Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications
Abstract
:1. Introduction
- Description of the development process of a novel biodegradable sensor based on the combination of the SF with different densities of CNFs;
- Presentation of the analyses of the piezoelectricity voltage levels that the proposed SF-based biosensor can generate with respect to the different CNF densities;
- Presentation of the analyses on the mechanical response of a developed SF-based sensor for different cyclic compressions;
- Presentation of the analyses of the proposed SF-based biosensor performance in the case of its practical implementation on real humans during different human motion activities.
2. Literature Review
3. Materials and Methods Used for Biosensors Development
3.1. Preparation of Silk Fibroin
3.2. Synthesis of Acid-Functionalized CNFs
3.3. Preparation of SF with CNFs Nanocomposite Sponges
3.4. Characterization of Material Combining SF and CNF Nanocomposites
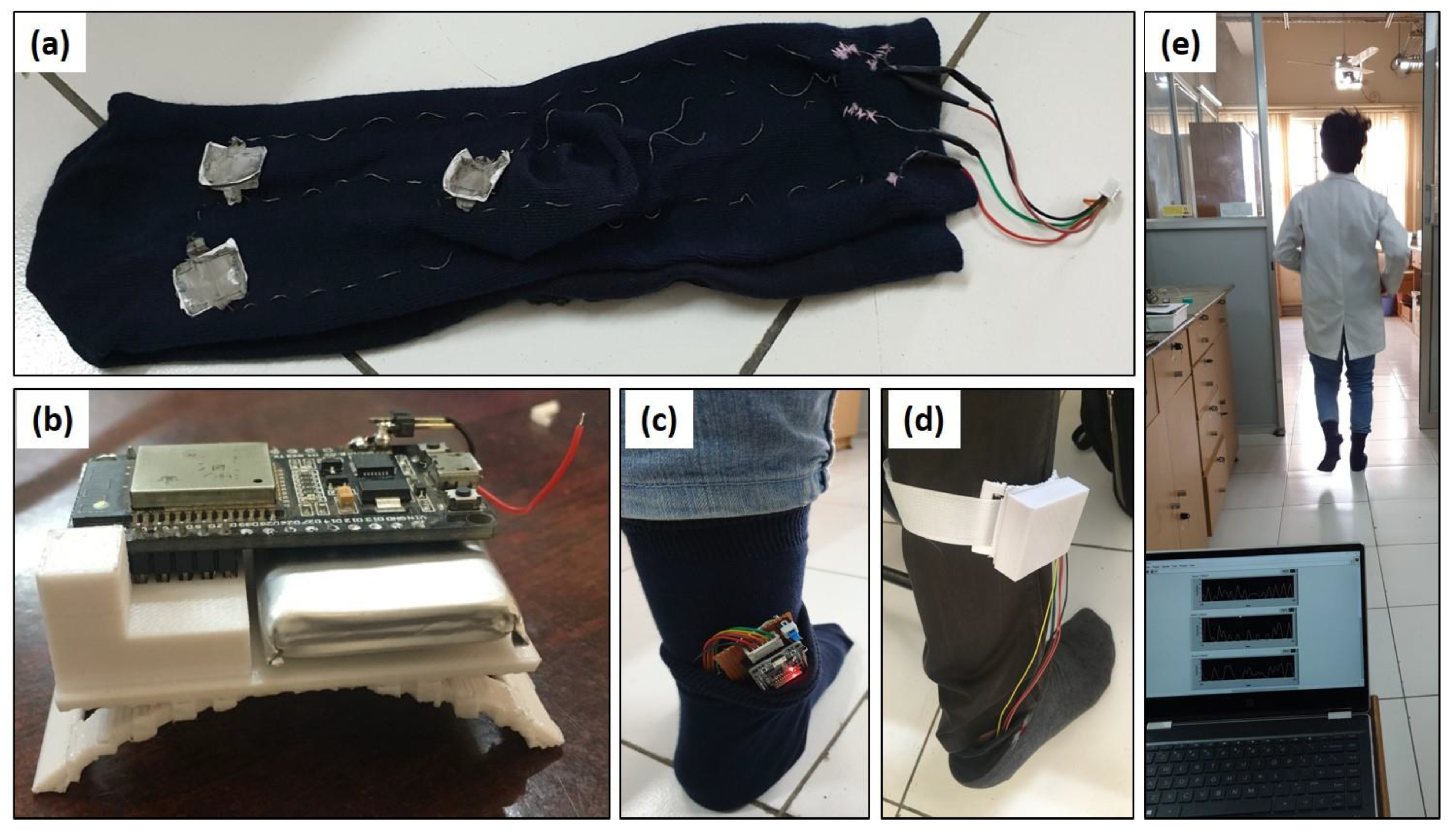
3.5. Wireless Monitoring of Human Gait
4. Results
4.1. Cross-Section Analysis
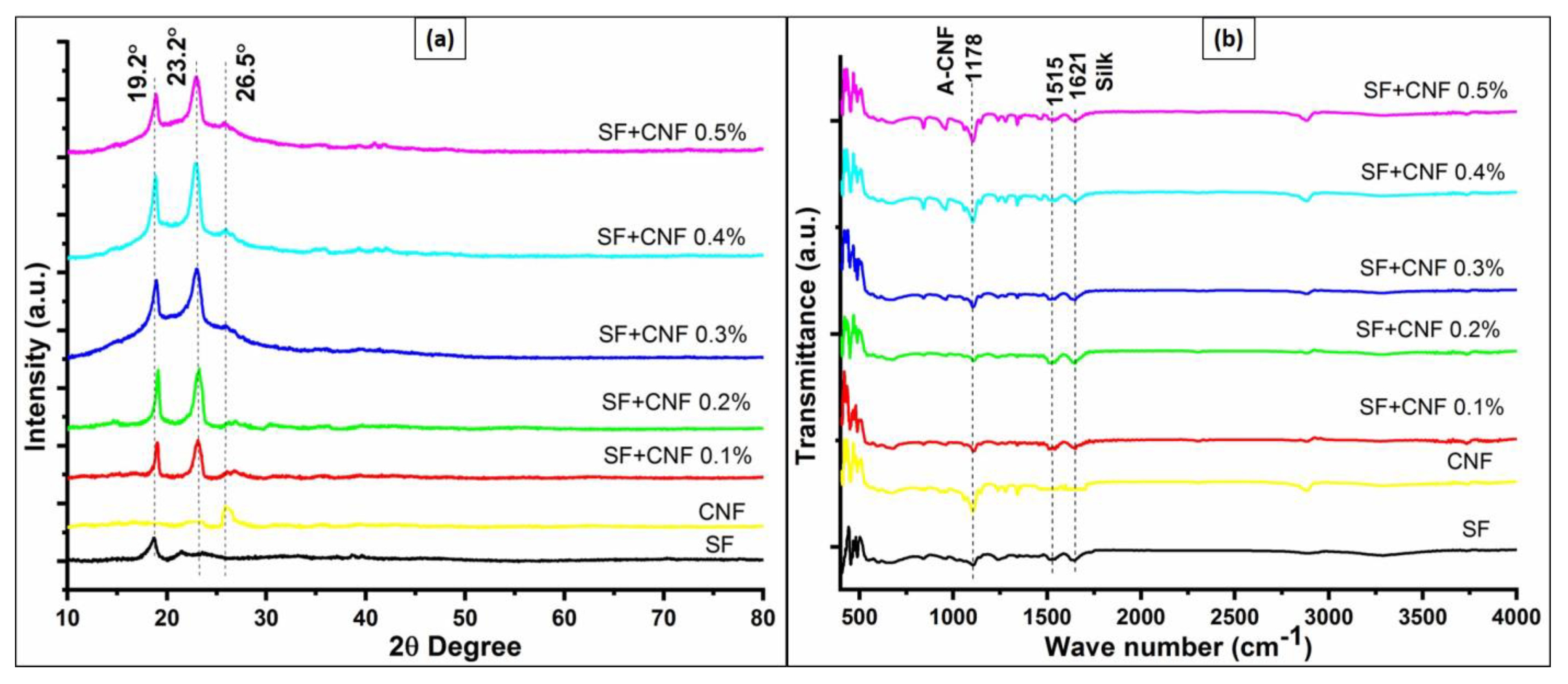
4.2. XRD Analysis of Materials Combining the SF and CNFs
4.3. Compression Analysis of Sponges
4.4. Dynamic Mechanical Analysis
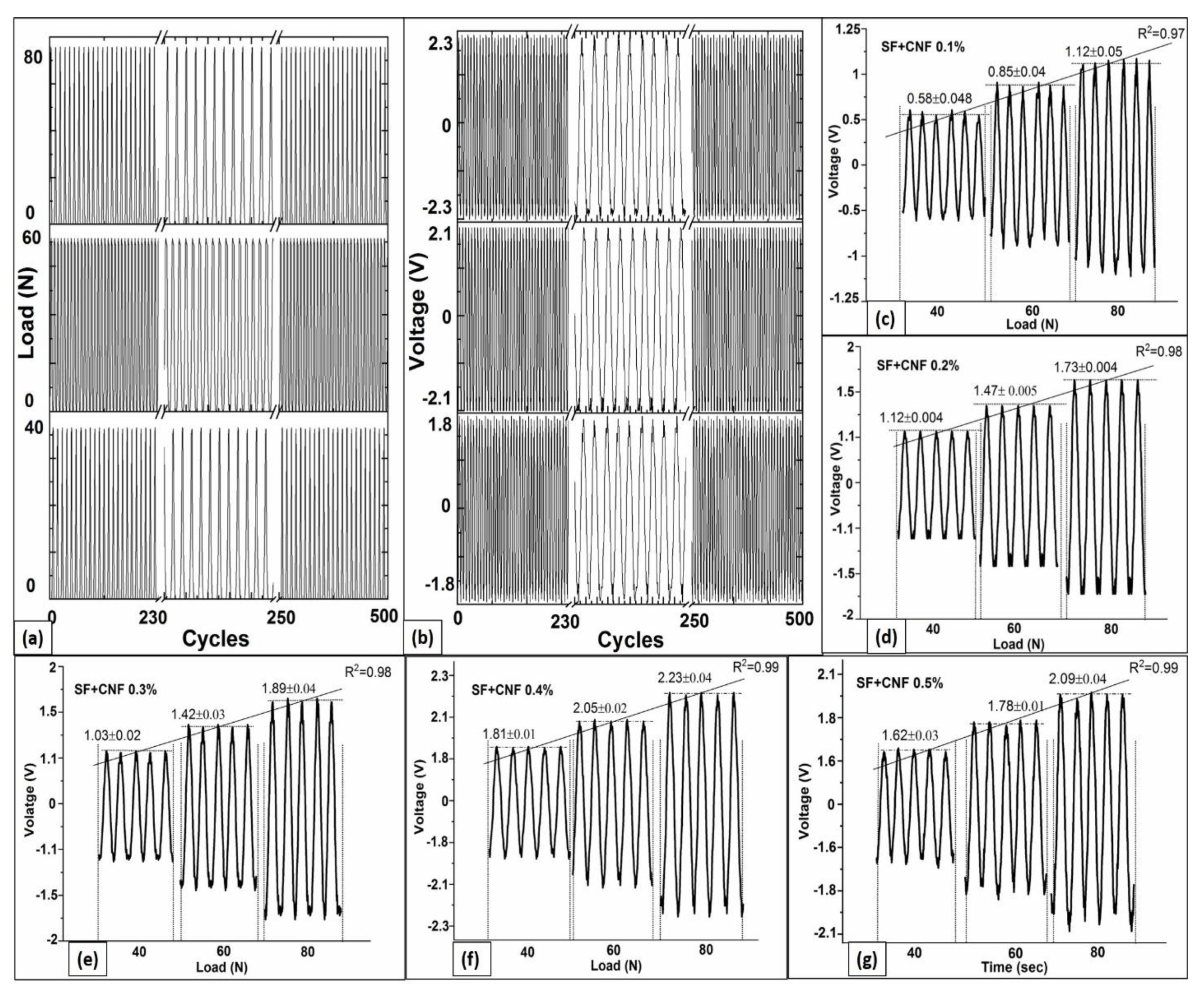
4.5. Piezoresponse under Cyclic Loading
4.6. Continuous Cyclic Load and Voltage Response Analyses
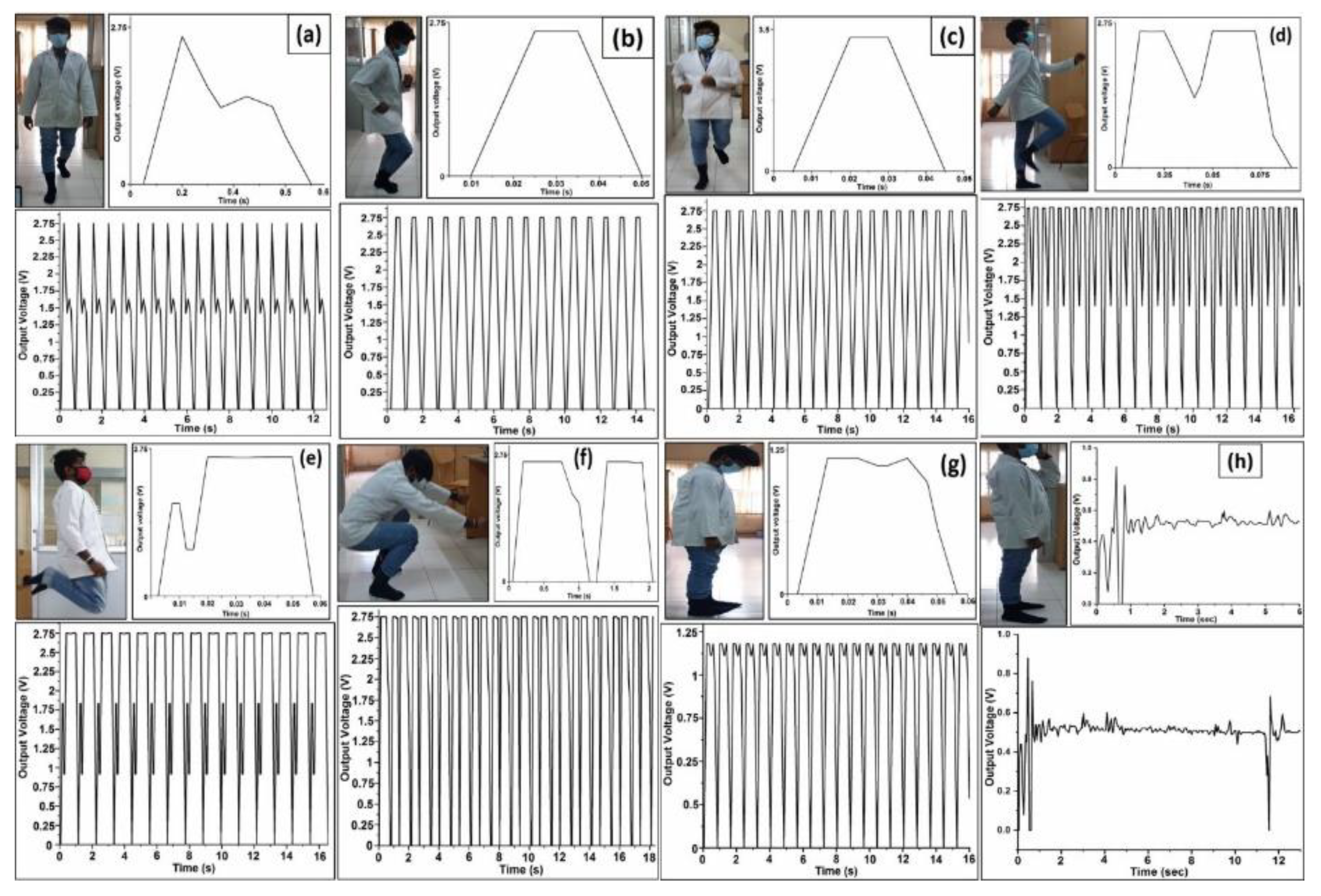
4.7. Testing of Various Human Motions
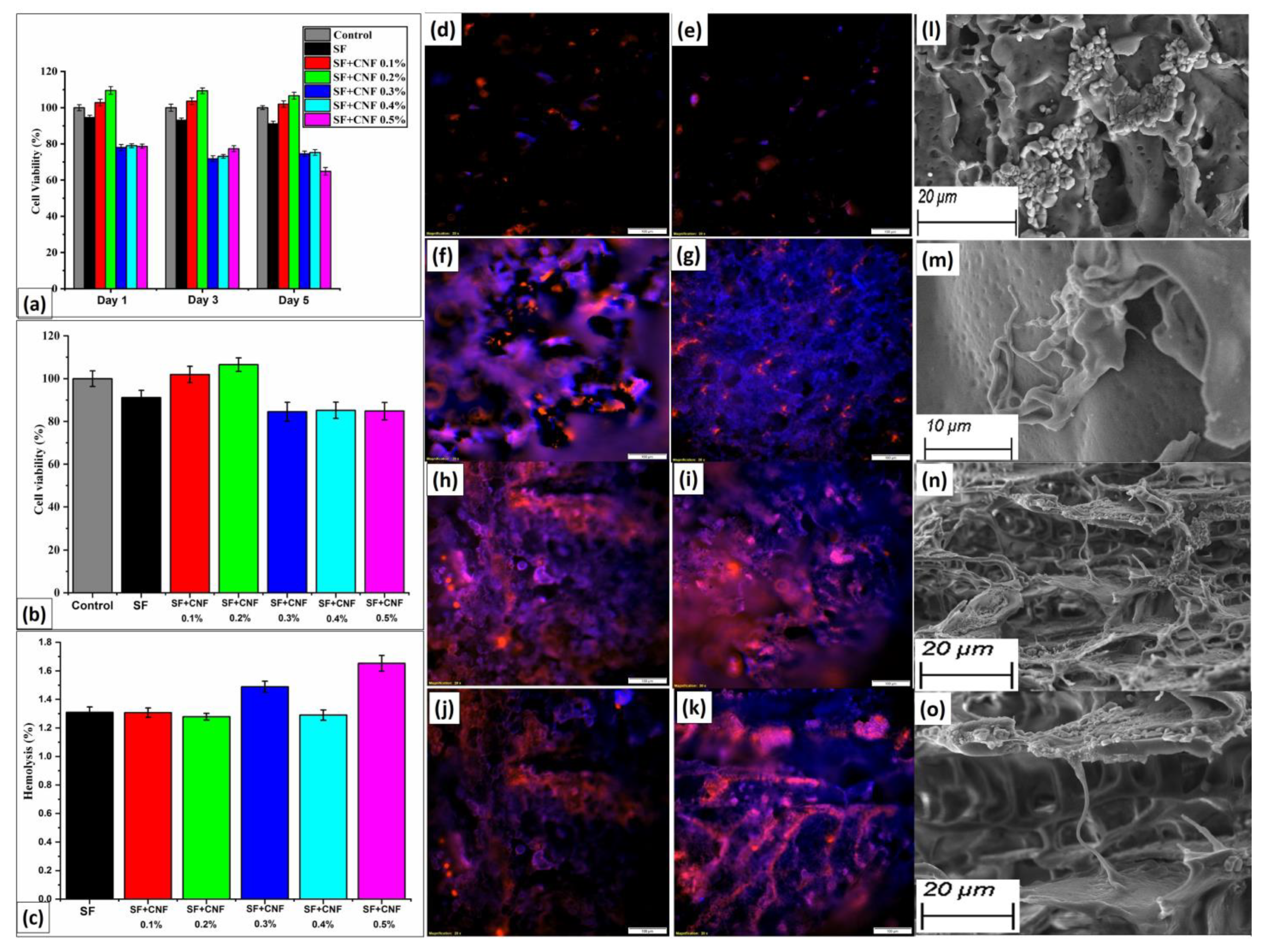
