Novel Dual-Signal SiO2-COOH@MIPs Electrochemical Sensor for Highly Sensitive Detection of Chloramphenicol in Milk
Abstract
1. Introduction
2. Experimental Section
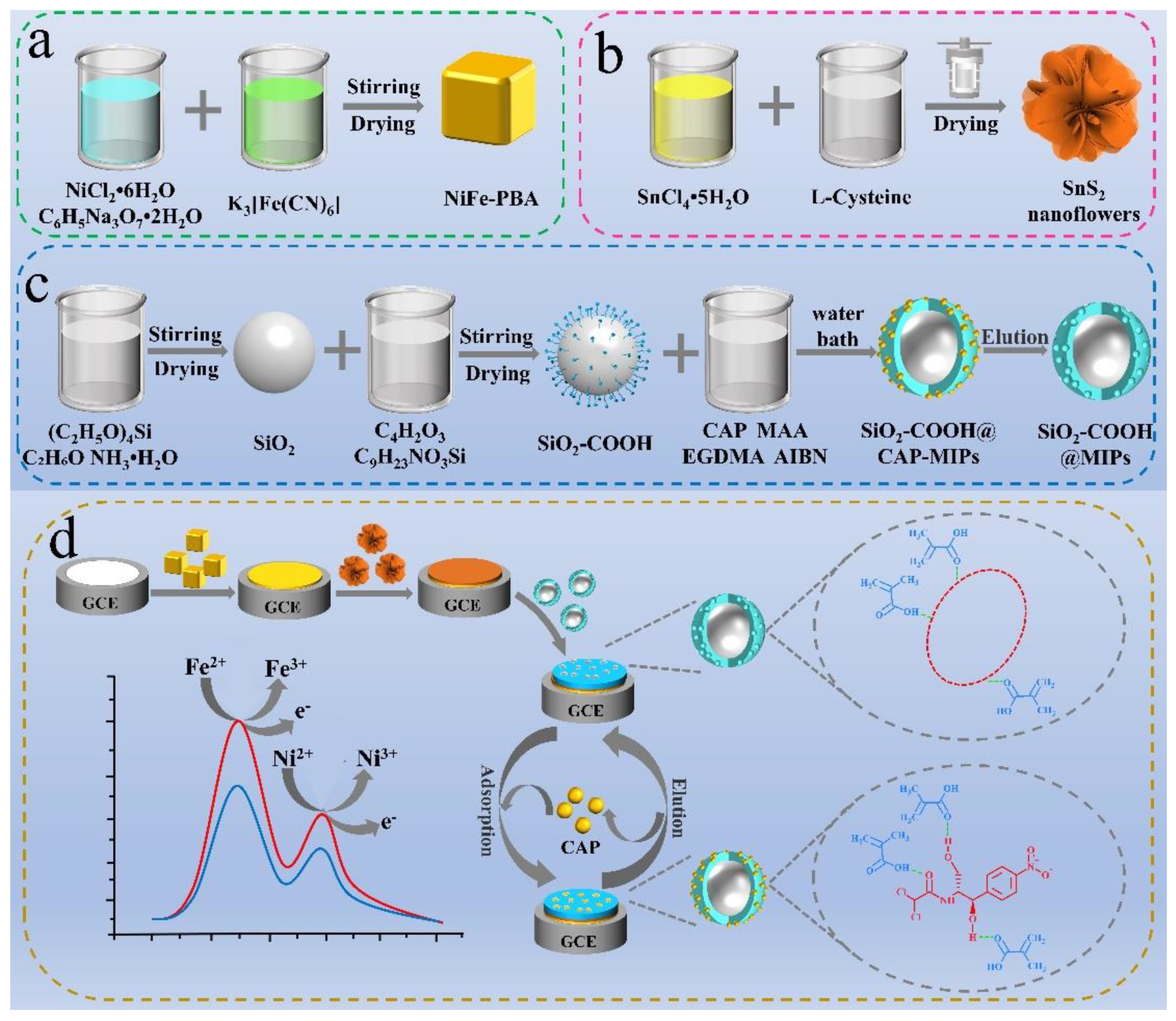
Preparation of SiO2-COOH@MIPs/SnS2/NiFe-PBA/GCE Sensor
3. Results and Discussion
3.1. Characterization of the Prepared Materials
3.1.1. NiFe-PBA
3.1.2. SnS2 Nanoflowers
3.1.3. SiO2-COOH@MIPs
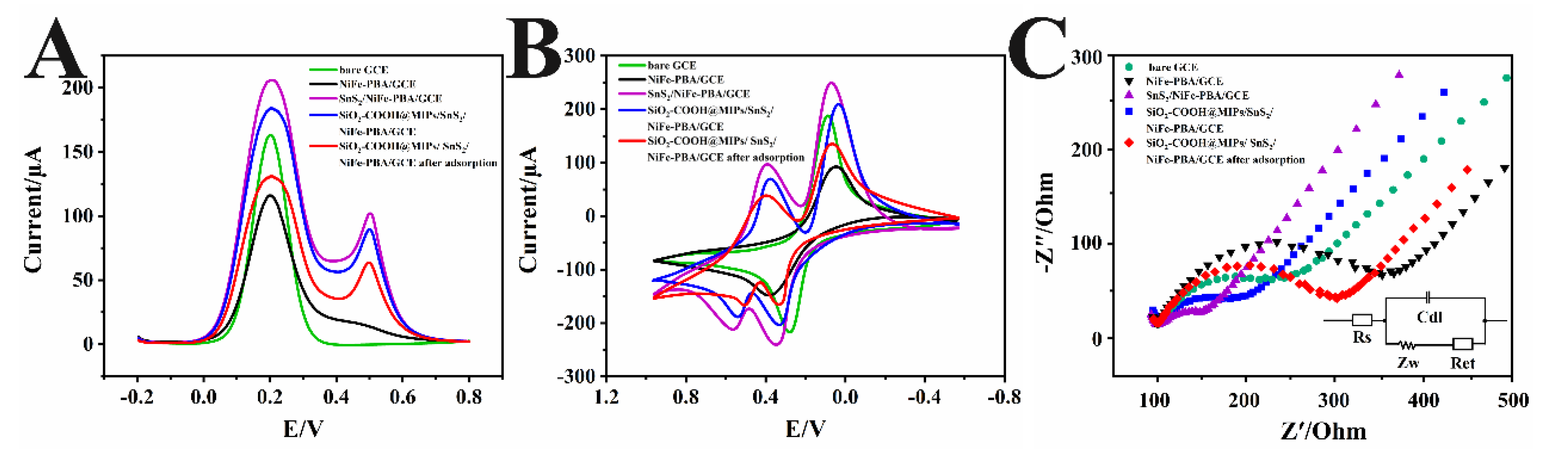
3.2. Electrochemical Investigation of SiO2-COOH@MIPs/SnS2/NiFe-PBA/GCE
3.3. Optimization of Conditions
3.3.1. Molar Ratio of CAP to MAA
3.3.2. Molar Ratio of CAP to EGDMA
3.3.3. Adsorption Time
3.3.4. Elution Times
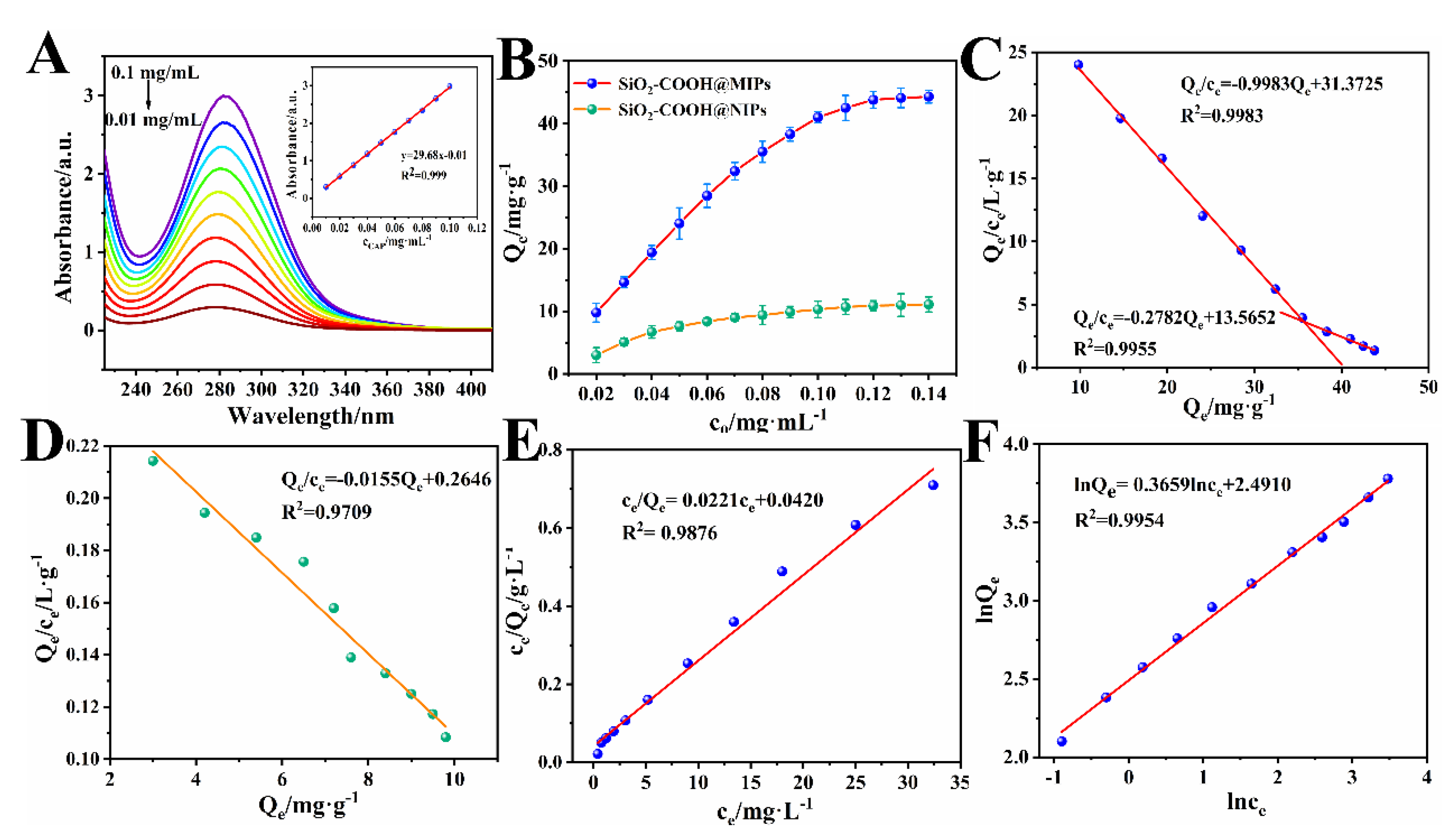
3.4. Adsorption Studies
3.4.1. Adsorption Isotherm Analysis
3.4.2. Adsorption Kinetics Analysis and Selective Adsorption Analysis
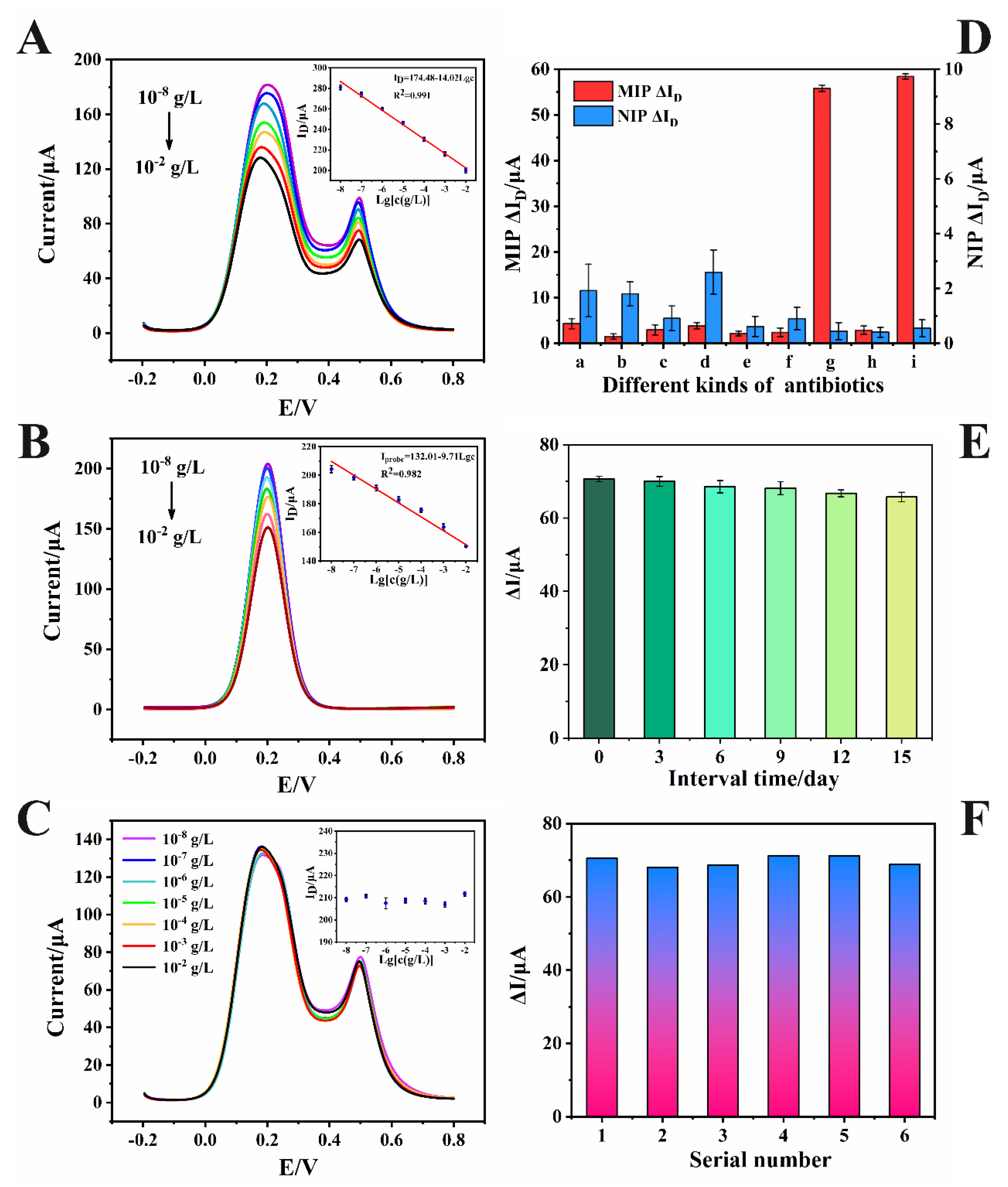
3.5. Capability Assessment of the SiO2-COOH@MIPs/SnS2/NiFe-PBA/GCE Sensor
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, R.; Wei, X.; Jiang, W.; Lu, Z.; Tang, Q.; Chen, Y.; Liu, Z.; Kang, J.; Ye, Y.; Liu, D. Photodegradation of chloramphenicol in micro-polluted water using a circulatory thin-layer inclined plate reactor. Chemosphere 2022, 291, 132883. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, V.; Abinaya, M.; Chen, S.-M. Ultrasonic assisted preparation of CoMoO4 nanoparticles modified electrochemical sensor for chloramphenicol determination. J. Solid State Chem. 2021, 302, 122392. [Google Scholar] [CrossRef]
- Wu, M.; Jing, T.; Tian, J.; Qi, H.; Shi, D.; Zhao, C.; Chen, T.; Zhao, Z.; Zhang, P.; Guo, Z. Synergistic effect of silver plasmon resonance and p-n heterojunction enhanced photoelectrochemical aptasensing platform for detecting chloramphenicol. Adv. Compos. Hybrid Mater. 2022, 5, 2247–2259. