Water Stress Index Detection Using a Low-Cost Infrared Sensor and Excess Green Image Processing
Abstract
1. Introduction
2. Material and Methods
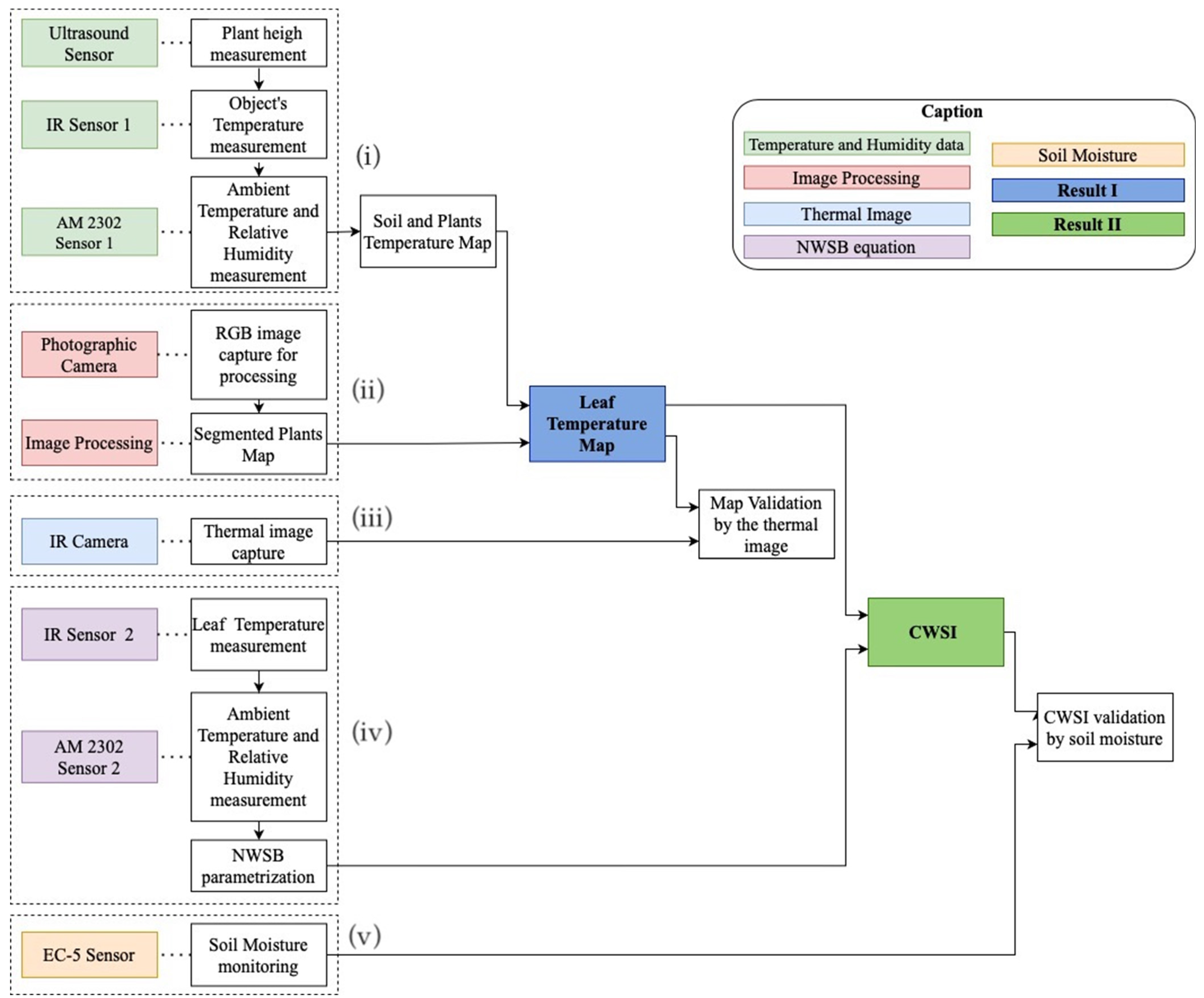
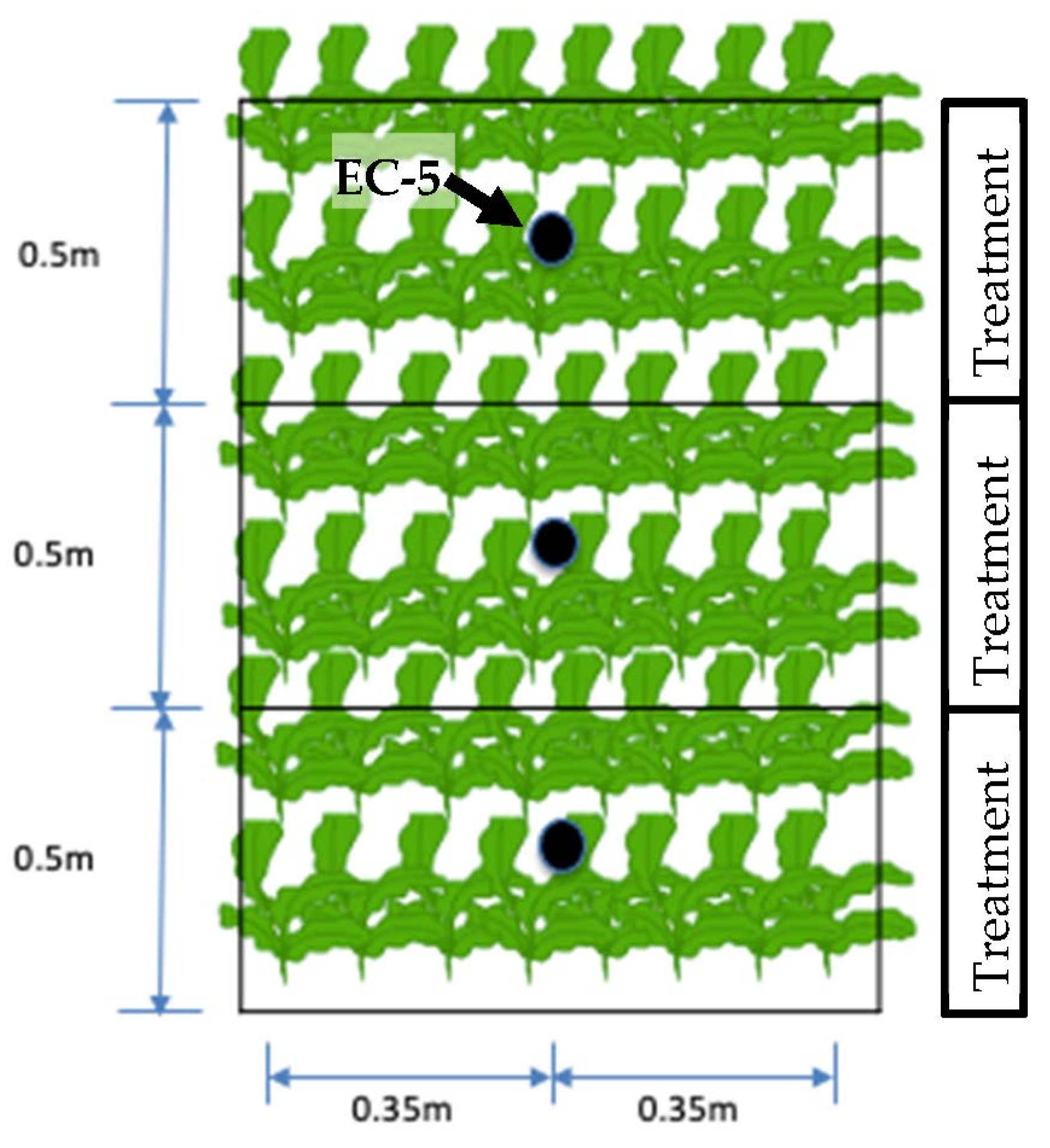
2.1. Instrumentation
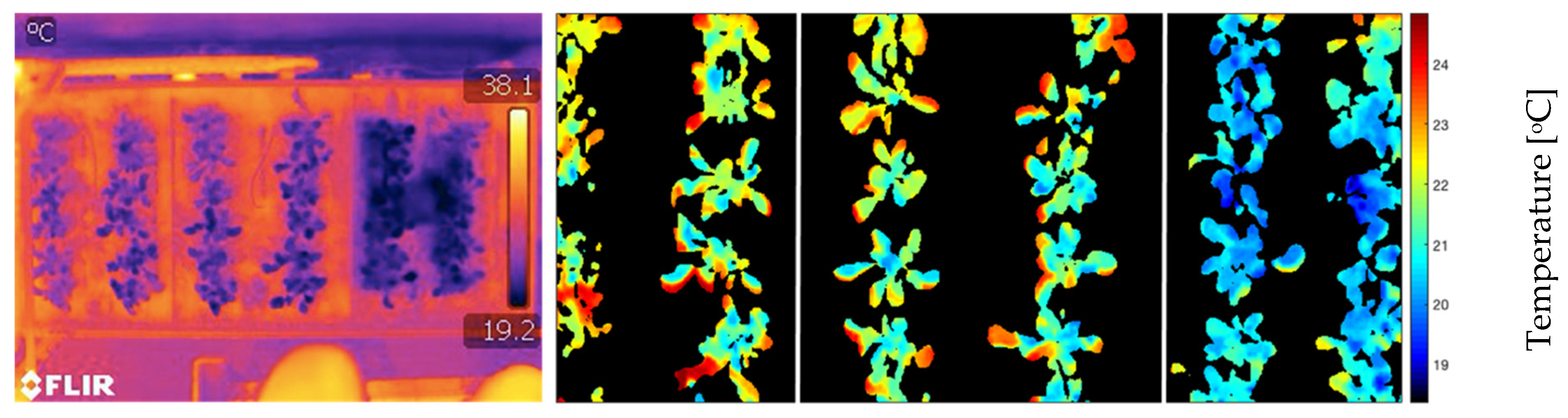
2.2. Soil/Plant Thermal Map
2.3. Image Processing
2.4. Leaf Temperature Map Obtaining
2.5. Leaf Temperature Map Validation
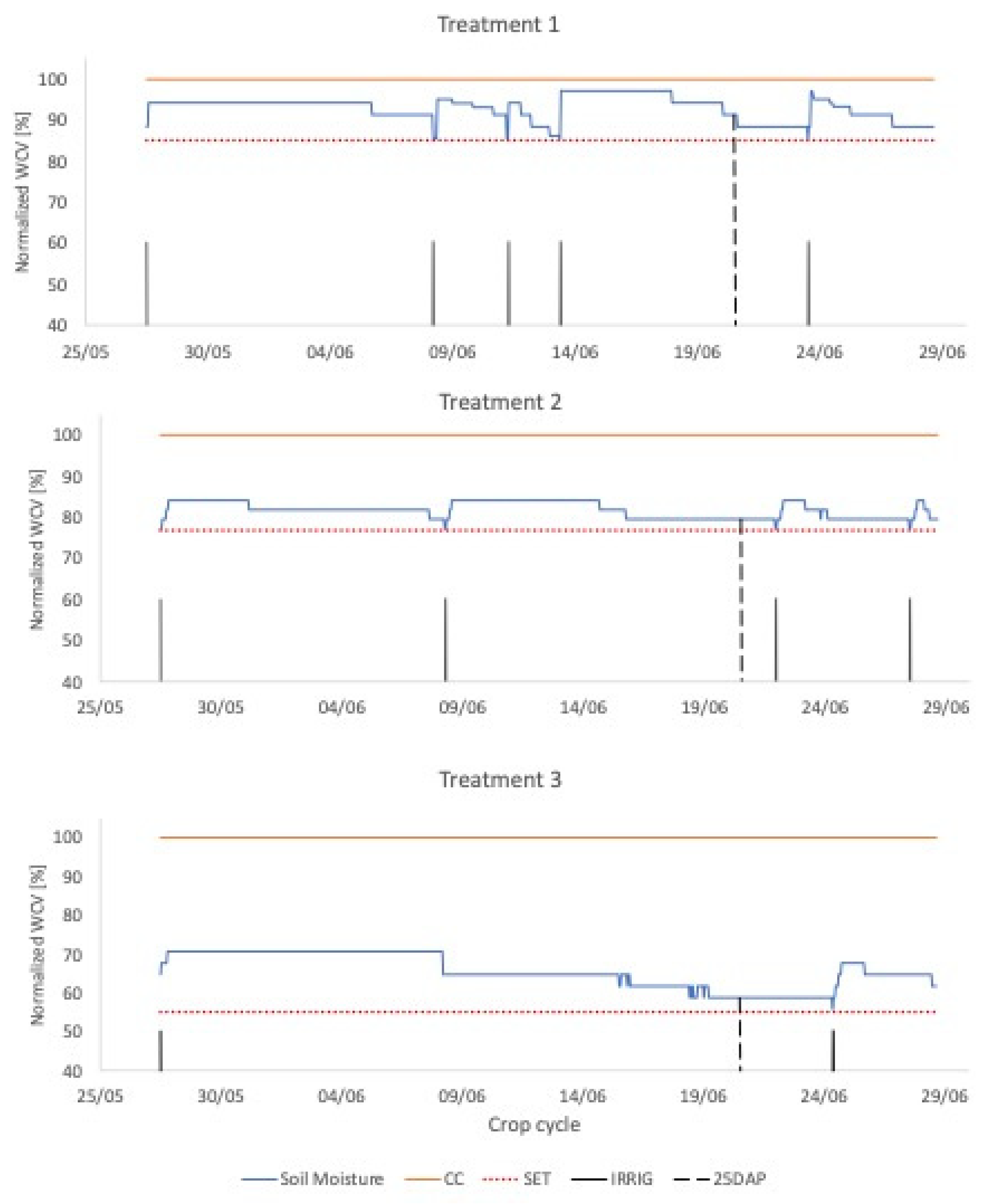
2.6. Parameterization of the Non-Water-Stressed Baseline
2.7. Crop Water Stress Index Calculation
2.8. Leaf Temperature Map Validation
2.9. Non-Water-Stressed Baseline Equation
3. Results and Discussion
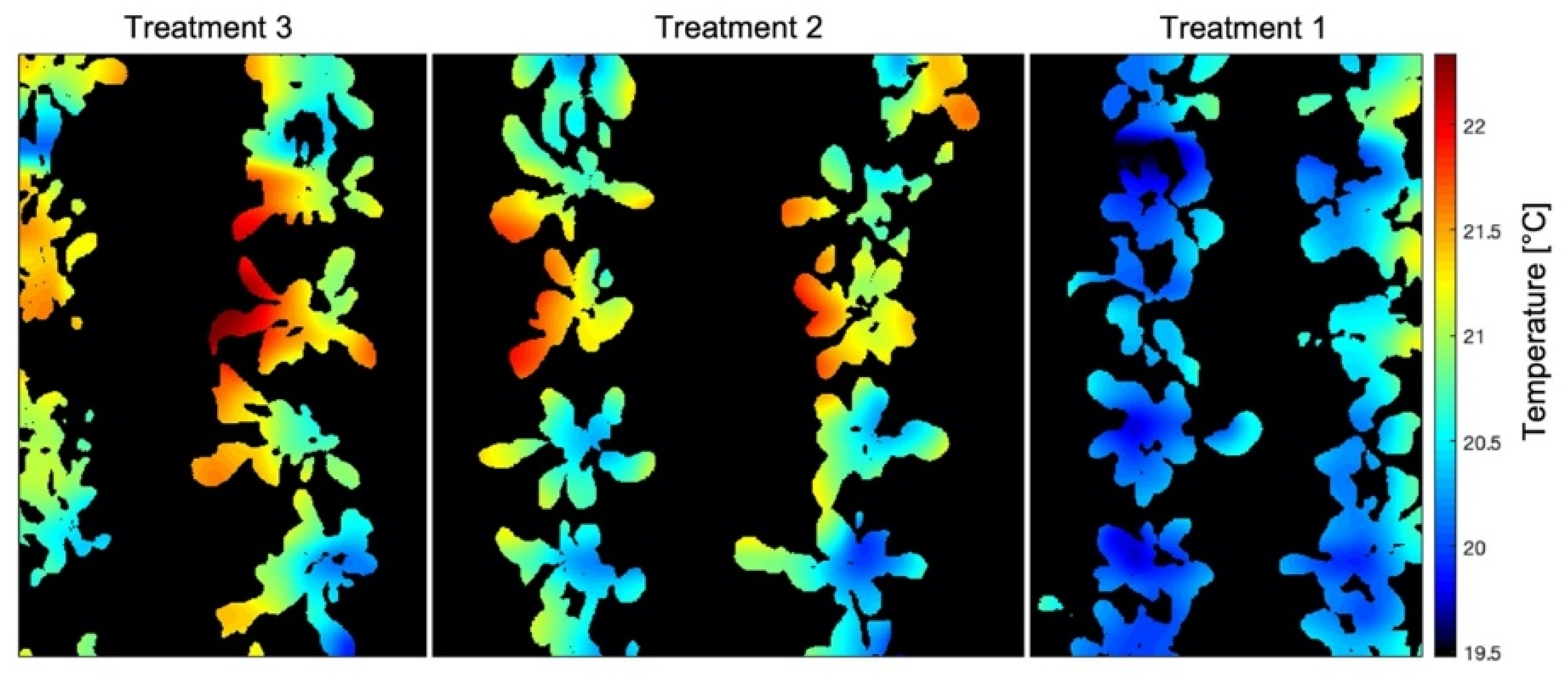
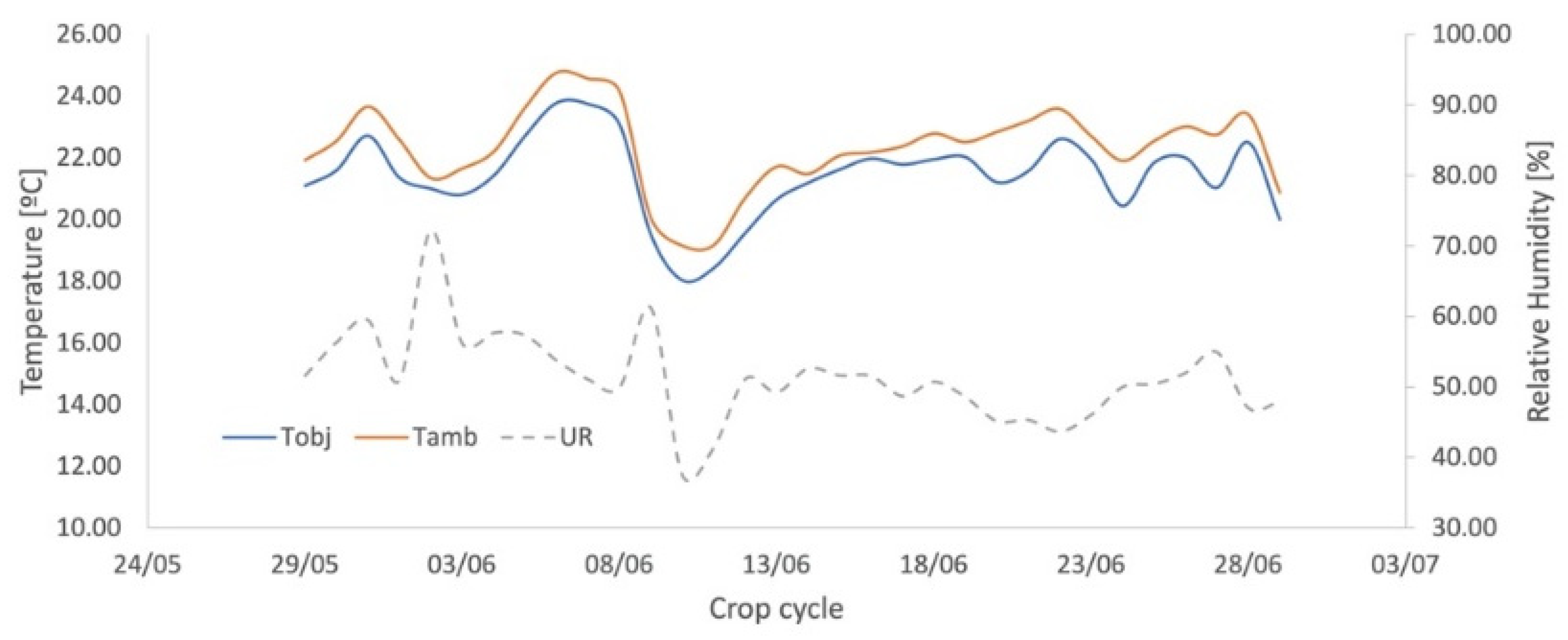
3.1. Leaf Temperature Map
3.2. Image Processing
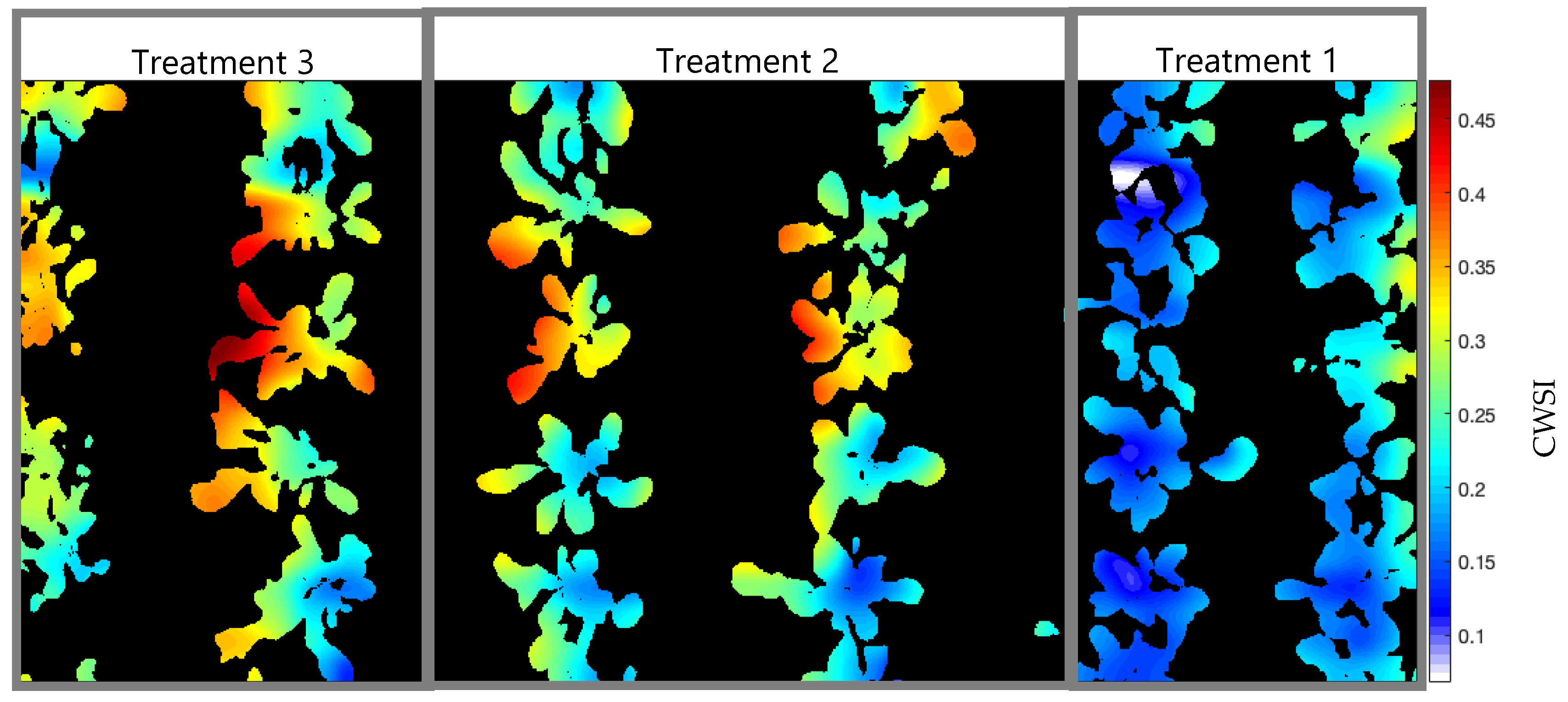
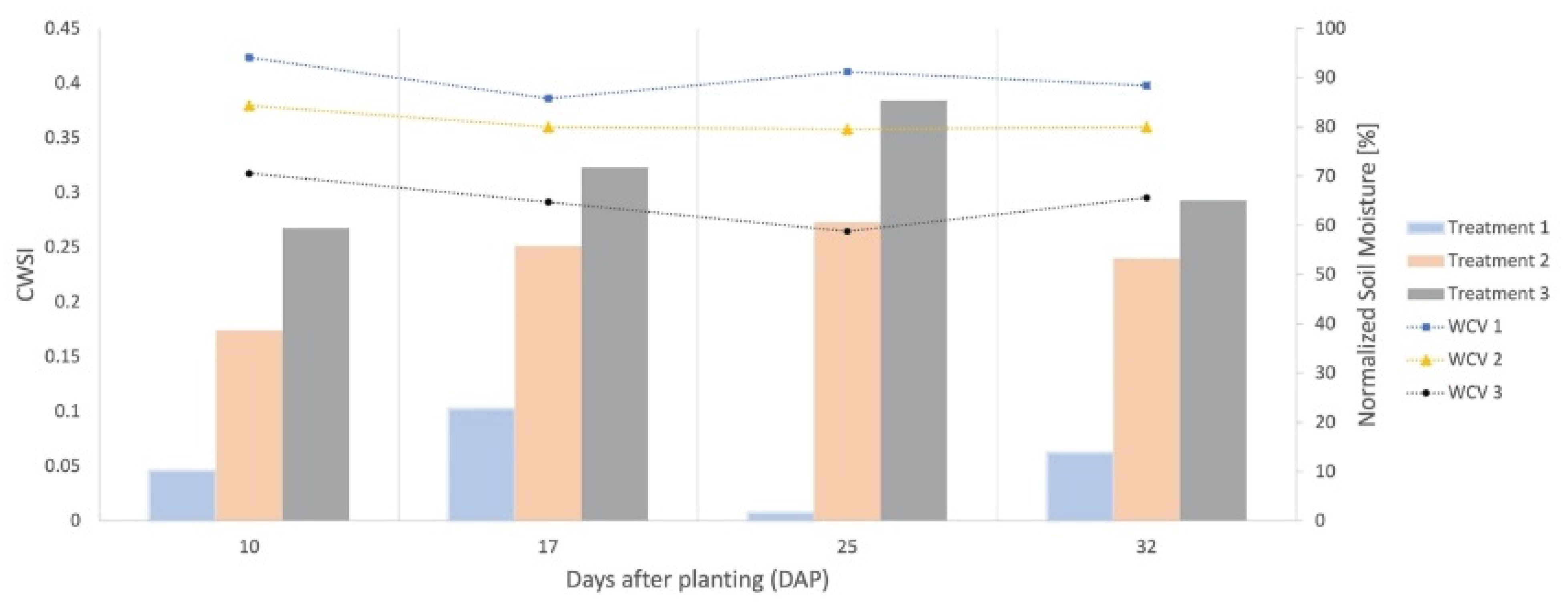
3.3. Crop Water Stress Index
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rassini, J.B. Manejo Da Água de Irrigação. In Irrigação e Fertilização em Fruteiras e Hortaliças; Embrapa Informação Tecnológica: Brasília, Brazil, 2011; pp. 156–232. [Google Scholar]
- Albiero, D. Agricultural Robotics: A Promising Challenge. Curr. Agric. Res. J. 2019, 7, 1–3. [Google Scholar] [CrossRef]
- Albiero, D. Robots and AI: Illusions and Social Dilemmas; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Abioye, E.A.; Abidin, M.S.Z.; Mahmud, M.S.A.; Buyamin, S.; Ishak, M.H.I.; Rahman, M.K.I.A.; Otuoze, A.O.; Onotu, P.; Ramli, M.S.A. A Review on Monitoring and Advanced Control Strategies for Precision Irrigation. Comput. Electron. Agric. 2020, 173, 105441. [Google Scholar] [CrossRef]
- Xavier, R.S.; Galvão, C.B.; Rodrigues, R.L.; Garcia, A.P.; Albiero, D. Mechanical Properties of Lettuce (Lactuca Sativa L.) for Horticultural Machinery Design. Sci. Agric. 2022, 79, 2022. [Google Scholar] [CrossRef]
- Kacira, M.; Ling, P.P.; Short, T.H. Establishing crop water stress index (cwsi) threshold values for early, non–contact detection of plant water stress. Trans. ASAE 2002, 45, 775–780. [Google Scholar] [CrossRef]
- Idso, S.B. Non-Water-Stressed Baselines: A Key to Measuring and Interpreting Plant Water Stress. Agric. Meteorol. 1982, 27, 59–70. [Google Scholar] [CrossRef]
