Exploring the Role of the Connection Length of Screen-Printed Electrodes towards the Hydrogen and Oxygen Evolution Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical Measurements
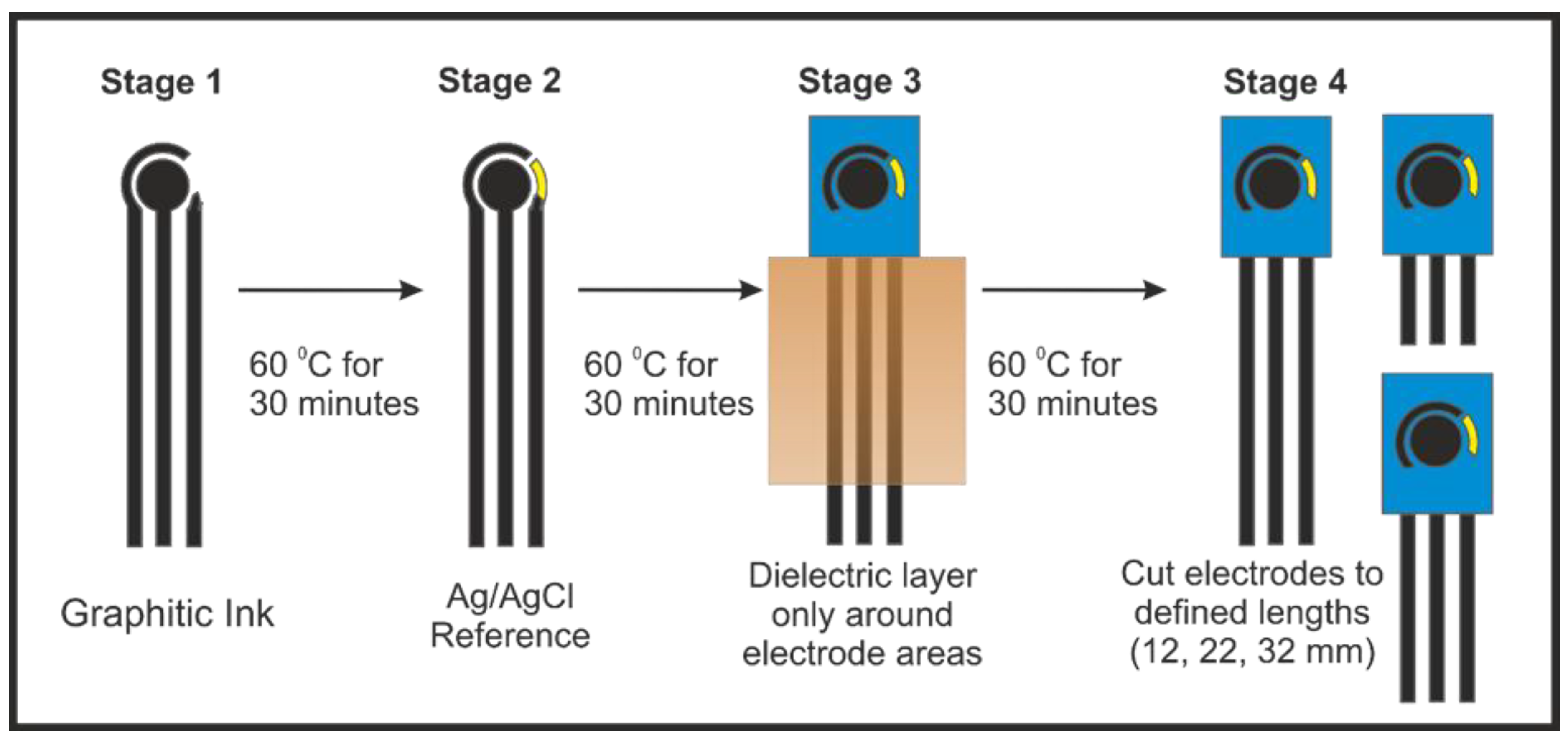
2.3. Screen-Printed Electrode Fabrication
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- COP26 The Glasgow Climate Pact. Available online: https://ukcop26.org/wp-content/uploads/2021/11/COP26-The-Glasgow-Climate-Pact.pdf (accessed on 26 November 2021).
