Novel Corrugated Long Period Grating Surface Balloon-Shaped Heterocore-Structured Plastic Optical Fibre Sensor for Microalgal Bioethanol Production
Abstract
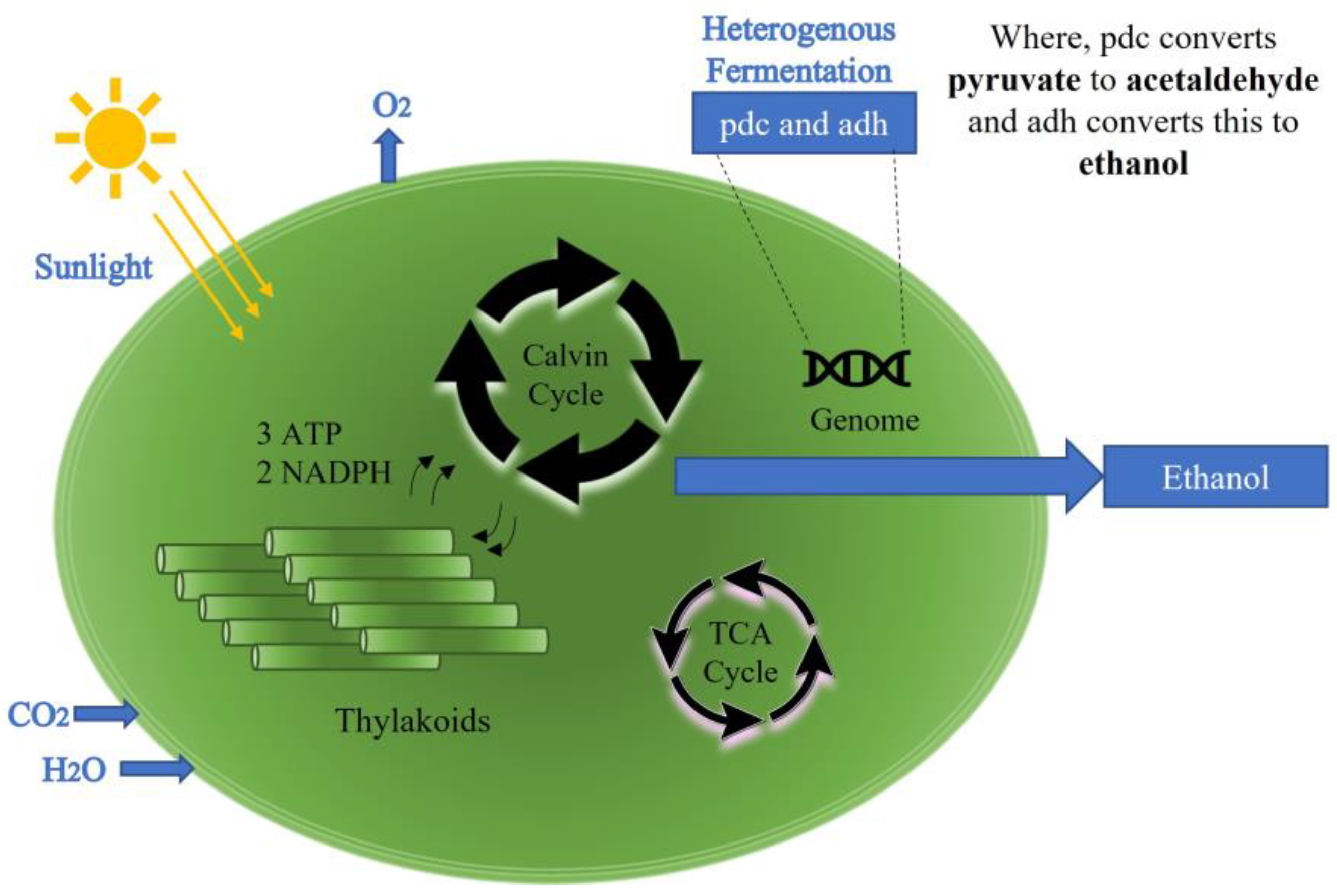
1. Introduction
2. Sensing Mechanism
3. Materials and Methods
3.1. Materials
- To make the BG11 solution, add 10 mL of concentrated BG11 Broth to 990 mL of distilled water and mix well.
- Divide the prepared solution in half and add the freeze-dried Synechocystis