- Waller, P.; Yitayew, M. Irrigation and Drainage Engineering; Springer: New York, NY, USA, 2016. [Google Scholar]
- García-Tejero, I.F.; Rubio, A.E.; Viñuela, I.; Hernández, A.; Gutiérrez-Gordillo, S.; Rodríguez-Pleguezuelo, C.R.; Durán-Zuazo, V.H. Thermal Imaging at Plant Level to Assess the Crop-Water Status in Almond Trees (Cv. Guara) under Deficit Irrigation Strategies. Agric. Water Manag. 2018, 208, 176–186. [Google Scholar] [CrossRef]
- Alsalam, B.H.Y.; Morton, K.; Campbell, D.; Gonzalez, F. Autonomous UAV with Vision Based On-Board Decision Making for Remote Sensing and Precision Agriculture. In Proceedings of the 2017 IEEE Aerospace Conference, Big Sky, MT, USA, 4–11 March 2017. [Google Scholar] [CrossRef]
- Çolak, Y.B.; Yazar, A.; Çolak, İ.; Akça, H.; Duraktekin, G. Evaluation of Crop Water Stress Index (CWSI) for Eggplant under Varying Irrigation Regimes Using Surface and Subsurface Drip Systems. Agric. Agric. Sci. Procedia 2015, 4, 372–382. [Google Scholar] [CrossRef]
- Camoglu, G.; Demirel, K.; Genc, L. Use of Infrared Thermography and Hyperspectral Data to Detect Effects of Water Stress on Pepper. Quant. Infrared Thermogr. J. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating Water Stress in Irrigated Olives: Correlation of Soil Water Status, Tree Water Status, and Thermal Imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Da Silva, C.J.; da Silva, C.A.; de Freitas, C.A.; Golynski, A.; da Silva, L.F.M.; Frizzone, J.A. Tomato Water Stress Index as a Function of Irrigation Depths. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 95–100. [Google Scholar] [CrossRef]
- Erdem, Y.; Erdem, T.; Orta, A.H.; Okursoy, H. Irrigation Scheduling for Watermelon with Crop Water Stress Index (Cwsi). J. Cent. Eur. Agric. 2006, 6, 449–460. [Google Scholar] [CrossRef]
- Fattahi, K.; Babazadeh, H.; Najafi, P.; Sedghi, H. Scheduling Maize Irrigation Based on Crop Water Stress Index (CWSI). Appl. Ecol. Environ. Res. 2018, 16, 7535–7549. [Google Scholar] [CrossRef]
- Ballester, C.; Jiménez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Usefulness of Thermography for Plant Water Stress Detection in Citrus and Persimmon Trees. Agric. For. Meteorol. 2013, 168, 120–129. [Google Scholar] [CrossRef]