4.8. Cell Viability
4.9. In Vitro Hemolysis Assay
4.10. Visualization of Cytotoxicity Analyses
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SF | Silk Fibroin |
| CNF | Carbon Nanofiber |
| SEM | Scanning Electron Microscope |
| FTIR | Fourier Transform Infrared Spectroscopy |
| PZT | Lead Zirconate Titanate |
| BaTiO3 | Barium Titanate |
| PVDF | Polyvinylidene Fluoride |
| PLA | Polylactic Acid |
| MTT | (3-(4,5-dimethyl thiazolyl-2)-2, 5-diphenyltetrazolium bromide) |
| SKM | Scanning Kelvin Probe Microscopy |
| AFM | Atomic Force Microscope |
| DI | Deionized Water |
| DMA | Dynamic Mechanical Analysis |
| SRF | Solid Rectangular Fixture |
| PEG | Polyethylene Glycol |
References
- Liu, X.; Zhao, C.; Zheng, B.; Guo, Q.; Duan, X.; Wulamu, A.; Zhang, D. Wearable devices for gait analysis in intelligent healthcare. Front. Comput. Sci. 2021, 3, 661676. [Google Scholar] [CrossRef]
- Gonçalves, H.R.; Rodrigues, A.; Santos, C.P. Gait monitoring system for patients with Parkinson’s disease. Expert Syst. Appl. 2021, 185, 115653. [Google Scholar] [CrossRef]
- Ghoussayni, S.; Stevens, C.; Durham, S.; Ewins, D. Assessment and validation of a simple automated method for the detection of gait events and intervals. Gait Posture 2004, 20, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Boulgouris, N.V.; Hatzinakos, D.; Plataniotis, K.N. Gait recognition: A challenging signal processing technology for biometric identification. IEEE Signal Process. Mag. 2005, 22, 78–90. [Google Scholar] [CrossRef]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. J. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry: Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar]