concentrate to one half. Reserve half of the BG11 solution to adjust the concentration of the microalgae mixture.
- Shake well to dissolve.
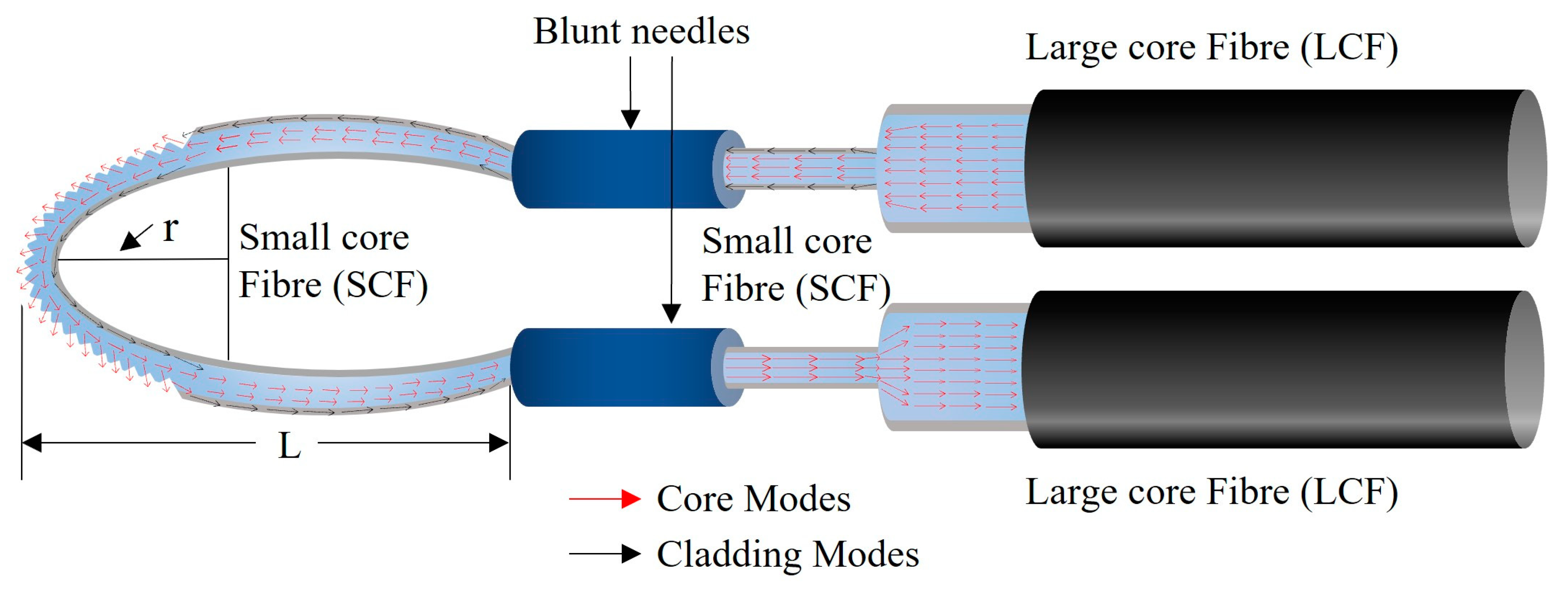
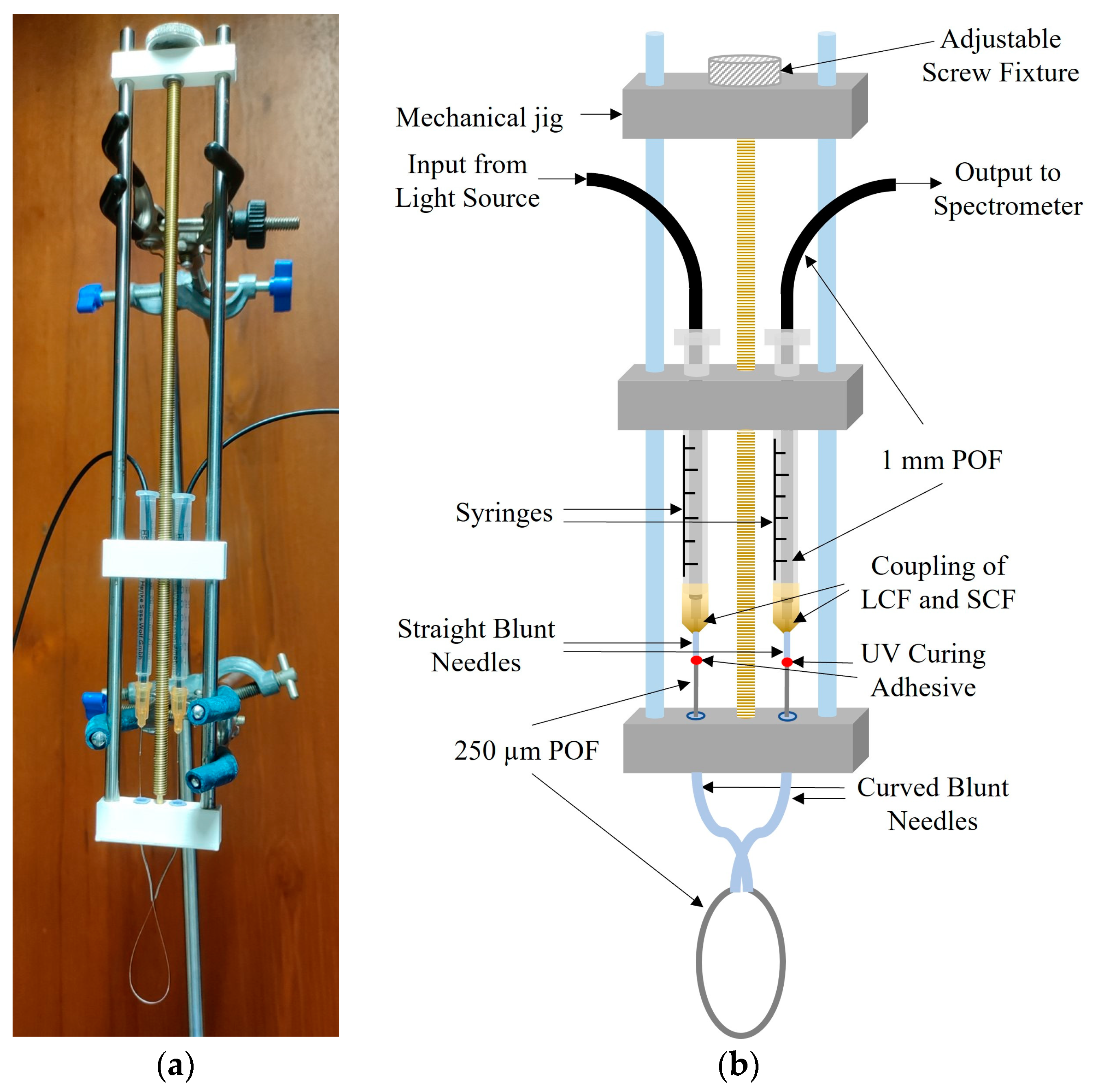
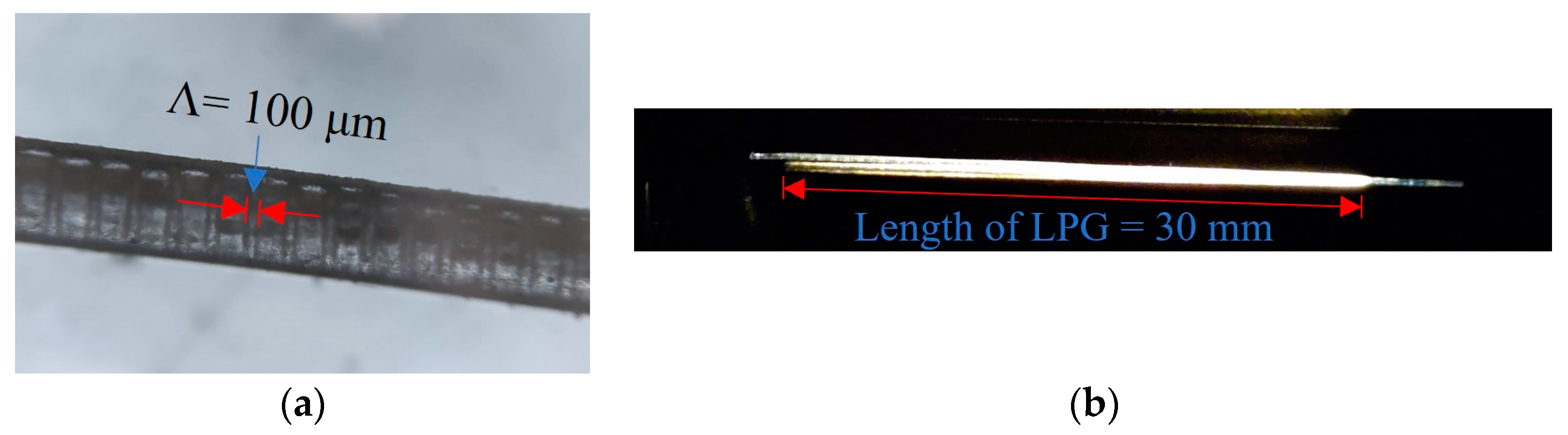
3.2. Sensor Fabrication
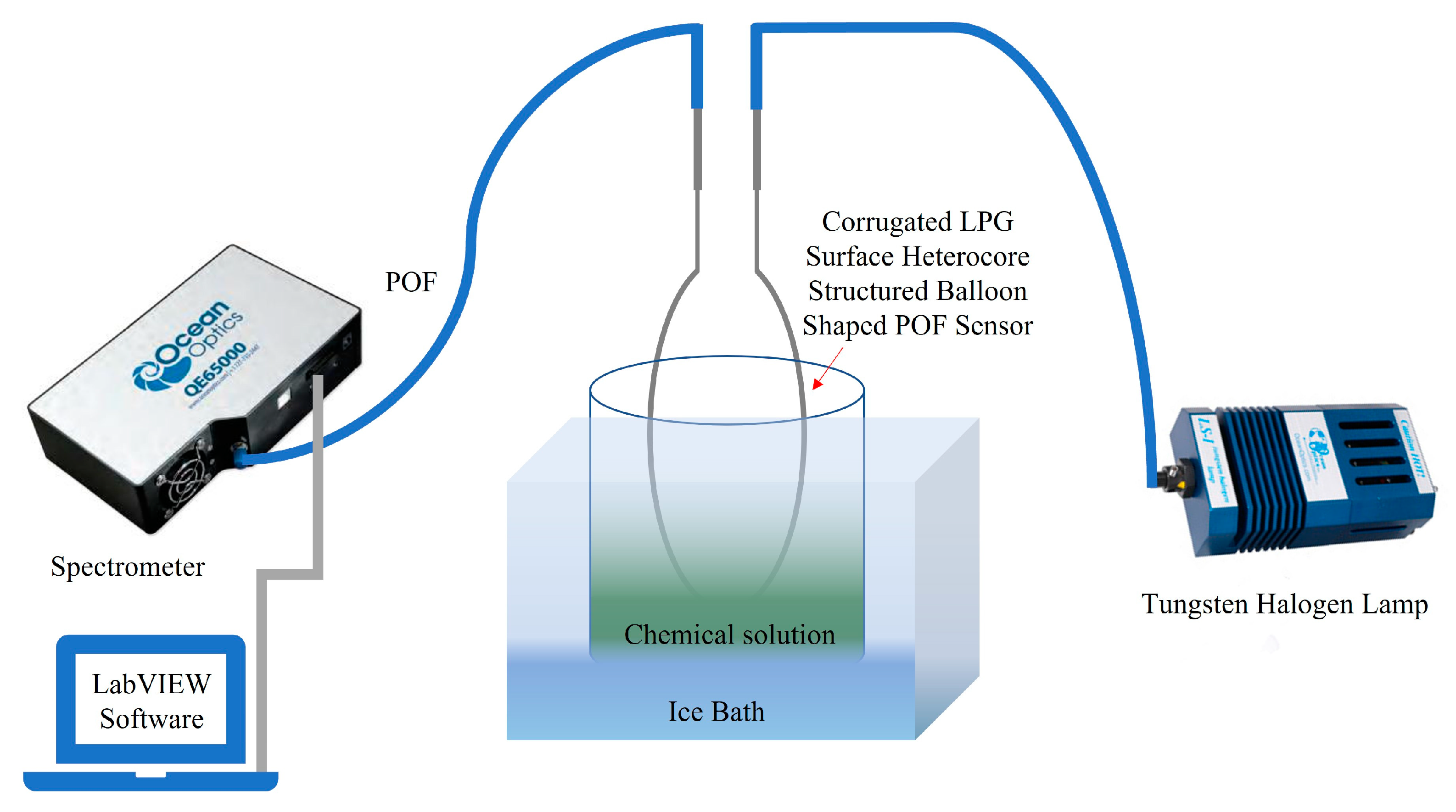
3.3. Experimental Setup and Sensor Calibration
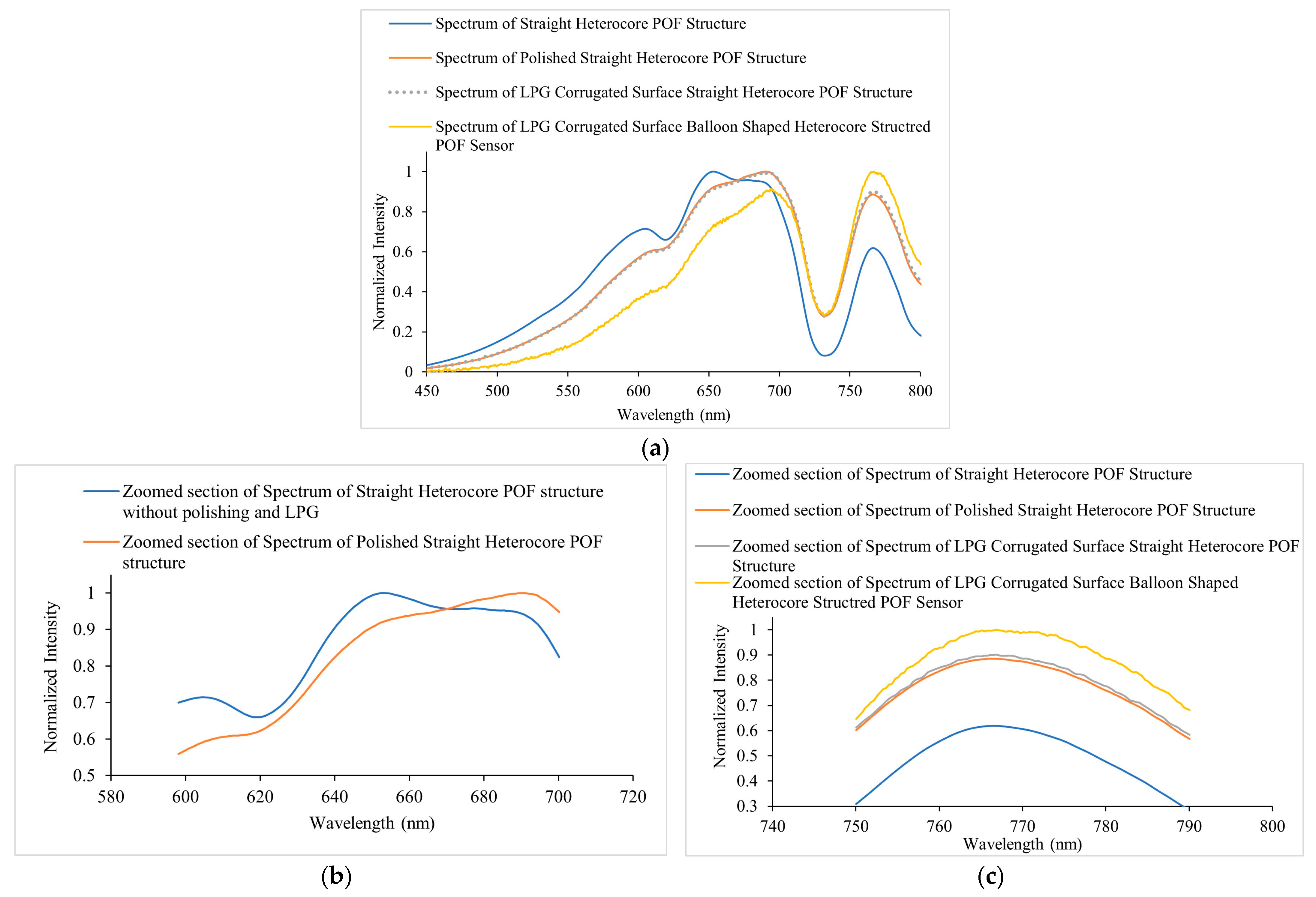
4. Experimental Results
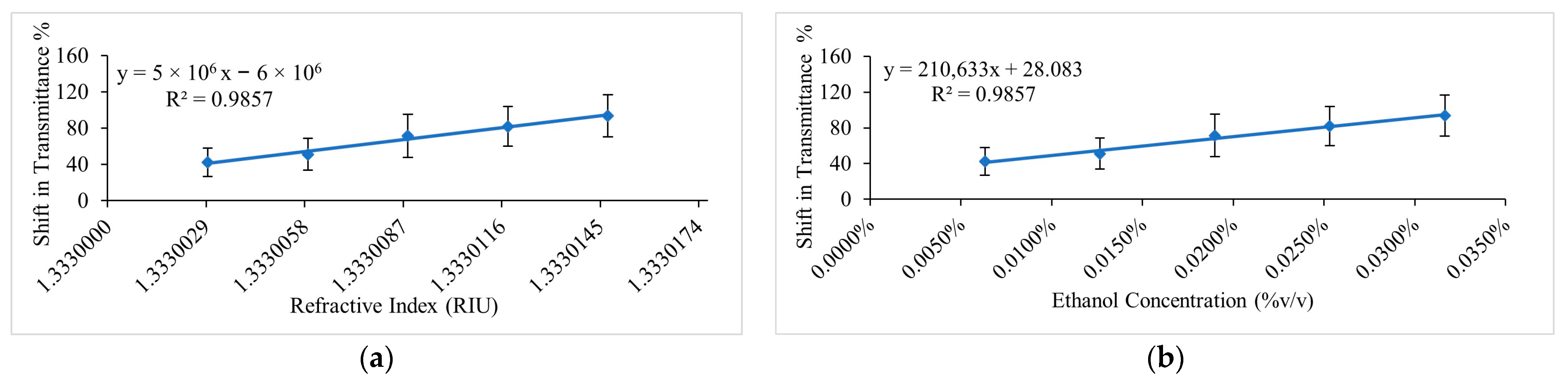