- Pantano, A.P.; Camparotto, L.B.; Meireles, E.J.L. Monitoramento Agrometeorológico para Regiões Cafeeiras do Estado de São Paulo: Janeiro/2010—Dezembro/2019 (Boletim técnico 224—IAC); IAC: Campinas, Brazil, 2021. [Google Scholar]
- De Freitas, C.; Kalliany, K.; Neto, F.B.; Grangeiro, C.; Silva, J.S. Desempenho Agronômico de Rúcula sob Diferentes Espaçamentos. Rev. Ciência Agronômica 2009, 40, 449–454. [Google Scholar]
- Adeyemi, O.; Grove, I.; Peets, S.; Domun, Y.; Norton, T. Dynamic Modelling of the Baseline Temperatures for Computation of the Crop Water Stress Index (CWSI) of a Greenhouse Cultivated Lettuce Crop. Comput. Electron. Agric. 2018, 153, 102–114. [Google Scholar] [CrossRef]
- Shirmohammadi, A.Z.; Kamgra, H.A.A.; Sepaskhah, A.R. Use of Crop Water Stress Index (CWSI) for Evaluation of Water Status and Irrigation Scheduling of Saffron. Iran. J. Hortic. Sci. Technol. 2006, 7, 23–32. [Google Scholar]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 4th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123869937. [Google Scholar]
- Jones, H.G.; Hutchinson, P.A.; May, T.; Jamali, H.; Deery, D.M. A Practical Method Using a Network of Fixed Infrared Sensors for Estimating Crop Canopy Conductance and Evaporation Rate. Biosyst. Eng. 2018, 165, 59–69. [Google Scholar] [CrossRef]
- Garcia, A.P.; Umezu, C.K.; Polania, E.C.M.; Dias Neto, A.F.; Rossetto, R.; Albiero, D. Sensor-Based Technologies in Sugarcane Agriculture. Sugar Tech. 2022, 24, 679–698. [Google Scholar] [CrossRef]
- Muangprathub, J.; Boonnam, N.; Kajornkasirat, S.; Lekbangpong, N.; Wanichsombat, A.; Nillaor, P. IoT and Agriculture Data Analysis for Smart Farm. Comput. Electron. Agric. 2019, 156, 467–474. [Google Scholar] [CrossRef]
- Mesas-Carrascosa, F.J.; Verdú Santano, D.; Meroño, J.E.; Sánchez de la Orden, M.; García-Ferrer, A. Open Source Hardware to Monitor Environmental Parameters in Precision Agriculture. Biosyst. Eng. 2015, 137, 73–83. [Google Scholar] [CrossRef]
- Flores, K.O.; Butaslac, I.M.; Gonzales, J.E.M.; Dumlao, S.M.G.; Reyes, R.S.J. Precision Agriculture Monitoring System Using Wireless Sensor Network and Raspberry Pi Local Server. In Proceedings of the IEEE Region 10 Annual International Conference, Proceedings/TENCON, Singapore, 22–25 November 2016; pp. 3018–3021. [Google Scholar] [CrossRef]