- Casadio, M.; Morasso, P.G.; Sanguineti, V. Direct measurement of ankle stiffness during quiet standing: Implications for control modelling and clinical application. Gait Posture 2005, 21, 410–424. [Google Scholar] [CrossRef]
- Uchino, K. Ferroelectric Devices; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yun, S.; Zhang, Y.; Xu, Q.; Liu, J.; Qin, Y. Recent advance in new-generation integrated devices for energy harvesting and storage. Nano Energy 2019, 60, 600–619. [Google Scholar] [CrossRef]
- Jain, A.; KJ, P.; Sharma, A.K.; Jain, A.; PN, R. Dielectric and piezoelectric properties of PVDF/PZT composites: A review. Polym. Eng. Sci. 2015, 55, 1589–1616. [Google Scholar] [CrossRef]
- Sencadas, V.; Ribeiro, C.; Heredia, A.; Bdikin, I.K.; Kholkin, A.L.; Lanceros-Méndez, S. Local piezoelectric activity of single poly (L-lactic acid) (PLLA) microfibers. Appl. Phys. A 2012, 109, 51–55. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Adhikary, P.; Jana, S.; Biswas, A.; Sencadas, V.; Gupta, S.D.; Mandal, D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy 2017, 36, 166–175. [Google Scholar] [CrossRef]