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, X.; Chen, H.; Li, J.; Yang, M.; Liu, J.; Sun, K.; Li, Z.; Deng, G. Fluorescence determination of chloramphenicol in milk powder using carbon dot decorated silver metal-organic frameworks. Microchim. Acta 2022, 189, 272. [Google Scholar] [CrossRef] [PubMed]
- Buledi, J.A.; Mahar, N.; Mallah, A.; Solangi, A.R.; Palabiyik, I.M.; Qambrani, N.; Karimi, F.; Vasseghian, Y.; Karimi-Maleh, H. Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: A potential method for environmental remediation. Food Chem. Toxicol. 2022, 161, 112843. [Google Scholar] [CrossRef]
- Buledi, J.A.; Shah, Z.-U.-H.; Mallah, A.; Solangi, A.R. Current Perspective and Developments in Electrochemical Sensors Modified with Nanomaterials for Environmental and Pharmaceutical Analysis. Curr. Anal. Chem. 2022, 18, 102–115. [Google Scholar] [CrossRef]
- Buledi, J.A.; Solangi, A.R.; Hyder, A.; Khand, N.H.; Memon, S.A.; Mallah, A.; Mahar, N.; Dragoi, E.N.; Show, P.; Behzadpour, M.; et al. Selective oxidation of amaranth dye in soft drinks through tin oxide decorated reduced graphene oxide nanocomposite based electrochemical sensor. Food Chem. Toxicol. 2022, 165, 113177. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, L.; Xu, X.; Song, S.; Liu, L.; Kuang, H.; Zhu, Y.; Xu, L.; Xu, C. Ultrasensitive paper sensor for simultaneous detection of alpha-amanitin and beta-amanitin by the production of monoclonal antibodies. Food Chem. 2022, 396, 133660. [Google Scholar] [CrossRef]
- Peng, J.; Liu, L.; Xu, L.; Song, S.; Kuang, H.; Cui, G.; Xu, C. Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Res. 2016, 10, 108–120. [Google Scholar] [CrossRef]