- Bellvert, J.; Zarco-Tejada, P.J.; Girona, J.; Fereres, E. Mapping Crop Water Stress Index in a ‘Pinot-Noir’ Vineyard: Comparing Ground Measurements with Thermal Remote Sensing Imagery from an Unmanned Aerial Vehicle. Precis. Agric. 2013, 15, 361–376. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color Indices for Weed Identification under Various Soil, Residue, and Lighting Conditions. Trans. Am. Soc. Agric. Eng. 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Perissini, I.C. Análise Experimental de Algoritmos de Constância de Cor e Segmentação Para Detecção de Mudas de Plantas. Master’s Thesis, São Paulo University, São Carlos, Brazil, 2 March 2018. [Google Scholar]
- Bailly, S.; Giordano, S.; Landrieu, L.; Chehata, N. Crop-Rotation Structured Classification Using Multi-Source Sentinel Images and LPIS for Crop Type Mapping. In Proceedings of the 2018 International Geoscience and Remote Sensing Symposium (IGARSS), Valencia, Spain, 22–27 July 2018; pp. 1950–1953. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Wheaton, A.; Cooley, N.; Moran, B. Automated Canopy Temperature Estimation via Infrared Thermography: A First Step towards Automated Plant Water Stress Monitoring. Comput. Electron. Agric. 2010, 73, 74–83. [Google Scholar] [CrossRef]
- Testi, L.; Goldhamer, D.A.; Iniesta, F.; Salinas, M. Crop Water Stress Index Is a Sensitive Water Stress Indicator in Pistachio Trees. Irrig. Sci. 2008, 26, 395–405. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the Stress-Degree-Day Parameter for Environmental Variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Albiero, D.; Maciel, A.J.D.S.; Milan, M.; de Almeida Monteiro, L.; Mion, R.L. Avaliação Da Distribuição de Sementes Por Uma Semeadora de Anel Interno Rotativo Utilizando Média Móvel Exponencial. Rev. Ciência Agronômica 2012, 43, 86–95. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vogt, H.H.; de Melo, R.R.; Daher, S.; Schmuelling, B.; Antunes, F.L.M.; dos Santos, P.A.; Albiero, D. Electric Tractor System for Family Farming: Increased Autonomy and Economic Feasibility for an Energy Transition. J. Energy Storage 2021, 40, 102744. [Google Scholar] [CrossRef]