4.1. Analysis of Ethanol Sensing in Water
4.2. Analysis of Ethanol Sensing in Ethanol-Producing Microalgal Mixtures
- Variation in microalgae concentration that occurs with the changing phases of the life cycle.
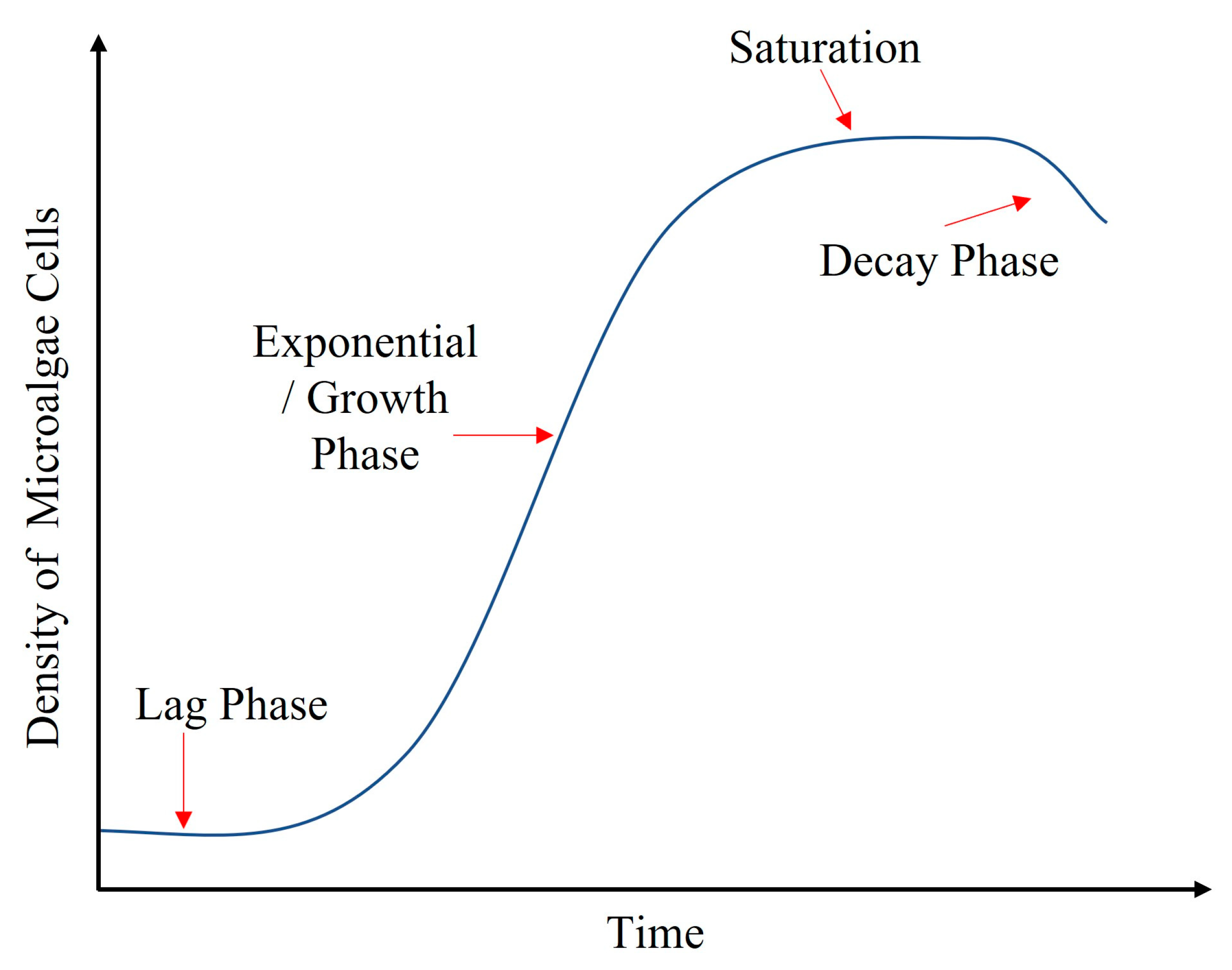
- The life cycle of microalgae cells in the mixture is explained in Figure 12 and is a change in the density of microalgae cells where:
- a.
- The lag phase is when the organisms adapt to the mixture’s environment and surroundings, i.e., pH, temperature, nutrients, and light.
- b.
- The exponential/growth stage is the phase of maximum growth where maximum cell division occurs, increasing the green pigment in the mixture.
- c.
- The death/decay phase is when microalgae cells start dying off and cause a change in the optimal pH of the mixture.
- Excreted products from the microalgae cells.
- The amount of light-harvesting pigments produced during growth.
