
- Evtugyn, G.A.; Budnikov, H.C.; Nikolskaya, E.B. Influence of Surface-Active Compounds on the Response and Sensitivity of Cholinesterase Biosensors for Inhibitor Determination. Analyst 1996, 121, 1911. [Google Scholar] [CrossRef]
- Lei, Y.; Mulchandani, P.; Wang, J.; Chen, W.; Mulchandani, A. Highly Sensitive and Selective Amperometric Microbial Biosensor for Direct Determination of p-Nitrophenyl-Substituted Organophosphate Nerve Agents. Environ. Sci. Technol. 2005, 39, 8853–8857. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X. Acetylcholinesterase Biosensor Based on Prussian Blue-Modified Electrode for Detecting Organophosphorous Pesticides. Biosens. Bioelectron. 2010, 25, 2611–2614. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Hu, H.; Shu, W.; Yang, L.; Zhang, J. Acetylcholinesterase Electrochemical Biosensors with Graphene-Transition Metal Carbides Nanocomposites Modified for Detection of Organophosphate Pesticides. PLoS ONE 2020, 15, e0231981. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent Advances in Nanomaterials-Based Electrochemical (Bio)Sensors for Pesticides Detection. TrAC Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Arduini, F.; Guidone, S.; Amine, A.; Palleschi, G.; Moscone, D. Acetylcholinesterase Biosensor Based on Self-Assembled Monolayer-Modified Gold-Screen Printed Electrodes for Organophosphorus Insecticide Detection. Sens. Actuators B Chem. 2013, 179, 201–208. [Google Scholar] [CrossRef]
- Selvolini, G.; Băjan, I.; Hosu, O.; Cristea, C.; Săndulescu, R.; Marrazza, G. DNA-Based Sensor for the Detection of an Organophosphorus Pesticide: Profenofos. Sensors 2018, 18, 2035. [Google Scholar] [CrossRef] [Green Version]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Khairy, M.; Ayoub, H.A.; Banks, C.E. Non-Enzymatic Electrochemical Platform for Parathion Pesticide Sensing Based on Nanometer-Sized Nickel Oxide Modified Screen-Printed Electrodes. Food Chem. 2018, 255, 104–111. [Google Scholar] [CrossRef]
- Istamboulie, G.; Andreescu, S.; Marty, J.-L.; Noguer, T. Highly Sensitive Detection of Organophosphorus Insecticides Using Magnetic Microbeads and Genetically Engineered Acetylcholinesterase. Biosens. Bioelectron. 2007, 23, 506–512. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Li, F.-Y.; Ndikuryayo, F.; Yang, W.-C.; Wang, H.-M. Cholinesterases and Engineered Mutants for the Detection of Organophosphorus Pesticide Residues. Sensors 2018, 18, 4281. [Google Scholar] [CrossRef]
- Ambreen, S.; Yasmin, A.; Aziz, S. Isolation and Characterization of Organophosphorus Phosphatases from Bacillus Thuringiensis MB497 Capable of Degrading Chlorpyrifos, Triazophos and Dimethoate. Heliyon 2020, 6, e04221. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.C.; Scott, J.R.; Kauffman, M.A.; Hodgins, S.M.; diTargiani, R.C.; Hughes, J.H.; Sarricks, E.P.; Saturday, G.A.; Hamilton, T.A.; Cerasoli, D.M. Identification and Characterization of Novel Catalytic Bioscavengers of Organophosphorus Nerve Agents. Chem. Biol. Interact. 2013, 203, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Su, H.; Qu, X.; Ju, P.; Cui, L.; Ai, S. Acetylcholinesterase Biosensor Based on 3-Carboxyphenylboronic Acid/Reduced Graphene Oxide–Gold Nanocomposites Modified Electrode for Amperometric Detection of Organophosphorus and Carbamate Pesticides. Sens. Actuators B Chem. 2011, 160, 1255–1261. [Google Scholar] [CrossRef]
- Hu, H.; Yang, L. Development of Enzymatic Electrochemical Biosensors for Organophosphorus Pesticide Detection. J. Environ. Sci. Health Part B 2021, 56, 168–180. [Google Scholar] [CrossRef]
- Zhai, R.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Zhang, Y.; Wang, J.; Jin, M.; Xu, D.; et al. Enzyme Inhibition Methods Based on Au Nanomaterials for Rapid Detection of Organophosphorus Pesticides in Agricultural and Environmental Samples: A Review. J. Adv. Res. 2022, 37, 61–74. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-Based Electrochemical Sensor for the Detection of Nitrophenols and Organophosphates. Sens. Actuators B Chem. 2018, 257, 570–578. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y. A Lanthanide-Based Ratiometric Fluorescent Biosensor for the Enzyme-Free Detection of Organophosphorus Pesticides. Anal. Methods 2021, 13, 2005–2010. [Google Scholar] [CrossRef]