- Kumar, N.; Poddar, A.; Shankar, V.; Ojha, C.S.P.; Adeloye, A.J. Crop Water Stress Index for Scheduling Irrigation of Indian Mustard (Brassica Juncea) Based on Water Use Efficiency Considerations. J. Agron. Crop. Sci 2020, 206, 148–159. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping Canopy Conductance and CWSI in Olive Orchards Using High Resolution Thermal Remote Sensing Imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Agam, N.; Cohen, Y.; Berni, J.A.J.; Alchanatis, V.; Kool, D.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. An Insight to the Performance of Crop Water Stress Index for Olive Trees. Agric. Water Manag. 2013, 118, 79–86. [Google Scholar] [CrossRef]
- Jackson, R.D.; Kustas, W.P.; Choudhury, B.J. A Reexamination of the Crop Water Stress Index. Irrig. Sci. 1988, 9, 309–317. [Google Scholar] [CrossRef]
- Gilman, K.L. Pistachio Yields and Nut Quality Determination and the Relationship Between Soil Characteristics. Master’s Thesis, California State University, Long Beach, CA, USA, 2021. [Google Scholar]
- Sudianto, A.; Jamaludin, Z.; Abdul Rahman, A.A.; Novianto, S.; Muharrom, F. Smart Temperature Measurement System for Milling Process Application Based on MLX90614 Infrared Thermometer Sensor with Arduino. J. Adv. Res. Appl. Mech. 2020, 72, 10–24. [Google Scholar] [CrossRef]
- Martínez, J.; Egea, G.; Agüera, J.; Pérez-Ruiz, M. A Cost-Effective Canopy Temperature Measurement System for Precision Agriculture: A Case Study on Sugar Beet. Precis. Agric. 2017, 18, 95–110. [Google Scholar] [CrossRef]
- Fisher, D.K.; Kebede, H. A Low-Cost Microcontroller-Based System to Monitor Crop Temperature and Water Status. Comput. Electron. Agric. 2010, 74, 168–173. [Google Scholar] [CrossRef]
- Gintsioudis, I.; Skoufogianni, E.; Bartzialis, D.; Giannoulis, K.; Danalatos, N. Diurnal Variations in Leaf—Air Temperature and Vapor Pressure Deficit of Sunlit and Shaded Kenaf Leaves. CEUR Workshop Proc. 2020, 2761, 574–579. [Google Scholar]
- Osroosh, Y.; Hhot, L.R.; Peters, R.T. Econimical thermal-RGB imaging system for monitoring agricultural crops. Comput. Electron. Agric. 2018, 147, 34–43. [Google Scholar] [CrossRef]