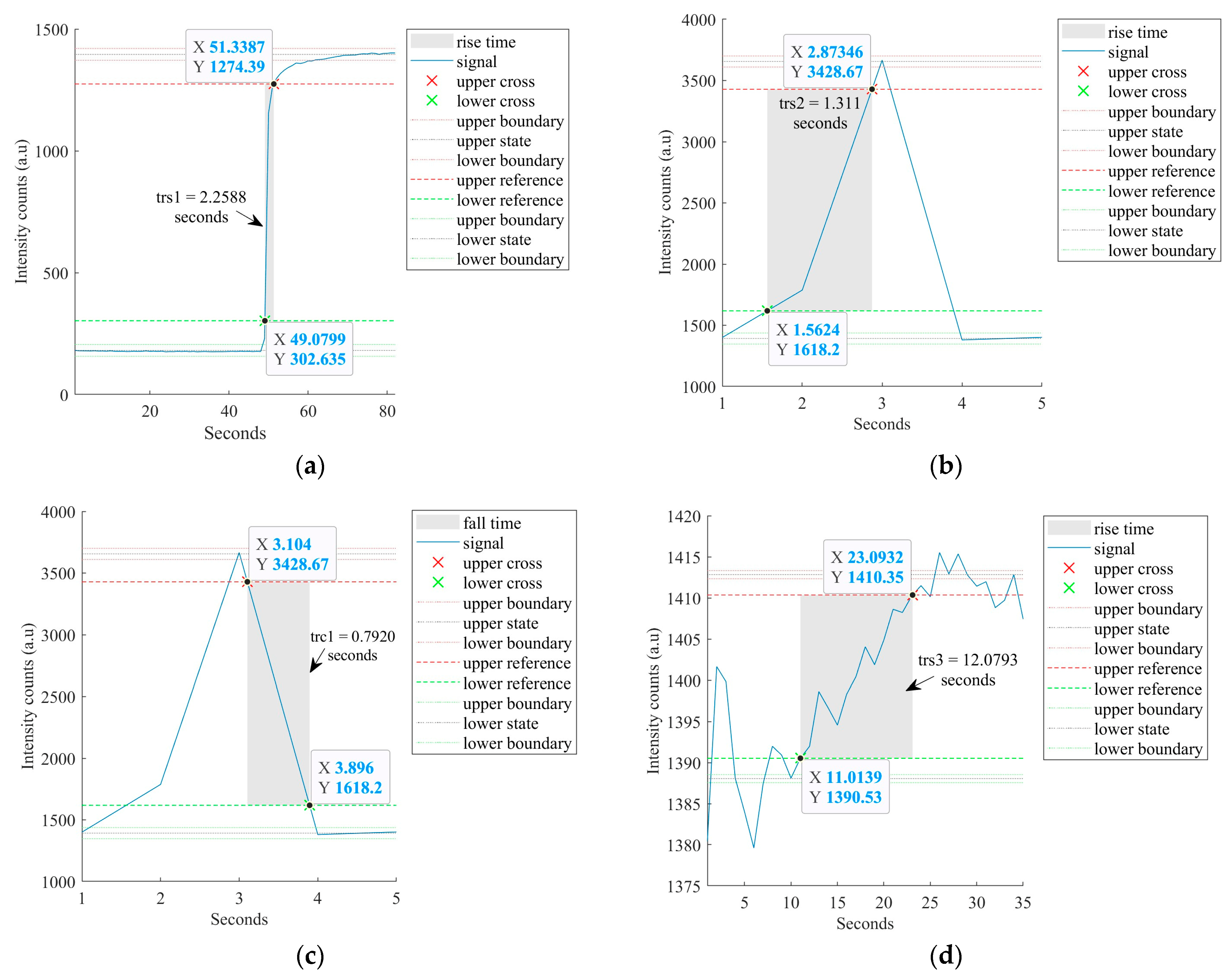
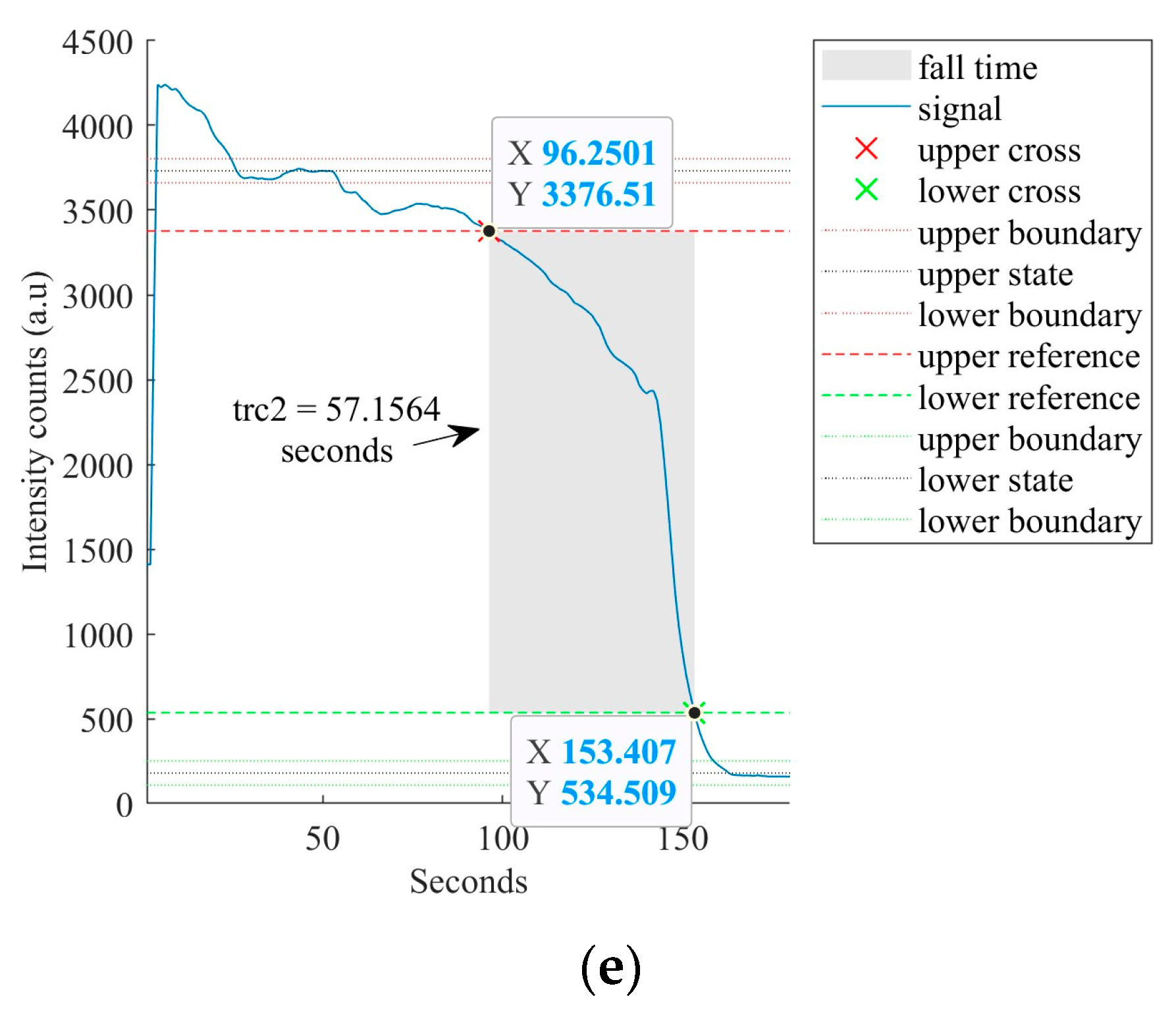
4.3. Sensor’s Response and Recovery Time Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harun, R.; Yip, J.W.S.; Thiruvenkadam, S.; Ghani, W.A.W.A.K.; Cherrington, T.; Danquah, M.K. Algal Biomass Conversion to Bioethanol–A Step-by-Step Assessment. Biotechnol. J. 2014, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental Sustainability of Biofuels: A Review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Godbole, V.; Pal, M.K.; Gautam, P. A Critical Perspective on the Scope of Interdisciplinary Approaches Used in Fourth-Generation Biofuel Production. Algal Res. 2021, 58, 102436. [Google Scholar] [CrossRef]
- Dexter, J.; Armshaw, P.; Sheahan, C.; Pembroke, J.T. The State of Autotrophic Ethanol Production in Cyanobacteria. J. Appl. Microbiol. 2015, 119, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Pembroke, J.T.; Quinn, L.; O’Riordan, H.; Sheahan, C.; Armshaw, P. Ethanol Production in Cyanobacteria: Impact of Omics of the Model Organism Synechocystis on Yield Enhancement. In Cyanobacteria: Omics and Manipulation; Caister Academic Press: Poole, UK, 2017; pp. 199–217. ISBN 978-1-910190-55-5. [Google Scholar]
- Lopes da Silva, T.; Passarinho, P.C.; Galriça, R.; Zenóglio, A.; Armshaw, P.; Pembroke, J.T.; Sheahan, C.; Reis, A.; Gírio, F. Evaluation of the Ethanol Tolerance for Wild and Mutant Synechocystis Strains by Flow Cytometry. Biotechnol. Rep. 2018, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Moura, P.; Reis, A.; Oliveira, C.; Gírio, F. Microalgae for Biofuels: The Portuguese Experience. Watch Letter 2015, 32, 1–10. [Google Scholar]
- Numata, Y.; Shinohara, Y.; Tanaka, H. Quantitative Analysis of Ethanol–Methanol–Water Ternary Solutions Using Raman Spectroscopy. Spectrosc. Lett. 2019, 52, 306–311. [Google Scholar] [CrossRef]
- Fernanbes, E.N.; Reis, B.F. Automatic Flow Procedure for the Determination of Ethanol in Wine Exploiting Multicommutation and Enzymatic Reaction with Detection by Chemiluminescence. J. AOAC Int. 2004, 87, 920–926. [Google Scholar] [CrossRef]
- Castritius, S.; Kron, A.; Schäfer, T.; Rädle, M.; Harms, D. Determination of Alcohol and Extract Concentration in Beer Samples Using a Combined Method of Near-Infrared (NIR) Spectroscopy and Refractometry. J. Agric. Food Chem. 2010, 58, 12634–12641. [Google Scholar] [CrossRef]
- Seo, H.B.; Kim, H.J.; Lee, O.K.; Ha, J.H.; Lee, H.Y.; Jung, K.H. Measurement of Ethanol Concentration Using Solvent Extraction and Dichromate Oxidation and Its Application to Bioethanol Production Process. J. Ind. Microbiol. Biotechnol. 2009, 36, 285–292. [Google Scholar] [CrossRef]