- Liu, N.; Liu, R.; Zhang, J. CRISPR-Cas12a-mediated label-free electrochemical aptamer-based sensor for SARS-CoV-2 antigen detection. Bioelectrochemistry 2022, 146, 108105. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yue, F.; Shen, Z.; Xu, D.; Fang, H.; Chen, W.; Wang, Z.; Li, P.; Guo, Y.; et al. Dual enzyme electrochemiluminescence sensor based on in situ synthesis of ZIF-67@AgNPs for the detection of IMP in fresh meat. LWT 2022, 165, 113658. [Google Scholar] [CrossRef]
- Yang, H.; Song, H.; Suo, Z.; Li, F.; Jin, Q.; Zhu, X.; Chen, Q. A molecularly imprinted electrochemical sensor based on surface imprinted polymerization and boric acid affinity for selective and sensitive detection of P-glycoproteins. Anal. Chim. Acta 2022, 1207, 339797. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Mostafiz, B.; Bigdeli, S.A.; Banan, K.; Afsharara, H.; Hatamabadi, D.; Mousavi, P.; Hussain, C.M.; Keçili, R.; Ghorbani-Bidkorbeh, F. Molecularly imprinted polymer-carbon paste electrode (MIP-CPE)-based sensors for the sensitive detection of organic and inorganic environmental pollutants: A review. Trends Environ. Anal. 2021, 32, e00144. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 2022, 305, 102693. [Google Scholar] [CrossRef]
- Soomro, A.N.; Shaikh, H.; Malik, M.I.; Buledi, J.A.; Qazi, S.; Solangi, A. Fluorene intercalated graphene oxide based CoQ10 imprinted polymer composite as a selective platform for electrochemical sensing of CoQ10. RSC Adv. 2022, 12, 31639–31649. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Xia, Y.; Liu, Y.; Zhao, F.; Zeng, B. Antifouling ionic liquid doped molecularly imprinted polymer-based ratiometric electrochemical sensor for highly stable and selective detection of zearalenone. Anal. Chim. Acta 2022, 1210, 339884. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Solangi, A.R.; Buledi, J.A.; Khand, N.H.; Junejo, B.; Memon, A.F.; Ameen, S.; Bhatti, A.; Show, P.L.; Vasseghian, Y.; et al. Plant extract-based green fabrication of nickel ferrite (NiFe(2)O(4)) nanoparticles: An operative platform for non-enzymatic determination of pentachlorophenol. Chemosphere 2022, 294, 133760. [Google Scholar] [CrossRef]