- Giménez-Gallego, J.; González-Teruel, J.D.; Soto-Valles, F.; Jiménez-Buendía, M.; Navarro-Hellín, H. Intelligent thermal image-based sensor for affordable measurement of crop canopy temperature. Agric. Water Manag. 2021, 188, 106319. [Google Scholar] [CrossRef]
- Zhou, Z.; Majeed, Y.; Naranjo, G.D.; Gambacorta, E.M.T. Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications. Comput. Electron. Agric. 2021, 182, 106019. [Google Scholar] [CrossRef]
- Parihar, G.; Saha, S.; Giri, L.I. Application of infrared thermography for irrigation scheduling of horticulture plants. Smart Agric. Technol. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, J.; Lv, Y.; Liu, X.; Wang, H.; Liu, S. Improving the performance in crop water deficit diagnosis with canopy temperature spatial distribution information measured by thermal imaging. Agric. Water Manag. 2021, 246, 106699. [Google Scholar] [CrossRef]
- Katimbo, A.; Rudnick, D.R.; DeJonge, K.C.; Lo, T.H.; Qiao, X.; Franz, T.E.; Nakabuye, H.N.; Duanm, J. Crop water stress index computation approaches and their sensitivity to soil water dynamics. Agric. Water Manag. 2022, 266, 107575. [Google Scholar] [CrossRef]
- Barbosa Da Silva, B.; Ramana Rao, T.V. The CWSI Variations of a Cotton Crop in a Semi-Arid Region of Northeast Brazil. J. Arid Environ. 2005, 62, 649–659. [Google Scholar] [CrossRef]
- DeJonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of canopy temperature-based water stress indices for maize. Agric. Water Manag. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Hornero, A.; Mottus, M.; Penuelas, J.; González-Dugo, V.; Jiménez, J.C.; Suárez, L.; Alonso, L.; Zarco-Tejada, P.J. Early Diagnosis of Vegetation Health from High-Resolution Hyperspectral and Thermal Imagery: Lessons Learned from Empirical Relationships and Radiative Transfer Modelling. Curr. For. Rep. 2019, 5, 169–183. [Google Scholar] [CrossRef]
- Quebrajo, L.; Perez-Ruiz, M.; Pérez-Urrestarazu, L.; Martínez, G.; Egea, G. Linking Thermal Imaging and Soil Remote Sensing to Enhance Irrigation Management of Sugar Beet. Biosyst. Eng. 2018, 165, 77–87. [Google Scholar] [CrossRef]