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Li, X.; Lei, H.; Xie, L.; Wang, N.; Zhang, W.; Cao, R. Metalloporphyrins as catalytic models for studying hydrogen and oxygn evolution and oxygen reduction reactions. Acc. Chem. Res. 2022, 55, 878–892. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Bui, V.Q.; Kumar, A.; Bui, H.T.; Lee, J.; Hwang, Y.; Le, H.M.; Lee, H. Boosting electrocatalytic HER activity of 3D interconnected CoSP via metal doping: Active and stable electrocatalysts for pH-universal hydrogen generation. Chem. Mater. 2020, 32, 9591–9601. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Arias-Pinedo, O.M.; Cardenas Riojas, A.A.; Pastor, E.; López, E.O.; Perez, G.; Archanjo, B.S.; Baena-Moncada, A.M. Hierarchical Porous Carbon-PtPd Catalysts and Their Activity toward Oxygen Reduction Reaction. ACS Omega 2022, 7, 20860–20871. [Google Scholar] [CrossRef]
- McKenzie, K.J.; Marken, F. Electrochemical characterization of hydrous ruthenium oxide nanoparticle decorated boron-doped diamond electrodes. Electrochem. Solid-State Lett. 2002, 5, E47. [Google Scholar] [CrossRef]
- Gondolini, A.; Sangiorgi, N.; Sangiorgi, A.; Sanson, A. Photoelectrochemical hydrogen production by screen-printed copper oxide electrodes. Energies 2021, 14, 2942. [Google Scholar] [CrossRef]
- Díaz-Coello, S.; Palenzuela, J.A.; Afonso, M.M.; Pastor, E.; García, G. WC modified with ionic liquids for the hydrogen evolution reaction in alkaline solution. J. Electroanal. Chem. 2021, 880, 114878. [Google Scholar] [CrossRef]
- Amiripour, F.; Azizi, S.N.; Ghasemi, S. Nano P zeolite modified with Au/Cu bimetallic nanoparticles for enhanced hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 605–617. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E. High hydrogen production rate on RuS2@ MoS2 hybrid nanocatalyst by PEM electrolysis. Int. J. Hydrog. Energy 2019, 44, 4398–4405. [Google Scholar] [CrossRef]