- Memon, A.F.; Ameen, S.; Khand, N.H.; Qambrani, N.; Buledi, J.A.; Junejo, B.; Solangi, A.R.; Taqvi, S.I.H.; Dragoi, E.N.; Zare, N.; et al. Electrochemical monitoring of bisphenol-s through nanostructured tin oxide/Nafion/GCE: A solution to environmental pollution. Chemosphere 2022, 303, 135170. [Google Scholar] [CrossRef]
- Qambrani, N.; Buledi, J.A.; Khand, N.H.; Solangi, A.R.; Ameen, S.; Jalbani, N.S.; Khatoon, A.; Taher, M.A.; Moghadam, F.H.; Shojaei, M.; et al. Facile Synthesis of NiO/ZnO nanocomposite as an effective platform for electrochemical determination of carbamazepine. Chemosphere 2022, 303, 135270. [Google Scholar] [CrossRef] [PubMed]
- Guari, Y.; Cahu, M.; Félix, G.; Sene, S.; Long, J.; Chopineau, J.; Devoisselle, J.-M.; Larionova, J. Nanoheterostructures based on nanosized Prussian blue and its Analogues: Design, properties and applications. Coord. Chem. Rev. 2022, 461, 214497. [Google Scholar] [CrossRef]
- Chen, J.; Wei, L.; Mahmood, A.; Pei, Z.; Zhou, Z.; Chen, X.; Chen, Y. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, C.; Chen, C.; Wang, J.; Kong, Y.; Liu, T.; Ying, S.; Yi, F.-Y. CoFeP nanocube-arrays based on Prussian blue analogues for accelerated oxygen evolution electrocatalysis. J. Power Sources 2022, 520, 230884. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sensors Actuat. B-Chem. 2022, 359, 131612. [Google Scholar] [CrossRef]