- Çolak, Y.B.; Yazar, A.; Alghory, A.; Tekin, S. Evaluation of crop water stress index and leaf water potential for differentially irrigated quinoa with surface and subsurface drip systems. Irrig. Sci. 2021, 39, 81–100. [Google Scholar] [CrossRef]
- Cohen, V.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of leaf water potential by thermal imagery and spatial analysis. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef]
- Mastrorilli, M.; Katerji, N.; Losavio, N.; Rana, G. Comparison of water stress indicators for soybean. Acta Hortic. 1993, 335, 359–364. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Moosavi, S.M.; Bahador, F. Quantifying water stress of safflower (Carthamus tinctorius L.) cultivars by crop water stress index under different irrigation regimes. Heliyon 2022, 8, e09010. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy Temperature as a Crop Water Stress Indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Ciezkowski, W.; Szporak-Wasilewska, S.; Kleniewska, M.L.; Jóźwiak, J.; Gnatowski, T.; Dabrowski, P.; Góraj, M.; SzatyLowicz, J.; Ignar, S.; Chormański, J.L. Remotely Sensed Land Surface Temperature-Based Water Stress Index for Wetland Habitats. Remote Sens. 2020, 12, 631. [Google Scholar] [CrossRef]
- Gontia, N.K.; Tiwari, K.N. Development of Crop Water Stress Index of Wheat Crop for Scheduling Irrigation Using Infrared Thermometry. Agric. Water Manag. 2008, 95, 1144–1152. [Google Scholar] [CrossRef]
- Alchanatis, V.; Cohen, Y.; Cohen, S.; Moller, M.; Sprinstin, M.; Meron, M.; Tsipris, J.; Saranga, Y.; Sela, E. Evaluation of Different Approaches for Estimating and Mapping Crop Water Status in Cotton with Thermal Imaging. Precis. Agric. 2010, 11, 27–41. [Google Scholar] [CrossRef]
- Holt, C.C. Forecasting Seasonals and Trends by Exponentially Weighted Moving Averages. Int. J. Forecast. 2004, 20, 5–10. [Google Scholar] [CrossRef]

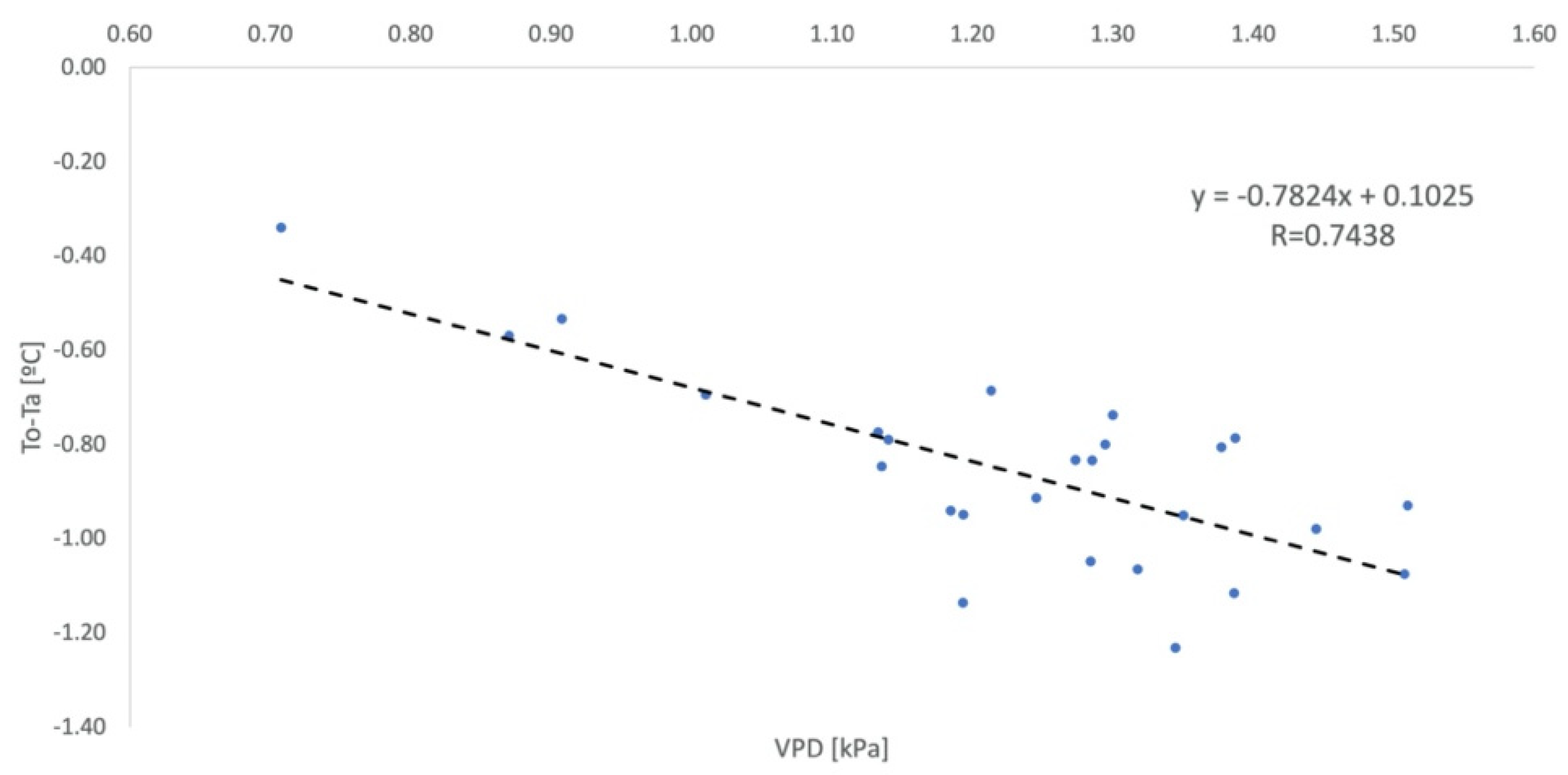
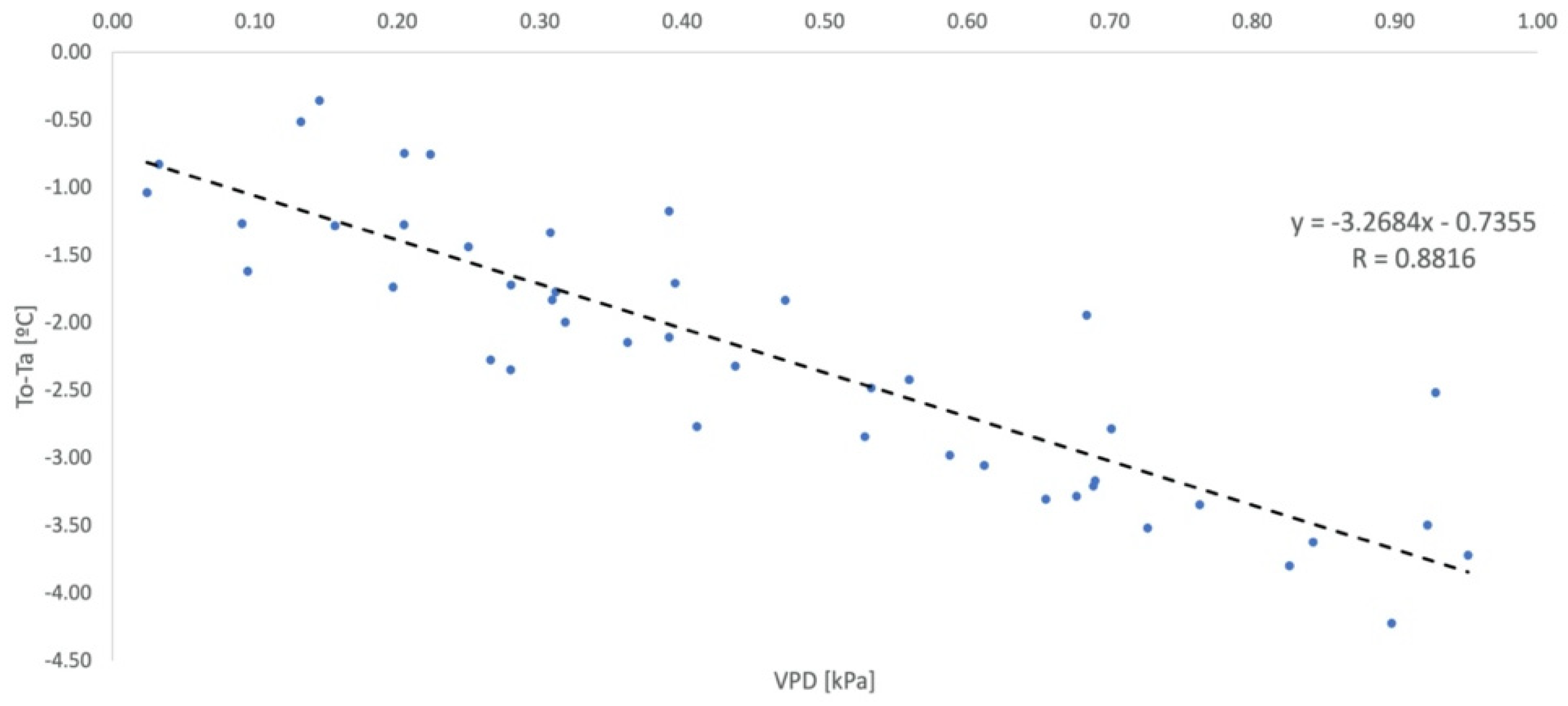






| Authors | Crop | NWSB |
|---|---|---|
| Idso [7] | Lettuce | Y = −2.96x + 4.18 |
| Erdem [15] | Watermelon | Y = −1.20x + 0.47 |
| Fattahi [16] | Maize | Y = −2.81x − 1.35 |
| Bellvert [28] | Grape | Y = −1.71x + 2.54 |