- Karan, S.K.; Maiti, S.; Kwon, O.; Paria, S.; Maitra, A.; Si, S.K.; Khatua, B.B. Nature driven spider silk as high energy conversion efficient bio-piezoelectric nanogenerator. Nano Energy 2018, 49, 655–666. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Structural origins of silk piezoelectricity. Adv. Funct. Mater. 2011, 21, 779–785. [Google Scholar] [CrossRef]
- Tulachan, B.; Meena, S.K.; Rai, R.K.; Mallick, C.; Kusurkar, T.S.; Teotia, A.K.; Das, M. Electricity from the silk cocoon membrane. Sci. Rep. 2014, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Perez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Mechanical properties of single-brin silkworm silk. J. Appl. Polym. Sci. 2000, 75, 1270–1277. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect silk: One name, many materials. Annu. Rev. Entomol. 2010, 55, 171–188. [Google Scholar] [CrossRef]
- Lotz, B.; Cesari, F.C. The chemical structure and the crystalline structures of Bombyx mori silk fibroin. Biochimie 1979, 61, 205–214. [Google Scholar] [CrossRef]
- Sencadas, V.; Garvey, C.; Mudie, S.; Kirkensgaard, J.J.; Gouadec, G.; Hauser, S. Electroactive properties of electrospun silk fibroin for energy harvesting applications. Nano Energy 2019, 66, 104106. [Google Scholar] [CrossRef]
- Su, M.; Kim, B. Silk fibroin-carbon nanotube composites based fiber substrated wearable triboelectric nanogenerator. ACS Appl. Nano Mater. 2020, 3, 9759–9770. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, W.D.; Jia, J.; Tang, C.Y.; Wang, S.; Yu, P.; Yang, W. Structure-regenerated silk fibroin with boosted piezoelectricity for disposable and biodegradable oral healthcare device. Nano Energy 2022, 103, 107787. [Google Scholar] [CrossRef]