- Sharma, K.; Patial, S.; Singh, P.; Khan, A.A.P.; Saini, V.; Nadda, A.K.; Hussain, C.M.; Nguyen, V.-H.; Nguyen, C.C.; Hac Nguyen, T.B.; et al. Strategies and perspectives of tailored SnS2 photocatalyst for solar driven energy applications. Sol. Energy 2022, 231, 546–565. [Google Scholar] [CrossRef]
- Zhu, Q.; Gu, D.; Liu, Z.; Huang, B.; Li, X. Au-modified 3D SnS2 nano-flowers for low-temperature NO2 sensors. Sensors Actuat. B-Chem. 2021, 349, 130775. [Google Scholar] [CrossRef]
- Niu, Q.; Bao, C.; Cao, X.; Liu, C.; Wang, H.; Lu, W. Ni-Fe PBA hollow nanocubes as efficient electrode materials for highly sensitive detection of guanine and hydrogen peroxide in human whole saliva. Biosens. Bioelectron. 2019, 141, 111445. [Google Scholar] [CrossRef]
- Qin, M.; Ren, W.; Jiang, R.; Li, Q.; Yao, X.; Wang, S.; You, Y.; Mai, L. Highly Crystallized Prussian Blue with Enhanced Kinetics for Highly Efficient Sodium Storage. ACS Appl. Mater. Interfaces 2021, 13, 3999–4007. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Yang, H.; Zhang, S.; Song, H.; Zhu, X. Preparation and evaluation of magnetic graphene oxide molecularly imprinted polymers (MIPs-GO-Fe3O4@SiO2) for the analysis and separation of tripterine. React. Funct. Polym. 2021, 169, 105055. [Google Scholar] [CrossRef]
- He, Y.; Tan, S.; Abd Ei-Aty, A.M.; Hacimuftuoglu, A.; She, Y. Magnetic molecularly imprinted polymers for the detection of aminopyralid in milk using dispersive solid-phase extraction. RSC Adv. 2019, 9, 29998–30006. [Google Scholar] [CrossRef]
- Garcia, S.M.; Wong, A.; Khan, S.; Sotomayor, M. A simple, sensitive and efficient electrochemical platform based on carbon paste electrode modified with Fe3O4@MIP and graphene oxide for folic acid determination in different matrices. Talanta 2021, 229, 122258. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Guan, W.; Chang, J.; Xu, L.; Guo, J.; Wei, G. Biosorption of Zn(II) by live and dead cells of Streptomyces ciscaucasicus strain CCNWHX 72-14. J. Hazard. Mater. 2010, 179, 151–159. [Google Scholar] [CrossRef]