- Gannavarapu, K.P.; Ganesh, V.; Thakkar, M.; Mitra, S.; Dandamudi, R.B. Nanostructured Diatom-ZrO2 Composite as a Selective and Highly Sensitive Enzyme Free Electrochemical Sensor for Detection of Methyl Parathion. Sens. Actuators B Chem. 2019, 288, 611–617. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Sirajjuddin; Memon, S.S.; Willander, M. Amino Acid Assisted Growth of CuO Nanostructures and Their Potential Application in Electrochemical Sensing of Organophosphate Pesticide. Electrochim. Acta 2016, 190, 972–979. [Google Scholar] [CrossRef]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A Highly Efficient Organophosphorus Pesticides Sensor Based on CuO Nanowires-SWCNTs Hybrid Nanocomposite. Sens. Actuators B Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S. Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors 2018, 18, 773. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical Sensor Based on Polyaniline Nanofibers/Single Wall Carbon Nanotubes Composite for Detection of Malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable Metal-Organic Framework/Methylene Blue Composites-Based Homogeneous Electrochemical Strategy for Pesticide Assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Diouf, A.; Österlund, L.; Bouchikhi, B.; El Bari, N. Development of a Molecularly Imprinted Polymer Electrochemical Sensor and Its Application for Sensitive Detection and Determination of Malathion in Olive Fruits and Oils. Bioelectrochemistry 2020, 132, 107404. [Google Scholar] [CrossRef]
- De, C.; Samuels, T.A.; Haywood, T.L.; Anderson, G.A.; Campbell, K.; Fletcher, K.; Murray, D.H.; Obare, S.O. Dual Colorimetric and Electrochemical Sensing of Organothiophosphorus Pesticides by an Azastilbene Derivative. Tetrahedron Lett. 2010, 51, 1754–1757. [Google Scholar] [CrossRef]
- Guo, W.; Engelman, B.J.; Haywood, T.L.; Blok, N.B.; Beaudoin, D.S.; Obare, S.O. Dual Fluorescence and Electrochemical Detection of the Organophosphorus Pesticides—Ethion, Malathion and Fenthion. Talanta 2011, 87, 276–283. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Electrochemical Sensor for Organophosphate Pesticides and Nerve Agents Using Zirconia Nanoparticles as Selective Sorbents. Anal. Chem. 2005, 77, 5894–5901. [Google Scholar] [CrossRef]
- Cagnini, A.; Palchetti, I.; Lionti, I.; Mascini, M.; Turner, A.P.F. Disposable Ruthenized Screen-Printed Biosensors for Pesticides Monitoring. Sens. Actuators B Chem. 1995, 24, 85–89. [Google Scholar] [CrossRef]
- Narakathu, B.B.; Guo, W.; Obare, S.O.; Atashbar, M.Z. Novel Approach for Detection of Toxic Organophosphorus Compounds. Sens. Actuators B Chem. 2011, 158, 69–74. [Google Scholar] [CrossRef]
- Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. A Smart-Phone Based Ratiometric Nanoprobe for Label-Free Detection of Methyl Parathion. Sens. Actuators B Chem. 2020, 322, 128580. [Google Scholar] [CrossRef]
- Narakathu, B.B.; Guo, W.; Obare, S.O.; Atashbar, M.Z. Detection of Picomolar Levels of Toxic Organophosphorus Compounds by Electrochemical and Fluorescence Spectroscopy. Sens. Lett. 2011, 9, 907–909. [Google Scholar] [CrossRef]
- McAlpine, M.C.; Ahmad, H.; Wang, D.; Heath, J.R. Highly Ordered Nanowire Arrays on Plastic Substrates for Ultrasensitive Flexible Chemical Sensors. Nat. Mater. 2007, 6, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.; Ganesh, V. Selective and Sensitive Electrochemical Detection of Methyl Parathion Using Chemically Modified Overhead Projector Sheets as Flexible Electrodes. Sens. Actuators B Chem. 2016, 227, 169–177. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Walcarius, A.; Minteer, S.D.; Wang, J.; Lin, Y.; Merkoçi, A. Nanomaterials for Bio-Functionalized Electrodes: Recent Trends. J. Mater. Chem. B 2013, 1, 4878. [Google Scholar] [CrossRef]
- Bakker, E.; Qin, Y. Electrochemical Sensors. Anal. Chem. 2006, 78, 3965–3984. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry: Wang/Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 978-0-471-79030-3. [Google Scholar]
- Thapliyal, N.; Chiwunze, T.E.; Karpoormath, R.; Goyal, R.N.; Patel, H.; Cherukupalli, S. Research Progress in Electroanalytical Techniques for Determination of Antimalarial Drugs in Pharmaceutical and Biological Samples. RSC Adv. 2016, 6, 57580–57602. [Google Scholar] [CrossRef]