- Kim, K.N.; Chun, J.; Chae, S.A.; Ahn, C.W.; Kim, I.W.; Kim, S.W.; Baik, J.M. Silk fibroin-based biodegradable piezoelectric composite nanogenerators using lead-free ferroelectric nanoparticles. Nano Energy 2015, 14, 87–94. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Jun, K.W.; Kim, J.H.; Seung, W.C.; Kwon, O.H.; Oh, I.K. Silk nanofiber networked bio-triboelectric generator: Silk bio-TEG. Adv. Energy Mater. 2016, 6, 1502329. [Google Scholar] [CrossRef]
- Maiti, S.; Karan, S.K.; Lee, J.; Mishra, A.K.; Khatua, B.B.; Kim, J.K. Bio-waste onion skin as an innovative nature-driven piezoelectric material with high energy conversion efficiency. Nano Energy 2017, 42, 282–293. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sarathi, T.; Venkataraman, K.K.; Bhattacharyya, A. Enhanced piezoelectric properties of polyvinylidene fluoride nanofibers using carbon nanofiber and electrical poling. Mater. Lett. 2019, 255, 126515. [Google Scholar] [CrossRef]
- Pillai, M.M.; Kumar, G.S.; Houshyar, S.; Padhye, R.; Bhattacharyya, A. Effect of nanocomposite coating and biomolecule functionalization on silk fibroin based conducting 3D braided scaffolds for peripheral nerve tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102131. [Google Scholar] [CrossRef]