- Wojtyła, S.; Baran, T. Copper zinc oxide heterostructure nanoflowers for hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 27343–27353. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Parkhomenko, R.G.; Klyamer, D.D.; Shushanyan, A.D.; Asanov, I.P.; Morozova, N.B.; Basova, T.V. Bilayer structures based on metal phthalocyanine and palladium layers for selective hydrogen detection. Int. J. Hydrog. Energy 2017, 42, 28640–28646. [Google Scholar] [CrossRef]
- Rivera-Gavidia, L.M.; Luis-Sunga, M.; Bousa, M.; Vales, V.; Kalbac, M.; Arévalo, M.C.; García, G. S-and N-doped graphene-based catalysts for the oxygen evolution reaction. Electrochim. Acta 2020, 340, 135975. [Google Scholar] [CrossRef]
- Lee, J.; Murugappan, K.; Arrigan, D.W.; Silvester, D.S. Oxygen reduction voltammetry on platinum macrodisk and screen-printed electrodes in ionic liquids: Reaction of the electrogenerated superoxide species with compounds used in the paste of Pt screen-printed electrodes? Electrochim. Acta 2013, 101, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Comisso, N.; Armelao, L.; Cattarin, S.; Fasolin, S.; Mattarozzi, L.; Musiani, M.; Verlato, E. Deposition of FeOOH layers onto porous PbO2 by galvanic displacement and their use as electrocatalysts for oxygen evolution reaction. J. Electroanal. Chem. 2021, 880, 114844. [Google Scholar] [CrossRef]
- Gutiérrez-Ceron, C.; Oñate, R.; Zagal, J.H.; Pizarro, A.; Silva, J.F.; Castro-Castillo, C.; Rezende, M.C.; Flores, M.; Cortés-Arriagada, D.; Toro-Labbé, A.; et al. Molecular conductance versus inductive effects of axial ligands on the electrocatalytic activity of self-assembled iron phthalocyanines: The oxygen reduction reaction. Electrochim. Acta 2019, 327, 134996. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Shape-Dependent Electrocatalysis: Oxygen Reduction on Carbon-Supported Gold Nanoparticles. ChemElectroChem 2014, 1, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.G.M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Hurst, N.J.; Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Blanco, E.; Davies, T.J.; Banks, C.E. Electrochemical improvements can Be realized via shortening the length of screen-printed electrochemical platforms. Anal. Chem. 2021, 93, 16481–16488. [Google Scholar] [CrossRef]