- Ghanam, A.; Mohammadi, H.; Amine, A.; Haddour, N.; Buret, F. Chemical Sensors: Voltammetric and Amperometric Electrochemical Sensors. In Encyclopedia of Sensors and Biosensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 161–177. ISBN 978-0-12-822549-3. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells: Fundamentals, Technology and Applications; Wiley: Chichester, UK, 2003; ISBN 978-0-470-74151-1. [Google Scholar]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial Based Electrochemical Sensors for the Safety and Quality Control of Food and Beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef]
- Adarakatti, P.S.; Kempahanumakkagari, S.K. Modified Electrodes for Sensing. In Electrochemistry; Banks, C., McIntosh, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; Volume 15, pp. 58–95. ISBN 978-1-78801-373-4. [Google Scholar]
- Wang, Q.; Zhao, R.; Wang, S.; Guo, H.; Li, J.; Zhou, H.; Wang, X.; Wu, X.; Wang, Y.; Chen, W.; et al. A Highly Selective Electrochemical Sensor for Nifedipine Based on Layer-by-Layer Assembly Films from Polyaniline and Multiwalled Carbon Nanotube. J. Appl. Polym. Sci. 2016, 133, 43452. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Y.; Huang, J.; Xu, S.; Luo, J.; Liu, X. Electrochemical Sensor Coating Based on Electrophoretic Deposition of Au-Doped Self-Assembled Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 5926–5932. [Google Scholar] [CrossRef]
- Ma, Y.; Han, J.; Wang, M.; Chen, X.; Jia, S. Electrophoretic Deposition of Graphene-Based Materials: A Review of Materials and Their Applications. J. Mater. 2018, 4, 108–120. [Google Scholar] [CrossRef]
- Kim, J.; Bae, S. Fabrication of Ti/Ir-Ru Electrode by Spin Coating Method for Electrochemical Removal of Copper. Environ. Eng. Res. 2019, 24, 646–653. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Torralba-Cadena, L.; Espriu-Gascon, A.; Casas, I.; Bastos-Arrieta, J.; Florido, A. Strategies for Surface Modification with Ag-Shaped Nanoparticles: Electrocatalytic Enhancement of Screen-Printed Electrodes for the Detection of Heavy Metals. Sensors 2019, 19, 4249. [Google Scholar] [CrossRef] [Green Version]
- Rosenberger, A.G.; Dragunski, D.C.; Muniz, E.C.; Módenes, A.N.; Alves, H.J.; Tarley, C.R.T.; Machado, S.A.S.; Caetano, J. Electrospinning in the Preparation of an Electrochemical Sensor Based on Carbon Nanotubes. J. Mol. Liq. 2020, 298, 112068. [Google Scholar] [CrossRef]
- Gong, J.; Miao, X.; Zhou, T.; Zhang, L. An Enzymeless Organophosphate Pesticide Sensor Using Au Nanoparticle-Decorated Graphene Hybrid Nanosheet as Solid-Phase Extraction. Talanta 2011, 85, 1344–1349. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z. Nano-Composite ZrO2/Au Film Electrode for Voltammetric Detection of Parathion. Sens. Actuators B Chem. 2008, 133, 607–612. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.-D.; Chen, C.-H.; Yu, Y.-X. Electrochemical Determination of Methyl Parathion at a Pd/MWCNTs-Modified Electrode. Microchim. Acta 2010, 171, 57–62. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.-M.; Chen, T.-W.; Sundramoorthy, A.K. Methyl Parathion Detection in Vegetables and Fruits Using Silver@graphene Nanoribbons Nanocomposite Modified Screen Printed Electrode. Sci. Rep. 2017, 7, 46471. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Miao, X.; Wan, H.; Song, D. Facile Synthesis of Zirconia Nanoparticles-Decorated Graphene Hybrid Nanosheets for an Enzymeless Methyl Parathion Sensor. Sens. Actuators B Chem. 2012, 162, 341–347. [Google Scholar] [CrossRef]
- Caetano, K.d.S.; da Rosa, D.S.; Pizzolato, T.M.; dos Santos, P.A.M.; Hinrichs, R.; Benvenutti, E.V.; Dias, S.L.P.; Arenas, L.T.; Costa, T.M.H. MWCNT/Zirconia Porous Composite Applied as Electrochemical Sensor for Determination of Methyl Parathion. Microporous Mesoporous Mater. 2020, 309, 110583. [Google Scholar] [CrossRef]
- Sakdarat, P.; Chongsuebsirikul, J.; Thanachayanont, C.; Prichanont, S.; Pungetmongkol, P. Development of a Nonenzymatic Electrochemical Sensor for Organophosphate Pesticide Detection Using Copper (II) Oxide Nanorod Electrodes. J. Nanomater. 2021, 2021, 6623668. [Google Scholar] [CrossRef]