- Pillai, M.M.; Gopinathan, J.; Senthil Kumar, R.; Sathish Kumar, G.; Shanthakumari, S.; Sahanand, K.S.; Selvakumar, R. Tissue engineering of human knee meniscus using functionalized and reinforced silk-polyvinyl alcohol composite three-dimensional scaffolds: Understanding the in vitro and in vivo behavior. J. Biomed. Mater. Res. Part A 2018, 106, 1722–1731. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Sarathi, T.; Venkataraman, K.K.; Bhattacharyya, A. Piezoresistive nanocomposite films for foot strike data monitoring. Sens. Actuators A Phys. 2018, 284, 76–84. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Balu, R.; Knott, R.; de Campo, L.; Mata, J.P.; Rehm, C.; Choudhury, N.R. Structural evolution of photocrosslinked silk fibroin and silk fibroin-based hybrid hydrogels: A small angle and ultra-small angle scattering investigation. Int. J. Biol. Macromol. 2018, 114, 998–1007. [Google Scholar] [CrossRef]
- Ilki, B.; Petrovska, S.; Sergiienko, R.; Tomai, T.; Shibata, E.; Nakamura, T.; Zaulychnyy, Y. X-ray emission spectra of graphene nanosheets. J. Nanosci. Nanotechnol. 2012, 12, 8913–8919. [Google Scholar] [CrossRef]
- Li, G.; Zhou, P.; Shao, Z.; Xie, X.; Chen, X.; Wang, H.; Yu, T. The natural silk spinning process: A nucleation-dependent aggregation mechanism. Eur. J. Biochem. 2001, 268, 6600–6606. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jang, J. Poly (vinylidene fluoride)/NH2-treated graphene nanodot/reduced graphene oxide nanocomposites with enhanced dielectric performance for ultrahigh energy density capacitor. ACS Appl. Mater. Interfaces 2015, 7, 9668–9681. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Q.; Wu, R.; Meng, Z.; Patil, A.; Yu, R.; Liu, X.Y. From molecular reconstruction of mesoscopic functional conductive silk fibrous materials to remote respiration monitoring. Small 2020, 16, e2000203. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, H.; Yu, P.; Wang, H.; Liu, J. Enhancement of adhesion, mechanical strength and anti-corrosion by multilayer superhydrophobic coating embedded electroactive PANI/CNF nanocomposite. J. Polym. Res. 2018, 25, 151. [Google Scholar] [CrossRef]




| Material | Piezoelectricity (V) | Resistance (kΩ) | Thickness (mm) |
|---|---|---|---|
| SF | 0.1 ± 0.01 | 293 | 3.07 ± 0.14 |
| SF with CNFs densities equal to 0.1% | 1.2 ± 0.18 | 164 | 3.25 ± 0.19 |
| SF with CNFs densities equal to 0.2% | 1.87 ± 0.22 | 133 | 3.42 ± 0.13 |
| SF with CNFs densities equal to 0.3% | 1.92 ± 0.32 | 119 | 3.63 ± 0.15 |
| SF with CNFs densities equal to 0.4% | 2.2 ± 0.12 | 88 | 3.75 ± 0.11 |
| SF with CNFs densities equal to 0.5% | 2.01 ± 0.20 | 57 | 3.92 ± 0.12 |
| Samples | Piezovoltage (V) after 500 Test Cycles | Compressive Recovery Rate after 500 Test Cycles for 80 N Load (%) | ||
|---|---|---|---|---|
| Load | ||||
| 40 N | 60 N | 80 N | ||
| SF | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 95.1 ± 0.24 |
| SF with CNF densities equal to 0.1% | 0.45 ± 0.22 | 0.91 ± 0.18 | 1.23 ± 0.17 | 97.2 ± 0.26 |
| SF with CNF densities equal to 0.2% | 1.03 ± 0.20 | 1.42 ± 0.22 | 1.87 ± 0.12 | 97.7 ± 0.18 |
| SF with CNF densities equal to 0.3% | 1.05 ± 0.12 | 1.43 ± 0.21 | 1.91 ± 0.26 | 98.6 ± 0.19 |