- Niu, X.; Shi, L.; Li, X.; Pan, J.; Gu, R.; Zhao, H.; Qiu, F.; Yan, Y.; Lan, M. Simple anodization of home-made screen-printed carbon electrodes makes significant activity enhancement for hydrogen evolution: The synergistic effect of surface functional groups, defect sites, and hydrophilicity. Electrochim. Acta 2017, 235, 64–71. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/738549?lang=en®ion=GB (accessed on 2 March 2022).
- Galdino, F.E.; Foster, C.W.; Bonacin, J.A.; Banks, C.E. Exploring the electrical wiring of screen-printed configurations utilised in electroanalysis. Anal. Methods 2015, 7, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Li, K.; Wang, W.; Xie, Z.; Ding, L.; Kang, Z.; Wrubel, J.; Ma, Z.; Bender, G.; Yu, H.; et al. Tuning Catalyst Activation and Utilization Via Controlled Electrode Patterning for Low-Loading and High-Efficiency Water Electrolyzers. Small 2022, 18, 2107745–2107758. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, G.; Mo, J.; Li, Y.; Yu, S.; Cullen, D.A.; Retterer, S.T.; Toops, T.J.; Bender, G.; Pivovar, B.S.; et al. Novel thin/tunable gas diffusion electrodes with ultra-low catalyst loading for hydrogen evolution reactions in proton exchange membrane electrolyzer cells. Nano Energy 2018, 47, 434–441. [Google Scholar] [CrossRef]
- Yang, G.; Yu, S.; Kang, Z.; Li, Y.; Bender, G.; Pivovar, B.S.; Green, J.B.; Cullen, D.A.; Zhang, F.Y. Building Electron/Proton Nanohighways for Full Utilization of Water Splitting Catalysts. Adv. Energy Mater. 2020, 10, 1903871–1903881. [Google Scholar] [CrossRef]
- Mo, J.; Kang, Z.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Green, J.B.; Mench, M.M.; Zhang, F.-Y. Discovery of true electrochemical reactions for ultrahigh catalyst mass activity in water splitting. Sci. Adv. 2016, 2, e1600690–e1600700. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Huang, W.; Gong, Q.; Liu, C.; Liu, Z.; Li, Y.; Dai, H. Ultrathin WS2 Nanoflakes as a High-Performance Electrocatalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef]