- Tian, X.; Liu, L.; Li, Y.; Yang, C.; Zhou, Z.; Nie, Y.; Wang, Y. Nonenzymatic Electrochemical Sensor Based on CuO-TiO2 for Sensitive and Selective Detection of Methyl Parathion Pesticide in Ground Water. Sens. Actuators B Chem. 2018, 256, 135–142. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, Y.; Lu, L.; Ma, X.; Gong, L.; Huang, X.; Liu, G.; Yu, Y. CuO Nanoparticles Decorated 3D Graphene Nanocomposite as Non-Enzymatic Electrochemical Sensing Platform for Malathion Detection. J. Electroanal. Chem. 2018, 812, 82–89. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Butmee, P.; Tumcharern, G.; Saejueng, P.; Stankovic, D.; Ortner, A.; Jitcharoen, J.; Kalcher, K.; Samphao, A. A Direct and Sensitive Electrochemical Sensing Platform Based on Ionic Liquid Functionalized Graphene Nanoplatelets for the Detection of Bisphenol, A. J. Electroanal. Chem. 2019, 833, 370–379. [Google Scholar] [CrossRef]
- Mehmeti, E.; Stanković, D.M.; Hajrizi, A.; Kalcher, K. The Use of Graphene Nanoribbons as Efficient Electrochemical Sensing Material for Nitrite Determination. Talanta 2016, 159, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Xiao, F.; Liu, L.; Zhao, F.; Zeng, B. Sensitive Voltammetric Response of Methylparathion on Single-Walled Carbon Nanotube Paste Coated Electrodes Using Ionic Liquid as Binder. Sens. Actuators B Chem. 2008, 132, 34–39. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A. A Voltammetric Sensor for Diazinon Pesticide Based on Electrode Modified with TiO2 Nanoparticles Covered Multi Walled Carbon Nanotube Nanocomposite. J. Electroanal. Chem. 2017, 807, 1–9. [Google Scholar] [CrossRef]
- Du, D.; Ye, X.; Zhang, J.; Liu, D. Cathodic Electrochemical Analysis of Methyl Parathion at Bismuth-Film-Modified Glassy Carbon Electrode. Electrochim. Acta 2008, 53, 4478–4484. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Detection of Trace Levels of Organophosphate Pesticides Using an Electronic Tongue Based on Graphene Hybrid Nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bakytkarim, Y.; Tursynbolat, S.; Zeng, Q.; Huang, J.; Wang, L. Nanomaterial Ink for On-Site Painted Sensor on Studies of the Electrochemical Detection of Organophosphorus Pesticide Residuals of Supermarket Vegetables. J. Electroanal. Chem. 2019, 841, 45–50. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, H.; Li, X.; Liu, B.; Liu, R.; Komarneni, S. Nanocomposite of Halloysite Nanotubes/Multi-Walled Carbon Nanotubes for Methyl Parathion Electrochemical Sensor Application. Appl. Clay Sci. 2021, 200, 105907. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Zhang, J.; Cai, J.; Tu, H.; Zhang, A. Application of Multiwalled Carbon Nanotubes for Solid-Phase Extraction of Organophosphate Pesticide. Electrochem. Commun. 2008, 10, 85–89. [Google Scholar] [CrossRef]
- Du, D.; Ye, X.; Zhang, J.; Zeng, Y.; Tu, H.; Zhang, A.; Liu, D. Stripping Voltammetric Analysis of Organophosphate Pesticides Based on Solid-Phase Extraction at Zirconia Nanoparticles Modified Electrode. Electrochem. Commun. 2008, 10, 686–690. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, T.-F.; Wan, Y.-W.; Chen, S.-Y. Gold Nanoparticles-Carbon Nanotubes Modified Sensor for Electrochemical Determination of Organophosphate Pesticides. Microchim. Acta 2009, 165, 307–311. [Google Scholar] [CrossRef]
- Parham, H.; Rahbar, N. Square Wave Voltammetric Determination of Methyl Parathion Using ZrO2-Nanoparticles Modified Carbon Paste Electrode. J. Hazard. Mater. 2010, 177, 1077–1084. [Google Scholar] [CrossRef]
- Kang, T.-F.; Wang, F.; Lu, L.-P.; Zhang, Y.; Liu, T.-S. Methyl Parathion Sensors Based on Gold Nanoparticles and Nafion Film Modified Glassy Carbon Electrodes. Sens. Actuators B Chem. 2010, 145, 104–109. [Google Scholar] [CrossRef]
- Tan, X.; Li, B.; Zhan, G.; Li, C. Sensitive Voltammetric Determination of Methyl Parathion Using a Carbon Paste Electrode Modified with Mesoporous Zirconia. Electroanalysis 2010, 22, 151–154. [Google Scholar] [CrossRef]
- Du, D.; Liu, J.; Zhang, X.; Cui, X.; Lin, Y. One-Step Electrochemical Deposition of a Graphene-ZrO2 Nanocomposite: Preparation, Characterization and Application for Detection of Organophosphorus Agents. J. Mater. Chem. 2011, 21, 8032. [Google Scholar] [CrossRef]