- Castellari, M.; Sartini, E.; Spinabelli, U.; Riponi, C.; Galassi, S. Determination of Carboxylic Acids, Carbohydrates, Glycerol, Ethanol, and 5-HMF in Beer by High-Performance Liquid Chromatography and UV-Refractive Index Double Detection. J. Chromatogr. Sci. 2001, 39, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Choong, Y.M.; Su, N.W.; Lee, M.H. A Rapid Method for Determination of Ethanol in Alcoholic Beverages Using Capillary Gas Chromatography. J. Food Drug Anal. 2003, 11, 133–140. [Google Scholar] [CrossRef]
- Weatherly, C.A.; Woods, R.M.; Armstrong, D.W. Rapid Analysis of Ethanol and Water in Commercial Products Using Ionic Liquid Capillary Gas Chromatography with Thermal Conductivity Detection and/or Barrier Discharge Ionization Detection. J. Agric. Food Chem. 2014, 62, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.S.; Miller, C.A.; Altria, K.D.; Waterhouse, A.L. Development of a Rapid Method for the Analysis of Ethanol in Wines Using Capillary Electrophoresis. Am. J. Enol. Vitic. 1997, 48, 280–284. [Google Scholar] [CrossRef]
- Brereton, P.; Hasnip, S.; Bertrand, A.; Wittkowski, R.; Guillou, C. Analytical Methods for the Determination of Spirit Drinks. TrAC-Trends Anal. Chem. 2003, 22, 19–25. [Google Scholar] [CrossRef]
- Sumbhate, S.; Sumbhate, S.V.; Nayak, S.; Goupale, D.; Tiwari, A.; Jadon, R.S. Colorimetric Method for the Estimation of Ethanol in Alcoholic-Drinks. J. Anal. Technol. 2012, 1, 1–6. [Google Scholar]
- Memon, S.F.; Wang, R.; Strunz, B.; Chowdhry, B.S.; Pembroke, J.T.; Lewis, E. A Review of Optical Fibre Ethanol Sensors: Current State and Future Prospects. Sensors 2022, 22, 950. [Google Scholar] [CrossRef]
- Saad, H.; Rahman, M.K.A.; Ali, M.T. Tapered Plastic Optical Fiber Sensor for Detection of Ethanol Concentration in H2O. In Proceedings of the International Conference on Sensing Technology, ICST 2013, Wellington, New Zealand, 3–5 December 2013; pp. 559–564. [Google Scholar] [CrossRef]
- Memon, S.F.; Lewis, E.; Ali, M.M.; Pembroke, J.T.; Chowdhry, B.S. U-Bend Evanescent Wave Plastic Optical Fibre Sensor for Minute Level Concentration Detection of Ethanol Corresponding to Biofuel Production Rate. In Proceedings of the 2017 IEEE Sensors Applications Symposium, Glassboro, NJ, USA, 13–15 March 2017. [Google Scholar]
- Memon, S.F.; Ali, M.M.; Pembroke, J.T.; Chowdhry, B.S.; Lewis, E. Measurement of Ultralow Level Bioethanol Concentration for Production Using Evanescent Wave Based Optical Fiber Sensor. IEEE Trans. Instrum. Meas. 2017, 67, 780–788. [Google Scholar] [CrossRef]
- Khalaf, A.L.; Arasu, P.T.; Lim, H.N.; Paiman, S.; Yusof, N.A.; Mahdi, M.A.; Yaacob, M.H. Modified Plastic Optical Fiber with CNT and Graphene Oxide Nanostructured Coatings for Ethanol Liquid Sensing. Opt. Express 2017, 25, 5509–5520. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Z.; Zhang, C.; Gao, S.; Li, Z.; Qiu, H.; Li, C.; Yang, C.; Liu, M.; Liu, Y. A Novel U-Bent Plastic Optical Fibre Local Surface Plasmon Resonance Sensor Based on a Graphene and Silver Nanoparticle Hybrid Structure. J. Phys. D Appl. Phys. 2017, 50, 165105. [Google Scholar] [CrossRef]