- Scremin, J.; Joviano Dos Santos, I.V.; Hughes, J.P.; Ferrari, G.-M.A.; Valderrama, E.; Zheng, W.; Zhong, X.; Zhao, X.; Sartori, E.J.R.; Crapnell, R.D.; et al. Platinum nanoparticle decorated vertically aligned graphene screen-printed electrodes: Electrochemical characterisation and exploration towards the hydrogen evolution reaction. Nanoscale 2020, 12, 18214–18224. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Hughes, J.P.; Blanco, F.D.; Banks, C.E.; Rowley-Neale, S.J. Mass-producible 2D-WS 2 bulk modified screen printed electrodes towards the hydrogen evolution reaction. RSC Adv. 2019, 9, 25003–25011. [Google Scholar] [CrossRef] [Green Version]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.C.; Sawtell, D.A.G.; Kelly, P.J.; Banks, C.E. 2D nanosheet molybdenum disulphide (MoS2) modified electrodes explored towards the hydrogen evolution reaction. Nanoscale 2015, 7, 18152–18168. [Google Scholar] [CrossRef] [Green Version]
- Rowley-Neale, S.J.; Foster, C.W.; Smith, G.C.; Brownson, D.A.; Banks, C.E. Mass-producible 2D-MoSe2 bulk modified screen-printed electrodes provide significant electrocatalytic performances towards the hydrogen evolution reaction. Sustain. Energy Fuels 2017, 1, 74–83. [Google Scholar]
- Li, Q.; Yang, C.; Wu, L.; Wang, H.; Cui, X. Converting benzene into γ-graphyne and its enhanced electrochemical oxygen evolution performance. J. Mater. Chem. A 2019, 7, 5981–5990. [Google Scholar] [CrossRef]
- Vrubel, H.; Moehl, T.; Grätzel, M.; Hu, X. Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction. Chem. Commun. 2013, 49, 8985–8987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Laborda, E.; Tschulik, K.; Damm, C.; Molina, A.; Compton, R.G. Strong negative nanocatalysis: Oxygen reduction and hydrogen evolution at very small (2 nm) gold nanoparticles. Nanoscale 2014, 6, 11024–11030. [Google Scholar] [CrossRef]