- Wei, L.; Jiao, F.; Wang, Z.; Wu, L.; Dong, D.; Chen, Y. Enzyme-modulated photothermal immunoassay of chloramphenicol residues in milk and egg using a self-calibrated thermal imager. Food Chem. 2022, 392, 133232. [Google Scholar] [CrossRef]
- Yao, T.; Yao, S. Magnetic ionic liquid aqueous two-phase system coupled with high performance liquid chromatography: A rapid approach for determination of chloramphenicol in water environment. J. Chromatogr. A 2017, 1481, 12–22. [Google Scholar] [CrossRef]
- Kawano, S.-I.; Hao, H.-Y.; Hashi, Y.; Lin, J.-M. Analysis of chloramphenicol in honey by on-line pretreatment liquid chromatography–tandem mass spectrometry. Chin. Chem. Lett. 2015, 26, 36–38. [Google Scholar] [CrossRef]
- Chang, H.; Lv, J.; Zhang, H.; Zhang, B.; Wei, W.; Qiao, Y. Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens. Bioelectron. 2017, 87, 579–586. [Google Scholar] [CrossRef]
- Ma, P.; Guo, H.; Duan, N.; Ma, X.; Yue, L.; Gu, Q.; Wang, Z. Label free structure-switching fluorescence polarization detection of chloramphenicol with truncated aptamer. Talanta 2021, 230, 122349. [Google Scholar] [CrossRef]
- He, Z.-J.; Kang, T.-F.; Lu, L.-P.; Cheng, S.-Y. An electrochemiluminescence aptamer sensor for chloramphenicol based on GO-QDs nanocomposites and enzyme-linked aptamers. Electroanal. Chem. 2020, 860, 113870. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Zhai, H.; Shen, Z.; Han, J.; Yu, Y.; Fang, H.; Li, F.; Sun, X.; Guo, Y. Molecularly imprinted electrochemical sensor based on multi-walled carbon nanotubes for specific recognition and determination of chloramphenicol in milk. Microchem. J. 2022, 182, 107887. [Google Scholar] [CrossRef]