- Ma, J.-C.; Zhang, W.-D. Gold Nanoparticle-Coated Multiwall Carbon Nanotube-Modified Electrode for Electrochemical Determination of Methyl Parathion. Microchim. Acta 2011, 175, 309–314. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, H.; Zhang, A.; Du, D.; Lin, Y. Preparation and Characterization of Au-ZrO2-SiO2 Nanocomposite Spheres and Their Application in Enrichment and Detection of Organophosphorus Agents. J. Mater. Chem. 2012, 22, 4977. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, D.; Yu, Y.; Zhou, T.; Shi, G. Differential Pulse Voltammetric Determination of Methyl Parathion Based on Multiwalled Carbon Nanotubes-Poly(Acrylamide) Nanocomposite Film Modified Electrode. J. Hazard. Mater. 2012, 217–218, 315–322. [Google Scholar] [CrossRef]
- Xue, R.; Kang, T.-F.; Lu, L.-P.; Cheng, S.-Y. Electrochemical Sensor Based on the Graphene-Nafion Matrix for Sensitive Determination of Organophosphorus Pesticides. Anal. Lett. 2013, 46, 131–141. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly Sensitive Electrochemical Stripping Analysis of Methyl Parathion at MWCNTs-CeO2-Au Nanocomposite Modified Electrode. Sens. Actuators B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y.; Kim, H.; Yong, D.; Wang, L.; Han, X. A Highly Efficient ZrO2 Nanoparticle Based Electrochemical Sensor for the Detection of Organophosphorus Pesticides. Chin. J. Chem. 2015, 33, 1135–1139. [Google Scholar] [CrossRef]
- Fu, X.-C.; Zhang, J.; Tao, Y.-Y.; Wu, J.; Xie, C.-G.; Kong, L.-T. Three-Dimensional Mono-6-Thio-β-Cyclodextrin Covalently Functionalized Gold Nanoparticle/Single-Wall Carbon Nanotube Hybrids for Highly Sensitive and Selective Electrochemical Determination of Methyl Parathion. Electrochim. Acta 2015, 153, 12–18. [Google Scholar] [CrossRef]
- Shams, N.; Lim, H.N.; Hajian, R.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ibrahim, I.; Huang, N.M. Electrochemical Sensor Based on Gold Nanoparticles/Ethylenediamine-Reduced Graphene Oxide for Trace Determination of Fenitrothion in Water. RSC Adv. 2016, 6, 89430–89439. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Akilarasan, M.; Kogularasu, S.; Subramani, B. Nanocomposites Composed of Layered Molybdenum Disulfide and Graphene for Highly Sensitive Amperometric Determination of Methyl Parathion. Microchim. Acta 2017, 184, 725–733. [Google Scholar] [CrossRef]
- Huixiang, W.; Danqun, H.; Yanan, Z.; Na, M.; Jingzhou, H.; Miao, L.; Caihong, S.; Changjun, H. A Non-Enzymatic Electro-Chemical Sensor for Organophosphorus Nerve Agents Mimics and Pesticides Detection. Sens. Actuators B Chem. 2017, 252, 1118–1124. [Google Scholar] [CrossRef]
- Tunesi, M.M.; Kalwar, N.; Abbas, M.W.; Karakus, S.; Soomro, R.A.; Kilislioglu, A.; Abro, M.I.; Hallam, K.R. Functionalised CuO Nanostructures for the Detection of Organophosphorus Pesticides: A Non-Enzymatic Inhibition Approach Coupled with Nano-Scale Electrode Engineering to Improve Electrode Sensitivity. Sens. Actuators B Chem. 2018, 260, 480–489. [Google Scholar] [CrossRef]
- Govindasamy, M.; Rajaji, U.; Chen, S.-M.; Kumaravel, S.; Chen, T.-W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. Detection of Pesticide Residues (Fenitrothion) in Fruit Samples Based on Niobium Carbide@Molybdenum Nanocomposite: An Electrocatalytic Approach. Anal. Chim. Acta 2018, 1030, 52–60. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S.; Vural, T.; Bozdogan, B.; Denkbas, E.B. Sensitive Electrochemical Detection of Fenitrothion Pesticide Based on Self-Assembled Peptide-Nanotubes Modified Disposable Pencil Graphite Electrode. J. Electroanal. Chem. 2018, 809, 88–95. [Google Scholar] [CrossRef]
- Singh, M.; Kashyap, H.; Singh, P.K.; Mahata, S.; Rai, V.K.; Rai, A. AuNPs/Neutral Red-Biofunctionalized Graphene Nanocomposite for Nonenzymatic Electrochemical Detection of Organophosphate via NO2 Reduction. Sens. Actuators B Chem. 2019, 290, 195–202. [Google Scholar] [CrossRef]