- Ly, L.S.; Batchelor-McAuley, C.; Tschulik, K.; Kätelhön, E.; Compton, R.G. A critical evaluation of the interpretation of electrocatalytic nanoimpacts. J. Phys. Chem. C 2014, 118, 17756–17763. [Google Scholar] [CrossRef]

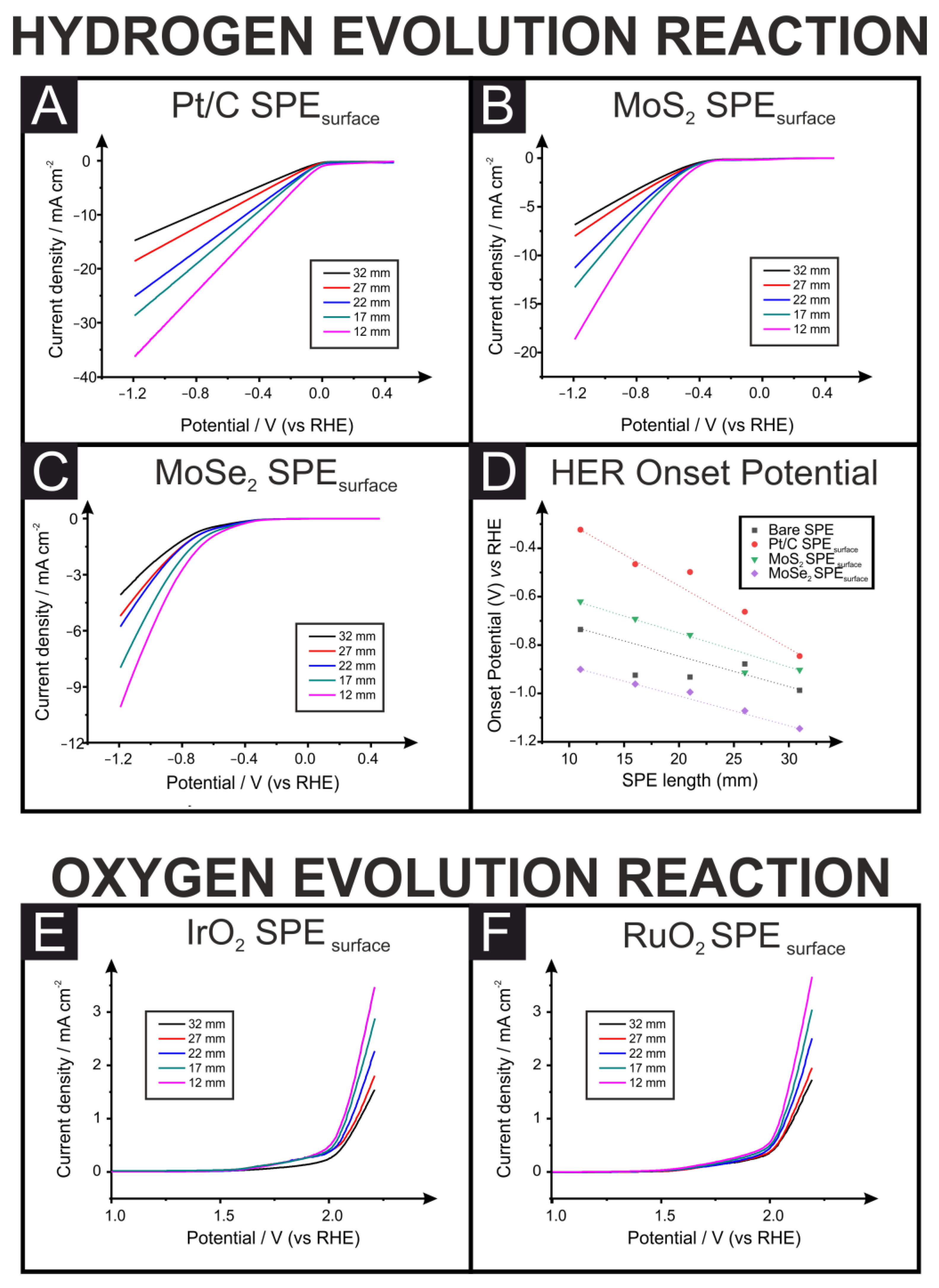

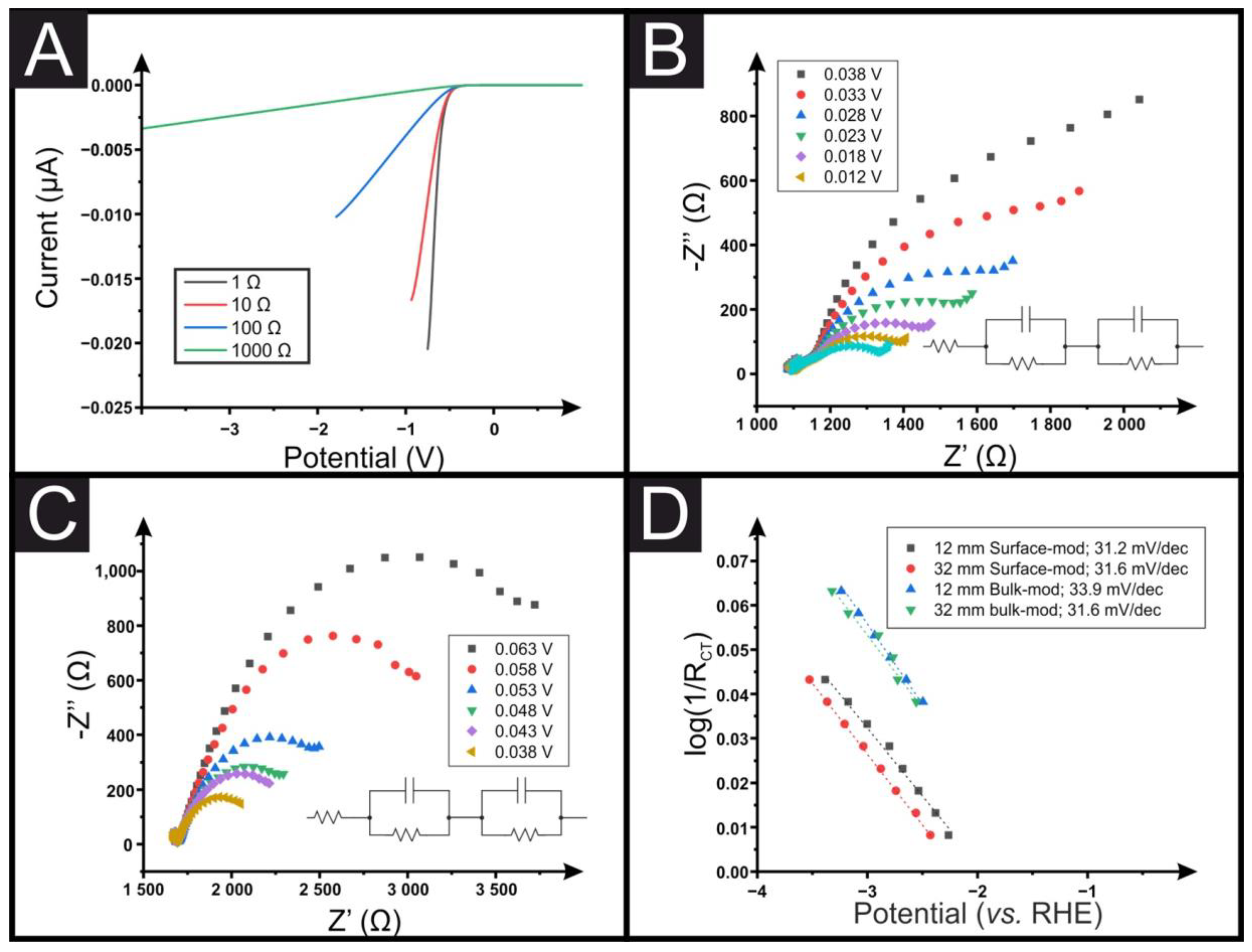
| Connection Length (mm). | Pt/C-SPEsurface Overpotential % | MoS2-SPEsurface Overpotential % | MoSe2-SPEsurface Overpotential % |
|---|---|---|---|
| 32 | 0.0 | 0.0 | 0.0 |
| 27 | −21.7 | 1.2 | −6.4 |
| 22 | −41.0 | −16.0 | −13.2 |
| 17 | −44.9 | −23.4 | −16.1 |
| 12 | −61.7 | −31.3 | −21.4 |
| Connection Length (mm). | RuO2-SPEsurface (V) | RuO2-SPEbulk (V) | IrO2-SPEsurface (V) | IrO2-SPEbulk (V) |
|---|---|---|---|---|
| 32 | 1.58 | 1.52 | 1.57 | 1.37 |
| 27 | 1.57 | 1.52 | 1.56 | 1.35 |
| 22 | 1.56 | 1.45 | 1.55 | 1.33 |
| 17 | 1.51 | 1.44 | 1.53 | 1.31 |
| 12 | 1.49 | 1.39 | 1.47 | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuamprakhon, P.; Ferrari, A.G.-M.; Crapnell, R.D.; Pimlott, J.L.; Rowley-Neale, S.J.; Davies, T.J.; Sawangphruk, M.; Banks, C.E. Exploring the Role of the Connection Length of Screen-Printed Electrodes towards the Hydrogen and Oxygen Evolution Reactions. Sensors 2023, 23, 1360. https://doi.org/10.3390/s23031360
Wuamprakhon P, Ferrari AG-M, Crapnell RD, Pimlott JL, Rowley-Neale SJ, Davies TJ, Sawangphruk M, Banks CE. Exploring the Role of the Connection Length of Screen-Printed Electrodes towards the Hydrogen and Oxygen Evolution Reactions. Sensors. 2023; 23(3):1360. https://doi.org/10.3390/s23031360
Chicago/Turabian StyleWuamprakhon, Phatsawit, Alejandro Garcia-Miranda Ferrari, Robert D. Crapnell, Jessica L. Pimlott, Samuel J. Rowley-Neale, Trevor J. Davies, Montree Sawangphruk, and Craig E. Banks. 2023. "Exploring the Role of the Connection Length of Screen-Printed Electrodes towards the Hydrogen and Oxygen Evolution Reactions" Sensors 23, no. 3: 1360. https://doi.org/10.3390/s23031360