- Fan, J.-P.; Xu, X.-K.; Xu, R.; Zhang, X.-H.; Zhu, J.-H. Preparation and characterization of molecular imprinted polymer functionalized with core/shell magnetic particles (Fe3O4 @SiO2@MIP) for the simultaneous recognition and enrichment of four taxoids in Taxus × media. Chem Eng. J. 2015, 279, 567–577. [Google Scholar] [CrossRef]
- Yue, F.; Li, H.; Kong, Q.; Liu, J.; Wang, G.; Li, F.; Yang, Q.; Chen, W.; Guo, Y.; Sun, X. Selection of broad-spectrum aptamer and its application in fabrication of aptasensor for detection of aminoglycoside antibiotics residues in milk. Sensor Actuat B-Chem. 2022, 351, 130959. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Meng, M.; Yan, Y. SiO2-coated molecularly imprinted sensor based on Si quantum dots for selective detection of catechol in river water. J. Environ. Chem. Eng. 2022, 10, 106850. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; He, W.; Chen, M.; Tu, W.; Zhu, M.; Gan, D.; Liu, S. Multifunctional stable PDA/RGO/MOFs&SiO2-COOH membrane with excellent flux and anti-fouling performance for the separation of organic dye and oil/water. Surf. Interfaces 2022, 33, 102183. [Google Scholar]
- Sun, J.; Guo, W.; Ji, J.; Li, Z.; Yuan, X.; Pi, F.; Zhang, Y.; Sun, X. Removal of patulin in apple juice based on novel magnetic molecularly imprinted adsorbent Fe3O4@SiO2@CS-GO@MIP. Lwt 2020, 118, 108854. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, Y.; Song, H.; Niu, Y.; Chen, X.; Liu, X.; Gao, R.; Wang, S. Fabrication of metal coordination-synergistic magnetic imprinted microspheres based on ligand-free Fe3O4-Cu for specific recognition of bovine hemoglobin. Talanta 2021, 233, 122496. [Google Scholar] [CrossRef]


| Method | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|
| ELISA | 1.0 × 10−2–1.0 × 102 | 9.0 × 10−3 | [34] |