- Zahirifar, F.; Rahimnejad, M.; Abdulkareem, R.A.; Najafpour, G. Determination of Diazinon in Fruit Samples Using Electrochemical Sensor Based on Carbon Nanotubes Modified Carbon Paste Electrode. Biocatal. Agric. Biotechnol. 2019, 20, 101245. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Caetano, F.R.; Papi, M.A.P.; Watanabe, E.Y.; Marcolino-Júnior, L.H.; Bergamini, M.F. Chemical Wet Oxidation of Carbon Nanotubes for Electrochemical Determination of Methyl Parathion. J. Anal. Chem. 2020, 75, 119–126. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, J.; Sun, J.; Gan, T.; Liu, Y. A Disposable Molecularly Imprinted Electrochemical Sensor for the Ultra-Trace Detection of the Organophosphorus Insecticide Phosalone Employing Monodisperse Pt-Doped UiO-66 for Signal Amplification. Analyst 2020, 145, 3245–3256. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Shimizu, F.M.; Machado, S.A.S.; Oliveira, O.N. Selective and Sensitive Multiplexed Detection of Pesticides in Food Samples Using Wearable, Flexible Glove-Embedded Non-Enzymatic Sensors. Chem. Eng. J. 2021, 408, 127279. [Google Scholar] [CrossRef]
- Ulloa, A.M.; Glassmaker, N.; Oduncu, M.R.; Xu, P.; Wei, A.; Cakmak, M.; Stanciu, L. Roll-to-Roll Manufactured Sensors for Nitroaromatic Organophosphorus Pesticides Detection. ACS Appl. Mater. Interfaces 2021, 13, 35961–35971. [Google Scholar] [CrossRef]
- Köksoy, B.; Akyüz, D.; Şenocak, A.; Durmuş, M.; Demirbas, E. Sensitive, Simple and Fast Voltammetric Determination of Pesticides in Juice Samples by Novel BODIPY-Phthalocyanine-SWCNT Hybrid Platform. Food Chem. Toxicol. 2021, 147, 111886. [Google Scholar] [CrossRef]
- Porto, L.S.; Ferreira, L.F.; Pio dos Santos, W.T.; Pereira, A.C. Determination of Organophosphorus Compounds in Water and Food Samples Using a Non-Enzymatic Electrochemical Sensor Based on Silver Nanoparticles and Carbon Nanotubes Nanocomposite Coupled with Batch Injection Analysis. Talanta 2022, 246, 123477. [Google Scholar] [CrossRef]
- Qader, B.; Hussain, I.; Baron, M.; Jimenez-Perez, R.; Gonzalez-Rodriguez, J.; Gil-Ramírez, G. A Molecular Imprinted Polymer Sensor for Biomonitoring of Fenamiphos Pesticide Metabolite Fenamiphos Sulfoxide. Electroanalysis 2021, 33, 1129–1136. [Google Scholar] [CrossRef]
- Alizadeh, T. High Selective Parathion Voltammetric Sensor Development by Using an Acrylic Based Molecularly Imprinted Polymer-Carbon Paste Electrode. Electroanalysis 2009, 21, 1490–1498. [Google Scholar] [CrossRef]
- Marx, S.; Zaltsman, A.; Turyan, I.; Mandler, D. Parathion Sensor Based on Molecularly Imprinted Sol-Gel Films. Anal. Chem. 2004, 76, 120–126. [Google Scholar] [CrossRef]
- Wu, B.; Hou, L.; Du, M.; Zhang, T.; Wang, Z.; Xue, Z.; Lu, X. A Molecularly Imprinted Electrochemical Enzymeless Sensor Based on Functionalized Gold Nanoparticle Decorated Carbon Nanotubes for Methyl-Parathion Detection. RSC Adv. 2014, 4, 53701–53710. [Google Scholar] [CrossRef]
- Xue, X.; Wei, Q.; Wu, D.; Li, H.; Zhang, Y.; Feng, R.; Du, B. Determination of Methyl Parathion by a Molecularly Imprinted Sensor Based on Nitrogen Doped Graphene Sheets. Electrochim. Acta 2014, 116, 366–371. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Electrochemical Determination of Methyl Parathion Using a Molecularly Imprinted Polymer-Ionic Liquid-Graphene Composite Film Coated Electrode. Sens. Actuators B Chem. 2013, 176, 818–824. [Google Scholar] [CrossRef]
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly Imprinted Polymers for the Determination of Organophosphorus Pesticides in Complex Samples. Talanta 2018, 176, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Kilele, J.C.; Chokkareddy, R.; Redhi, G.G. Ultra-Sensitive Electrochemical Sensor for Fenitrothion Pesticide Residues in Fruit Samples Using IL@CoFe2O4NPs@MWCNTs Nanocomposite. Microchem. J. 2021, 164, 106012. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, P.; Tang, J.; Zeng, Z.; Tang, D. Ultrasensitive Split-Type Electrochemical Sensing Platform for Sensitive Determination of Organophosphorus Pesticides Based on MnO2 Nanoflower-Electron Mediator as a Signal Transduction System. Anal. Bioanal. Chem. 2020, 412, 6939–6945. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in Electrochemical Sensors for Environmental Monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Ferrag, C.; Kerman, K. Grand Challenges in Nanomaterial-Based Electrochemical Sensors. Front. Sens. 2020, 1, 583822. [Google Scholar] [CrossRef]