| SF with CNF densities equal to 0.4% | 1.77 ± 0.18 | 2.03 ± 0.19 | 2.25 ± 0.24 | 99.1 ± 0.15 |
| SF with CNF densities equal to 0.5% | 1.54 ± 0.15 | 1.72 ± 0.20 | 2.01 ± 0.19 | 99.4 ± 0.18 |
| Volunteer Characteristics | Volunteer 1 | Volunteer 2 | Volunteer 3 |
|---|---|---|---|
| Age | 26 | 31 | 28 |
| Weight (kg) | 55 | 89 | 71 |
| Height (cm) | 148 | 159 | 151 |
| Gender | Male | Male | Female |
| Motion movements | Voltage (V) | ||
| Walking | 2.34 ± 0.11 | 2.51 ± 0.12 | 2.41 ± 0.11 |
| Jogging | 2.21 ± 0.19 | 2.42 ± 0.21 | 2.39 ± 0.21 |
| Running | 2.42 ± 0.05 | 2.72 ± 0.14 | 2.71 ± 0.12 |
| Marching | 2.12 ± 0.09 | 2.45 ± 0.04 | 2.41 ± 0.14 |
| Jumping | 2.65 ± 0.12 | 2.95 ± 0.03 | 2.87 ± 0.12 |
| Squatting | 2.62 ± 0.16 | 2.88 ± 0.09 | 2.91 ± 0.15 |
| Tapping | 1.11 ± 0.09 | 1.86 ± 0.09 | 2.10 ± 0.19 |
| Standing | 2.55 ± 0.08 | 2.87 ± 0.09 | 2.71 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathinasamy, S.K.; Maheswar, R.; Lorincz, J. Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications. Sensors 2023, 23, 1373. https://doi.org/10.3390/s23031373
Rathinasamy SK, Maheswar R, Lorincz J. Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications. Sensors. 2023; 23(3):1373. https://doi.org/10.3390/s23031373
Chicago/Turabian StyleRathinasamy, Senthil Kumar, Rajagopal Maheswar, and Josip Lorincz. 2023. "Silk Fibroin-Based Piezoelectric Sensor with Carbon Nanofibers for Wearable Health Monitoring Applications" Sensors 23, no. 3: 1373. https://doi.org/10.3390/s23031373