| Kumar [38] | Mustard | Y = −1.71x − 0.47 |
| Precision | Sensitivity | F-Score | Total Error | Accuracy | Processing Time [s] | |
|---|---|---|---|---|---|---|
| Mean | 88.79% | 84.83% | 86.54% | 9.96% | 90.04% | 1.50 |
| Standard error | 1.28% | 1.27% | 0.66% | 0.58% | 0.57% | 0.01 |
| Median | 89.96% | 85.61% | 87.14% | 9.99% | 90.01% | 0.50 |
| Standard deviation | 5.10% | 5.09% | 2.65% | 2.31% | 2.31% | 0.03 |
| Sample variance | 0.26% | 0.26% | 0.07% | 0.05% | 0.05% | 0.00 |
| kurtosis | 1.76 | 3.39 | 1.56 | 1.97 | 1.97 | −0.81 |
| Skew | −1.26 | −1.55 | −0.81 | −0.09 | 0.09 | −0.50 |
| Interval | 19.04% | 21.01% | 10.80% | 10.47% | 10.47% | 0.10 |
| Minimum | 75.29% | 70.53% | 80.72% | 4.54% | 84.99% | 0.44 |
| Maximum | 94.34% | 91.54% | 91.52% | 15.01% | 95.46% | 0.54 |
| Score | 16 | 16 | 15 | 16 | 15 | 15 |
| N | 2.72% | 2.71% | 1.41% | 1.23% | 1.23% | 0.02 |
| Stress Level | ψL [MPa] | CWSI | Reference |
|---|---|---|---|
| Well-irrigated vines | Bellvert [30] | ||
| Well-irrigated cotton | Cohen [58] | ||
| Well-irrigated quinoa | Çolak [57] | ||
| Well-irrigated soybean | Mastrorilli [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, R.L.d.; Garcia, A.P.; Umezu, C.K.; Camargo, A.P.d.; Soares, F.T.; Albiero, D. Water Stress Index Detection Using a Low-Cost Infrared Sensor and Excess Green Image Processing. Sensors 2023, 23, 1318. https://doi.org/10.3390/s23031318
Paulo RLd, Garcia AP, Umezu CK, Camargo APd, Soares FT, Albiero D. Water Stress Index Detection Using a Low-Cost Infrared Sensor and Excess Green Image Processing. Sensors. 2023; 23(3):1318. https://doi.org/10.3390/s23031318
Chicago/Turabian StylePaulo, Rodrigo Leme de, Angel Pontin Garcia, Claudio Kiyoshi Umezu, Antonio Pires de Camargo, Fabrício Theodoro Soares, and Daniel Albiero. 2023. "Water Stress Index Detection Using a Low-Cost Infrared Sensor and Excess Green Image Processing" Sensors 23, no. 3: 1318. https://doi.org/10.3390/s23031318
APA StylePaulo, R. L. d., Garcia, A. P., Umezu, C. K., Camargo, A. P. d., Soares, F. T., & Albiero, D. (2023). Water Stress Index Detection Using a Low-Cost Infrared Sensor and Excess Green Image Processing. Sensors, 23(3), 1318. https://doi.org/10.3390/s23031318