| HPLC | 12.3–2.2 × 103 | 0.14 | [35] |
| HPLC-MSIMS | 0.02–5 | 2.0 × 10−2 | [36] |
| Colorimetry | 8.5 × 10−3–4.1 | 8.5 × 10−3 | [37] |
| Fluorescence | 3.2 × 10−2–3.2 | 1.9 × 10−2 | [38] |
| ECL-enzyme-Apt | 3.2 × 10−4–0.32 | 3.2 × 10−4 | [39] |
| Electrochemistry-MIPs | 0.1–1.0 × 103 | 3.3 × 10−2 | [40] |
| Electrochemistry-MIPs | 1.0 × 10−2–1.0 × 104 | 3.3 × 10−3 | This work |
| Sample | Spiked (g/L) | Detected (g/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Milk | 0 | 0 | - | - |
| 1.0 × 10−7 | 0.981 × 10−7 | 98.1 | 2.19 | |
| 1.0 × 10−6 | 0.977 × 10−6 | 97.7 | 2.73 | |
| 1.0 × 10−5 | 1.041 × 10−5 | 104.1 | 3.44 | |
| 1.0 × 10−4 | 0.992 × 10−4 | 99.2 | 4.68 | |
| 1.0 × 10−3 | 1.027 × 10−3 | 102.7 | 4.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, L.; Liu, M.; Huang, J.; Li, F.; Zhang, Y.; Guo, Y.; Sun, X. Novel Dual-Signal SiO2-COOH@MIPs Electrochemical Sensor for Highly Sensitive Detection of Chloramphenicol in Milk. Sensors 2023, 23, 1346. https://doi.org/10.3390/s23031346
Geng L, Liu M, Huang J, Li F, Zhang Y, Guo Y, Sun X. Novel Dual-Signal SiO2-COOH@MIPs Electrochemical Sensor for Highly Sensitive Detection of Chloramphenicol in Milk. Sensors. 2023; 23(3):1346. https://doi.org/10.3390/s23031346
Chicago/Turabian StyleGeng, Lingjun, Mengyue Liu, Jingcheng Huang, Falan Li, Yanyan Zhang, Yemin Guo, and Xia Sun. 2023. "Novel Dual-Signal SiO2-COOH@MIPs Electrochemical Sensor for Highly Sensitive Detection of Chloramphenicol in Milk" Sensors 23, no. 3: 1346. https://doi.org/10.3390/s23031346
APA StyleGeng, L., Liu, M., Huang, J., Li, F., Zhang, Y., Guo, Y., & Sun, X. (2023). Novel Dual-Signal SiO2-COOH@MIPs Electrochemical Sensor for Highly Sensitive Detection of Chloramphenicol in Milk. Sensors, 23(3), 1346. https://doi.org/10.3390/s23031346