- Pathiraja, G.; Herr, D.J.C.; Rathnayake, H. Nanoscopic Insight into Sol-Gel Chemical Kinetics of Oriented Attachment Crystal Growth in Anisotropic Copper Hydroxide Nanowires. Cryst. Growth Des. 2022, 22, 2889–2902. [Google Scholar] [CrossRef]
- Wöhrle, D.; Suvorova, O.; Gerdes, R.; Bartels, O.; Lapok, L.; Baziakina, N.; Makarov, S.; Slodek, A. Efficient Oxidations and Photooxidations with Molecular Oxygen Using Metal Phthalocyanines as Catalysts and Photocatalysts. J. Porphyr. Phthalocyanines 2004, 8, 1020–1041. [Google Scholar] [CrossRef]
- İpek, Y.; Şener, M.K.; Koca, A. Electrochemical Pesticide Sensor Based on Langmuir-Blodgett Film of Cobalt Phthalocyanine-Anthraquinone Hybrid. J. Porphyr. Phthalocyanines 2015, 19, 708–718. [Google Scholar] [CrossRef]
- Akyüz, D.; Keleş, T.; Biyiklioglu, Z.; Koca, A. Electrochemical Pesticide Sensors Based on Electropolymerized Metallophthalocyanines. J. Electroanal. Chem. 2017, 804, 53–63. [Google Scholar] [CrossRef]
- Keleş, T.; Akyüz, D.; Biyiklioglu, Z.; Koca, A. Electropolymerization of Metallophthalocyanines Carrying Redox Active Metal Centers and Their Electrochemical Pesticide Sensing Application. Electroanalysis 2017, 29, 2125–2137. [Google Scholar] [CrossRef]
- Akyüz, D.; Koca, A. An Electrochemical Sensor for the Detection of Pesticides Based on the Hybrid of Manganese Phthalocyanine and Polyaniline. Sens. Actuators B Chem. 2019, 283, 848–856. [Google Scholar] [CrossRef]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef]
- Sreenivasan, S.V. Nanoimprint Lithography Steppers for Volume Fabrication of Leading-Edge Semiconductor Integrated Circuits. Microsyst. Nanoeng. 2017, 3, 17075. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.; Hsieh, H.; Lee, Y.-C. Contact Photolithography at Sub-Micrometer Scale Using a Soft Photomask. Micromachines 2019, 10, 547. [Google Scholar] [CrossRef] [Green Version]
- Altissimo, M. E-Beam Lithography for Micro-/Nanofabrication. Biomicrofluidics 2010, 4, 026503. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Takahashi, T.; Kim, S.; Heaney, Y.C.; Warner, J.; Chen, S.; Heller, M.J. A Programmable DNA Double-Write Material: Synergy of Photolithography and Self-Assembly Nanofabrication. ACS Appl. Mater. Interfaces 2017, 9, 22–28. [Google Scholar] [CrossRef]
- Jeerapan, I.; Poorahong, S. Review—Flexible and Stretchable Electrochemical Sensing Systems: Materials, Energy Sources, and Integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Segev-Bar, M.; Haick, H. Flexible Sensors Based on Nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef]
- Stefano, J.S.; Orzari, L.O.; Silva-Neto, H.A.; de Ataíde, V.N.; Mendes, L.F.; Coltro, W.K.T.; Longo Cesar Paixão, T.R.; Janegitz, B.C. Different Approaches for Fabrication of Low-Cost Electrochemical Sensors. Curr. Opin. Electrochem. 2022, 32, 100893. [Google Scholar] [CrossRef]
- Liang, G.; He, Z.; Zhen, J.; Tian, H.; Ai, L.; Pan, L.; Gong, W. Development of the Screen-Printed Electrodes: A Mini Review on the Application for Pesticide Detection. Environ. Technol. Innov. 2022, 28, 102922. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.d.l.E. Electrochemical (Bio)Sensors for Pesticides Detection Using Screen-Printed Electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Avuthu, S.G.R.; Wabeke, J.T.; Narakathu, B.B.; Maddipatla, D.; Arachchilage, J.S.; Obare, S.O.; Atashbar, M.Z. A Screen Printed Phenanthroline-Based Flexible Electrochemical Sensor for Selective Detection of Toxic Heavy Metal Ions. IEEE Sens. J. 2016, 16, 8678–8684. [Google Scholar] [CrossRef]
- Saeed, T.S.; Maddipatla, D.; Narakathu, B.B.; Albalawi, S.S.; Obare, S.O.; Atashbar, M.Z. Synthesis of a Novel Hexaazatriphenylene Derivative for the Selective Detection of Copper Ions in Aqueous Solution. RSC Adv. 2019, 9, 39824–39833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trojanowicz, M. Impact of Nanotechnology on Design of Advanced Screen-Printed Electrodes for Different Analytical Applications. TrAC Trends Anal. Chem. 2016, 84, 22–47. [Google Scholar] [CrossRef]