- Xi, X.; Xu, J.; Li, S.; Song, J.; Yang, W.; Sun, Y.; Jiang, S.; Han, Y.; Fan, X. An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis. Sensors 2020, 20, 991. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yu, F.; Cao, Y.; Zheng, J. Refractive Index Sensing Based on a Long Period Grating Imprinted on a Multimode Plastic Optical Fiber. IEEE Sens. J. 2019, 19, 7434–7439. [Google Scholar] [CrossRef]
- Teng, C.; Yu, F.; Zheng, J.; Jing, N.; Ding, Y. Refractive Index Sensor Based on a Multi-Notched Plastic Optical Fiber. Appl. Opt. 2017, 56, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.; Brambilla, G.; Ding, M.; Wang, P.; Wu, Q.; Semenova, Y. Investigation of Single-Mode-Multimode-Single-Mode and Single-Mode-Tapered-Multimode-Single-Mode Fiber Structures and Their Application for Refractive Index Sensing. JOSA B 2011, 28, 1180–1186. [Google Scholar] [CrossRef]
- Marfu’Ah; Amalia, N.R.; Hatta, A.M.; Pratama, D.Y. Multimode-Singlemode-Multimode Optical Fiber Sensor Coated with Novolac Resin for Detecting Liquid Phase Alcohol. AIP Conf. Proc. 2018, 1945, 020031. [Google Scholar]
- Zhu, W.; Huang, Q.; Wang, Y.; Lewis, E.; Yang, M. Enhanced Sensitivity of Heterocore Structure Surface Plasmon Resonance Sensors Based on Local Microstructures. Opt. Eng. 2018, 57, 076105. [Google Scholar] [CrossRef]
- Fernandez, L.; Yan, J.; Fonollosa, J.; Burgués, J.; Gutierrez, A.; Marco, S. A Practical Method to Estimate the Resolving Power of a Chemical Sensor Array: Application to Feature Selection. Front. Chem. 2018, 6, 209. [Google Scholar] [CrossRef]
- Bao, H.; Wu, B.; Liu, Y.; Zhou, Y.; Zheng, J. Investigation of Long Period Grating Imprinted on a Plastic Optical Fiber for Liquid Level Sensing. Opt.-Int. J. Light Electron Opt. 2022, 251, 168367. [Google Scholar] [CrossRef]

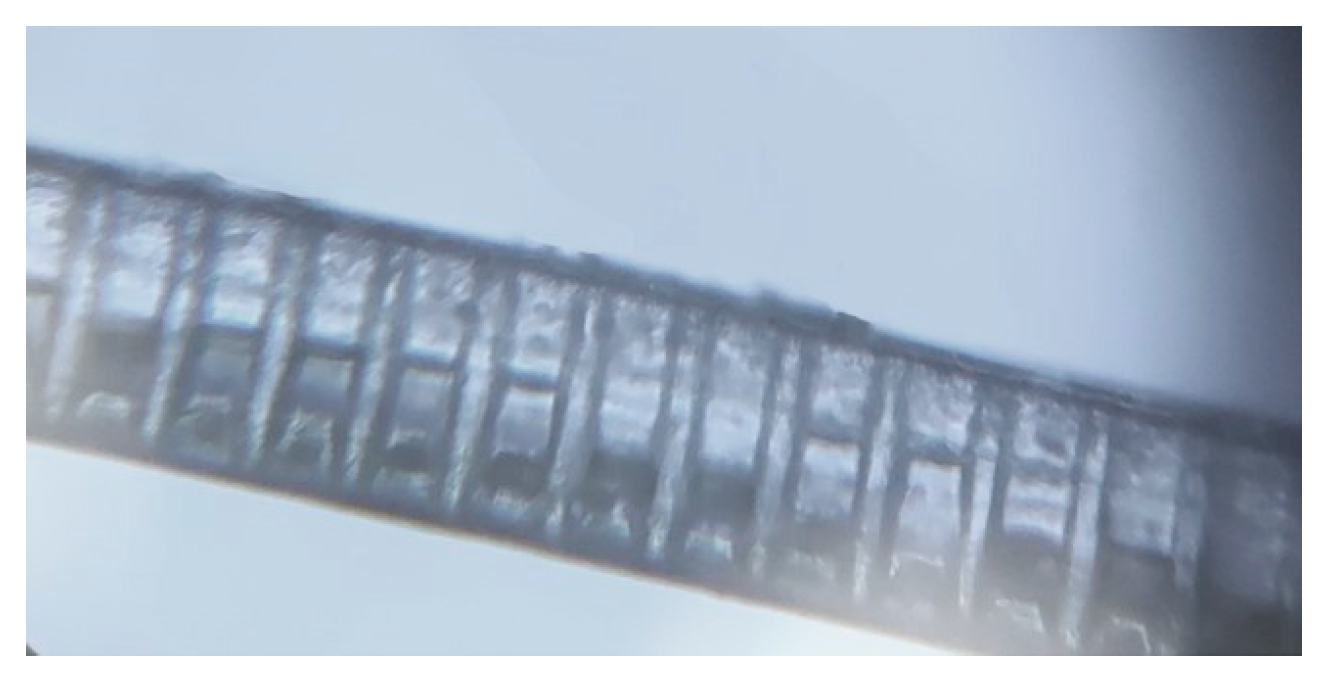



| Fibre Type | Diameter | Thickness of Cladding | RI of Core | RI of Cladding |
|---|---|---|---|---|
| DB-250 | 250 µm | - | 1.492 | 1.402 |
| DC-1000 | 1000 µm | 10 µm | 1.492 | 1.402 |
| Ethanol in %Volume/Volume (%v/v) | Refractive Index | Change in Refractive Index (Δn) |
|---|---|---|
| 0 | 1.333 | 0 |
| 0.00633 | 1.33300294673272 | 0.0000029 |
| 0.01266 | 1.33300589267715 | 0.0000058 |
| 0.01899 | 1.33300883783337 | 0.0000088 |
| 0.02532 | 1.33301178220152 | 0.0000117 |
| 0.03165 | 1.33301472578170 | 0.0000147 |
| 0.03798 | 1.33301766857403 | 0.0000176 |
| 0.04431 | 1.33302061057863 | 0.0000206 |
| 0.05064 | 1.33302355179560 | 0.0000235 |
| 0.05697 | 1.33302649222506 | 0.0000264 |
| 0.0633 | 1.33302943186713 | 0.0000294 |
| 0.1 | 1.33304949718124 | 0.0000494 |
| 0.2 | 1.33309900807336 | 0.0000990 |
| 0.3 | 1.33314853206077 | 0.0001485 |
| 0.4 | 1.33319806852963 | 0.0001980 |
| 0.5 | 1.33325129792262 | 0.0002512 |
| 0.6 | 1.33330085869122 | 0.0003008 |
| 0.7 | 1.33335043011084 | 0.0003504 |
| 0.8 | 1.33340011477828 | 0.0004001 |
| 0.9 | 1.33345339125821 | 0.0004533 |
| 1.0 | 1.33350299199426 | 0.0005029 |
| 2.0 | 1.33401115772126 | 0.0010111 |
| 3.0 | 1.33453202665564 | 0.0015320 |
| 4.0 | 1.33506565132369 | 0.0020656 |
| 5.0 | 1.33561589378843 | 0.0026158 |
| 6.0 | 1.33618289103629 | 0.0031828 |
| 7.0 | 1.33677060634193 | 0.0037706 |
| 8.0 | 1.33736793591301 | 0.0043679 |
| 9.0 | 1.33797864363640 | 0.0049786 |
| 10 | 1.33860298681633 | 0.0056029 |
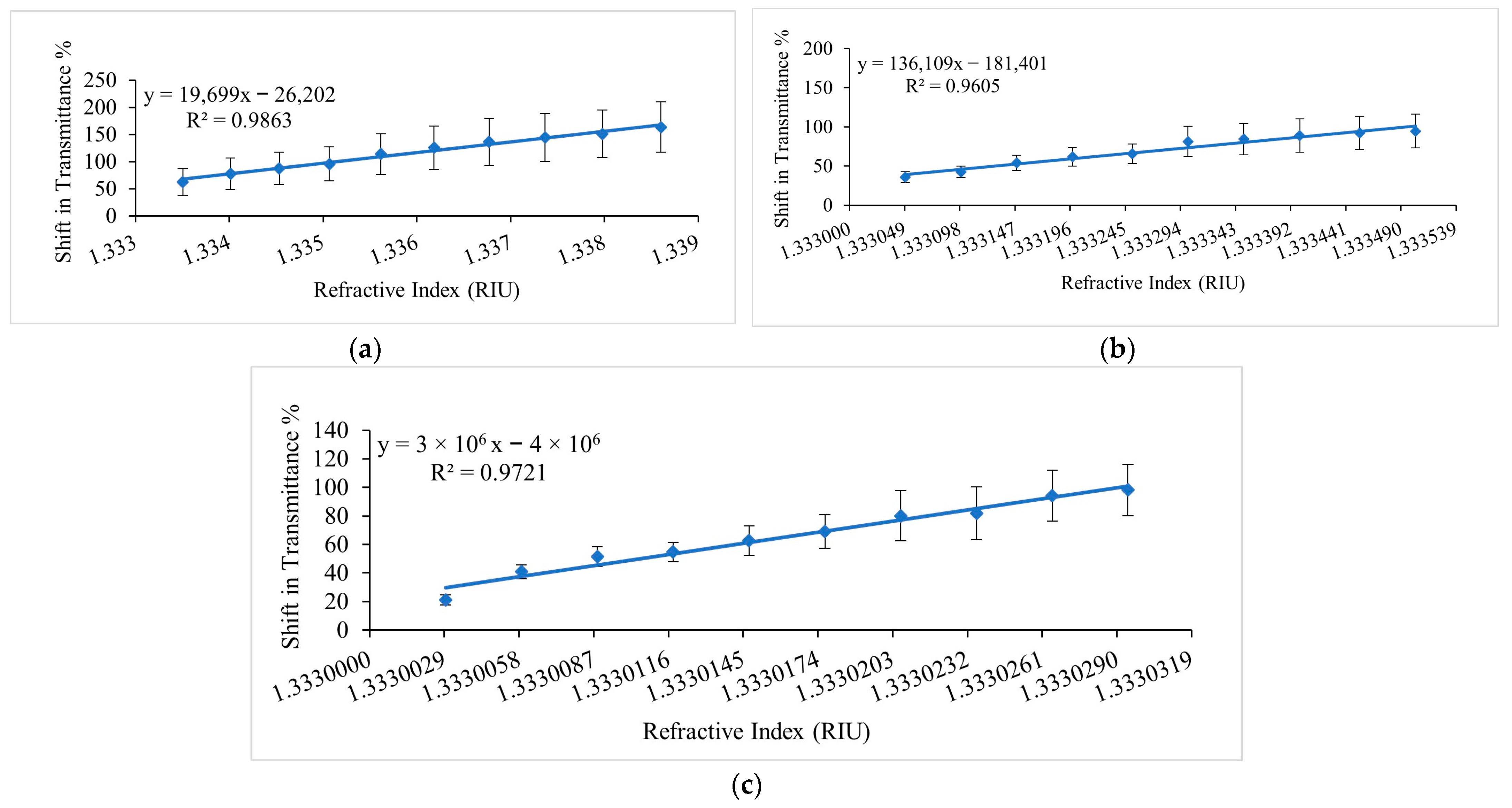
| Chemical Solution | Ethanol Concentration (%v/v) or RI Values | Sensitivity | Resolution | LOD |
|---|---|---|---|---|
| Ethanol–Water Solutions | 1 to 10 % v/v or 1.3335 to 1.3386 | 19,699 %/RIU | 7.9 × 10−6 RIU | 9.7 × 10−6 RIU |
| 0.1 to 1 %v/v or 1.333049 to 1.3335 | 131,609 %/RIU | |||
| 0.00633 % v/v to 0.0633 %v/v or 1.3330029 to 1.333029 | 3 × 106 %/RIU | |||
| Ethanol–Microalgae Mixtures | 0.00633 to 0.03165 %v/v or 1.3330029 to 1.3330147 (as a reference from ethanol-water solutions) | 210,632.8 %T/%v/v and 5 × 106 %/RIU | 2 × 10−4 %v/v and 9.4 × 10−6 RIU | 4.9 × 10−4 %v/v and 2.3 × 10−5 RIU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, S.F.; Wang, R.; Strunz, B.; Chowdhry, B.S.; Pembroke, J.T.; Lewis, E. Novel Corrugated Long Period Grating Surface Balloon-Shaped Heterocore-Structured Plastic Optical Fibre Sensor for Microalgal Bioethanol Production. Sensors 2023, 23, 1644. https://doi.org/10.3390/s23031644
Memon SF, Wang R, Strunz B, Chowdhry BS, Pembroke JT, Lewis E. Novel Corrugated Long Period Grating Surface Balloon-Shaped Heterocore-Structured Plastic Optical Fibre Sensor for Microalgal Bioethanol Production. Sensors. 2023; 23(3):1644. https://doi.org/10.3390/s23031644
Chicago/Turabian StyleMemon, Sanober Farheen, Ruoning Wang, Bob Strunz, Bhawani Shankar Chowdhry, J. Tony Pembroke, and Elfed Lewis. 2023. "Novel Corrugated Long Period Grating Surface Balloon-Shaped Heterocore-Structured Plastic Optical Fibre Sensor for Microalgal Bioethanol Production" Sensors 23, no. 3: 1644. https://doi.org/10.3390/s23031644
APA StyleMemon, S. F., Wang, R., Strunz, B., Chowdhry, B. S., Pembroke, J. T., & Lewis, E. (2023). Novel Corrugated Long Period Grating Surface Balloon-Shaped Heterocore-Structured Plastic Optical Fibre Sensor for Microalgal Bioethanol Production. Sensors, 23(3), 1644. https://doi.org/10.3390/s23031644