- Guan, W.-J.; Li, Y.; Chen, Y.-Q.; Zhang, X.-B.; Hu, G.-Q. Glucose Biosensor Based on Multi-Wall Carbon Nanotubes and Screen Printed Carbon Electrodes. Biosens. Bioelectron. 2005, 21, 508–512. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.; Santschi, C.; Martin, O. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [Green Version]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent Developments in Nanotechnology-Based Printing Electrode Systems for Electrochemical Sensors. Talanta 2021, 225, 121951. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive Silver Nanowires-Fenced Carbon Cloth Fibers-Supported Layered Double Hydroxide Nanosheets as a Flexible and Binder-Free Electrode for High-Performance Asymmetric Supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Bandodkar, A.J.; Parkhomovsky, S.; Wang, J. Stamp Transfer Electrodes for Electrochemical Sensing on Non-Planar and Oversized Surfaces. Analyst 2012, 137, 1570. [Google Scholar] [CrossRef]
- Ferreira, P.C.; Ataíde, V.N.; Silva Chagas, C.L.; Angnes, L.; Tomazelli Coltro, W.K.; Longo Cesar Paixão, T.R.; Reis de Araujo, W. Wearable Electrochemical Sensors for Forensic and Clinical Applications. TrAC Trends Anal. Chem. 2019, 119, 115622. [Google Scholar] [CrossRef]
- Muñoz, J.; Pumera, M. Accounts in 3D-Printed Electrochemical Sensors: Towards Monitoring of Environmental Pollutants. ChemElectroChem 2020, 7, 3404–3413. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Laschi, S.; Palchetti, I. Sustainable Printed Electrochemical Platforms for Greener Analytics. Front. Chem. 2020, 8, 644. [Google Scholar] [CrossRef]
- Ramiah Rajasekaran, P.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed Electrochemical Sensor-Integrated Transwell Systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef]
- Jyoti; Redondo, E.; Alduhaish, O.; Pumera, M. 3D-printed Electrochemical Sensor for Organophosphate Nerve Agents. Electroanalysis 2023, 35, e202200047. [Google Scholar] [CrossRef]
- Chekol, F.; Mehretie, S.; Hailu, F.A.; Tolcha, T.; Megersa, N.; Admassie, S. Roll-to-Roll Printed PEDOT/PSS/GO Plastic Film for Electrochemical Determination of Carbofuran. Electroanalysis 2019, 31, 1104–1111. [Google Scholar] [CrossRef]
- Cagnani, G.R.; Ibáñez-Redín, G.; Tirich, B.; Gonçalves, D.; Balogh, D.T.; Oliveira, O.N. Fully-Printed Electrochemical Sensors Made with Flexible Screen-Printed Electrodes Modified by Roll-to-Roll Slot-Die Coating. Biosens. Bioelectron. 2020, 165, 112428. [Google Scholar] [CrossRef]
- Liedert, C.; Rannaste, L.; Kokkonen, A.; Huttunen, O.-H.; Liedert, R.; Hiltunen, J.; Hakalahti, L. Roll-to-Roll Manufacturing of Integrated Immunodetection Sensors. ACS Sens. 2020, 5, 2010–2017. [Google Scholar] [CrossRef]
- Yuan, F.; Xia, Y.; Lu, Q.; Xu, Q.; Shu, Y.; Hu, X. Recent Advances in Inorganic Functional Nanomaterials Based Flexible Electrochemical Sensors. Talanta 2022, 244, 123419. [Google Scholar] [CrossRef]
- Park, T.H.; Shuler, M.L. Integration of Cell Culture and Microfabrication Technology. Biotechnol. Prog. 2003, 19, 243–253. [Google Scholar] [CrossRef]
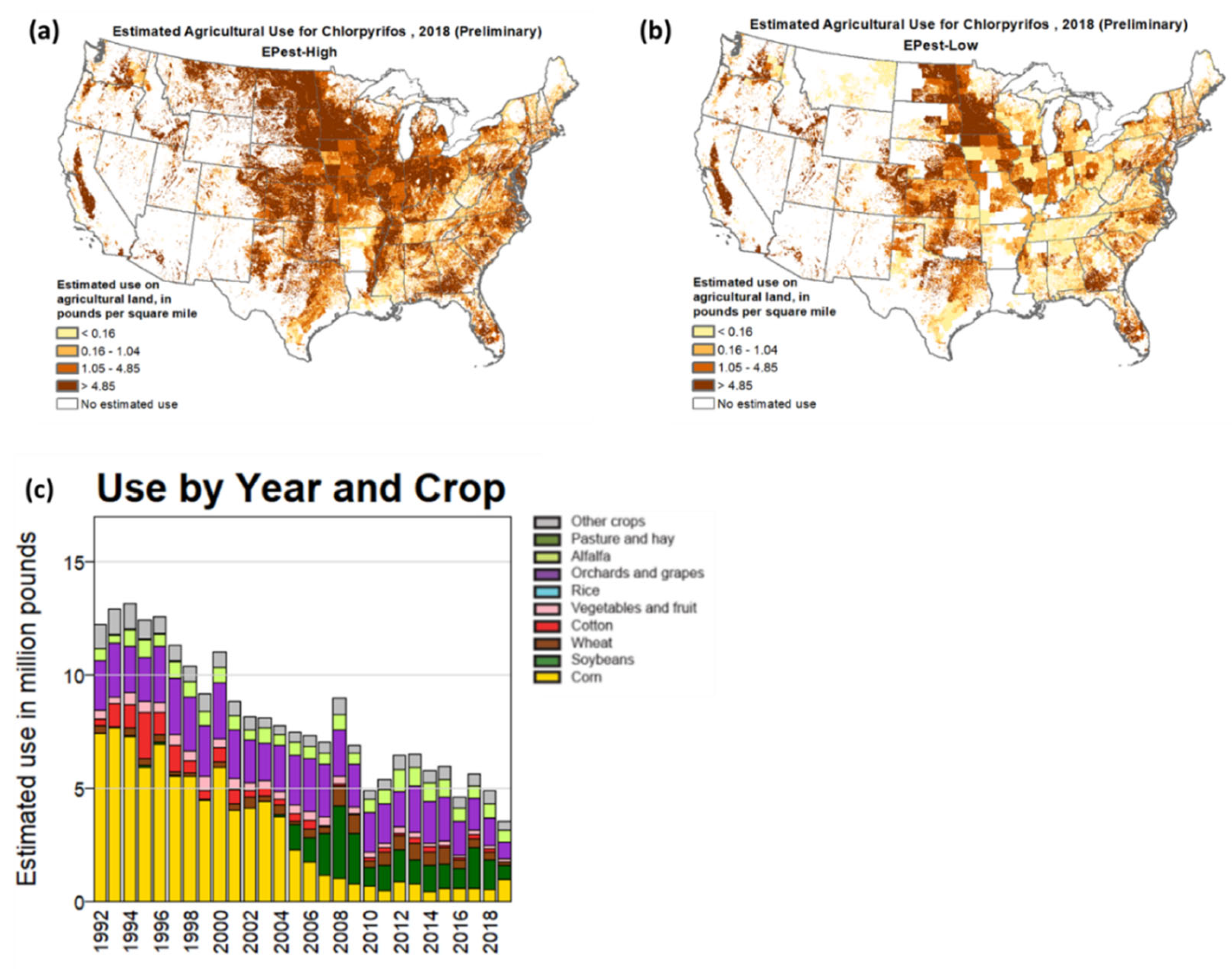
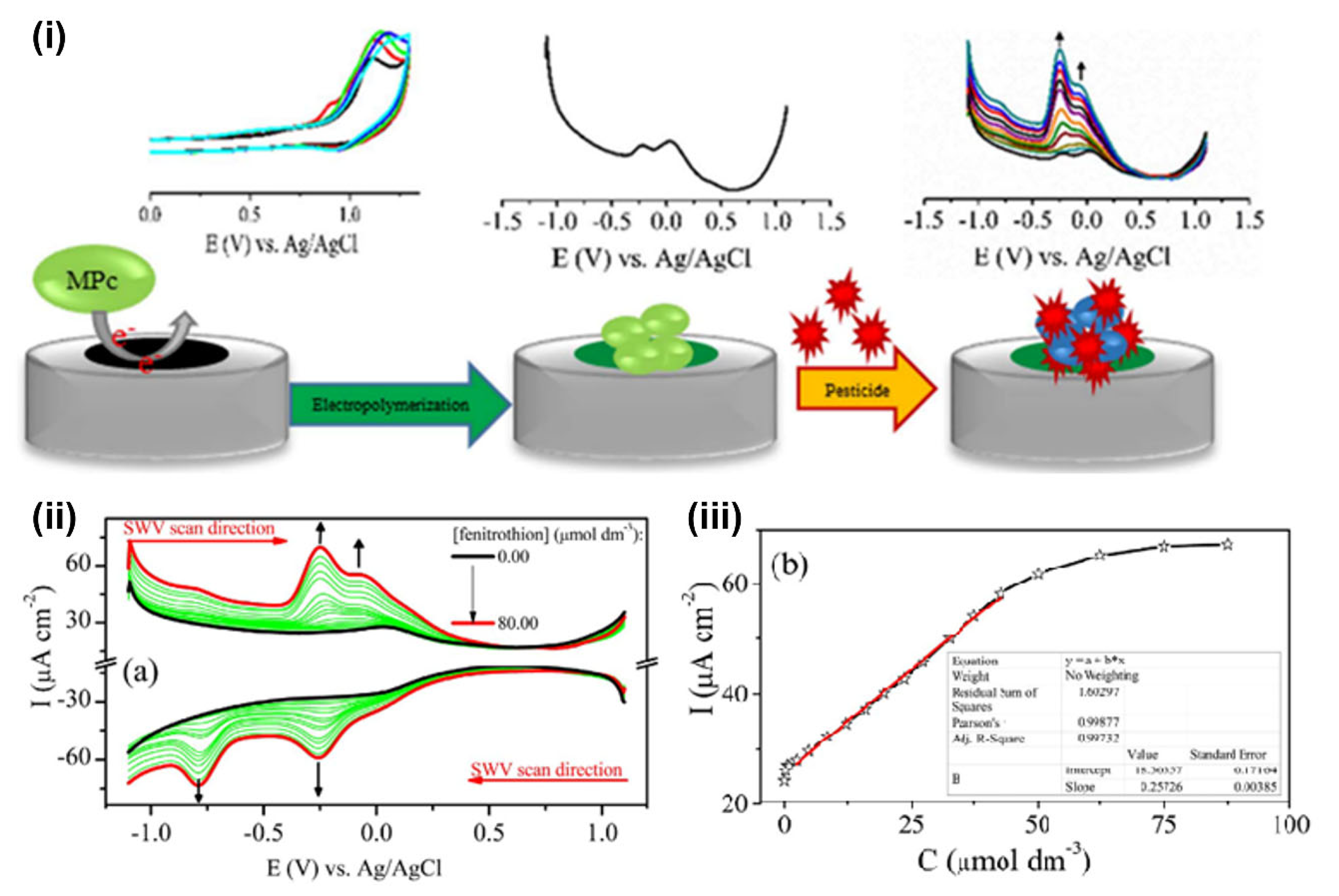
| OP Compound | Chemical Structure | Usage Range in 2012 (Millions of Pounds) |
|---|---|---|
| chlorpyrifos |  | 5–8 |
| acephate | 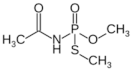 | 5–8 |
| malathion | 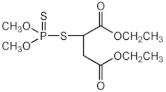 | 1–4 |
| naled | 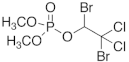 | 1–2 |
| phorate |  | 1–2 |
| dicrotophos |  | 1–2 |
| dimethoate |  | <1 |
| phosmet |  | <1 |
| ethoprophos | 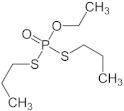 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathiraja, G.; Bonner, C.D.J.; Obare, S.O. Recent Advances of Enzyme-Free Electrochemical Sensors for Flexible Electronics in the Detection of Organophosphorus Compounds: A Review. Sensors 2023, 23, 1226. https://doi.org/10.3390/s23031226
Pathiraja G, Bonner CDJ, Obare SO. Recent Advances of Enzyme-Free Electrochemical Sensors for Flexible Electronics in the Detection of Organophosphorus Compounds: A Review. Sensors. 2023; 23(3):1226. https://doi.org/10.3390/s23031226
Chicago/Turabian StylePathiraja, Gayani, Chartanay D. J. Bonner, and Sherine O. Obare. 2023. "Recent Advances of Enzyme-Free Electrochemical Sensors for Flexible Electronics in the Detection of Organophosphorus Compounds: A Review" Sensors 23, no. 3: 1226. https://doi.org/10.3390/s23031226






