Exploring Coumarin-Based Boron Emissive Complexes as Temperature Thermometers in Polymer-Supported Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Starting Materials
2.2. Instrumentation
2.3. Synthetic Procedures
Synthesis of Compounds L1, L2 and L3
2.4. Photophysical Characterization
2.5. Kamlet–Taft Parameters
2.6. DFT Calculations
2.7. Preparation of Coumarin-Based Polymer Films
2.8. Temperature Variation Studies
3. Results and Discussion
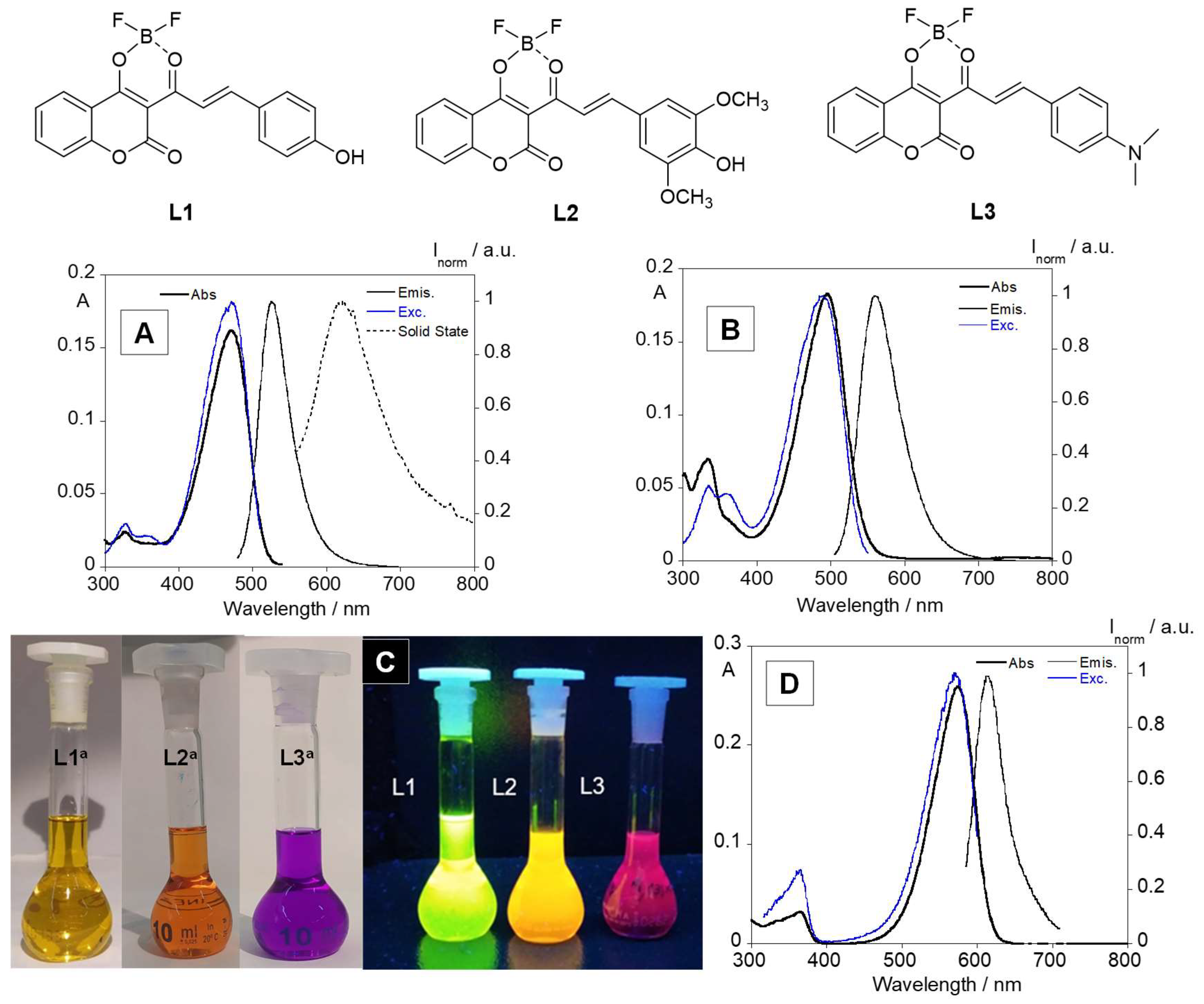
3.1. Photophysical Characterization
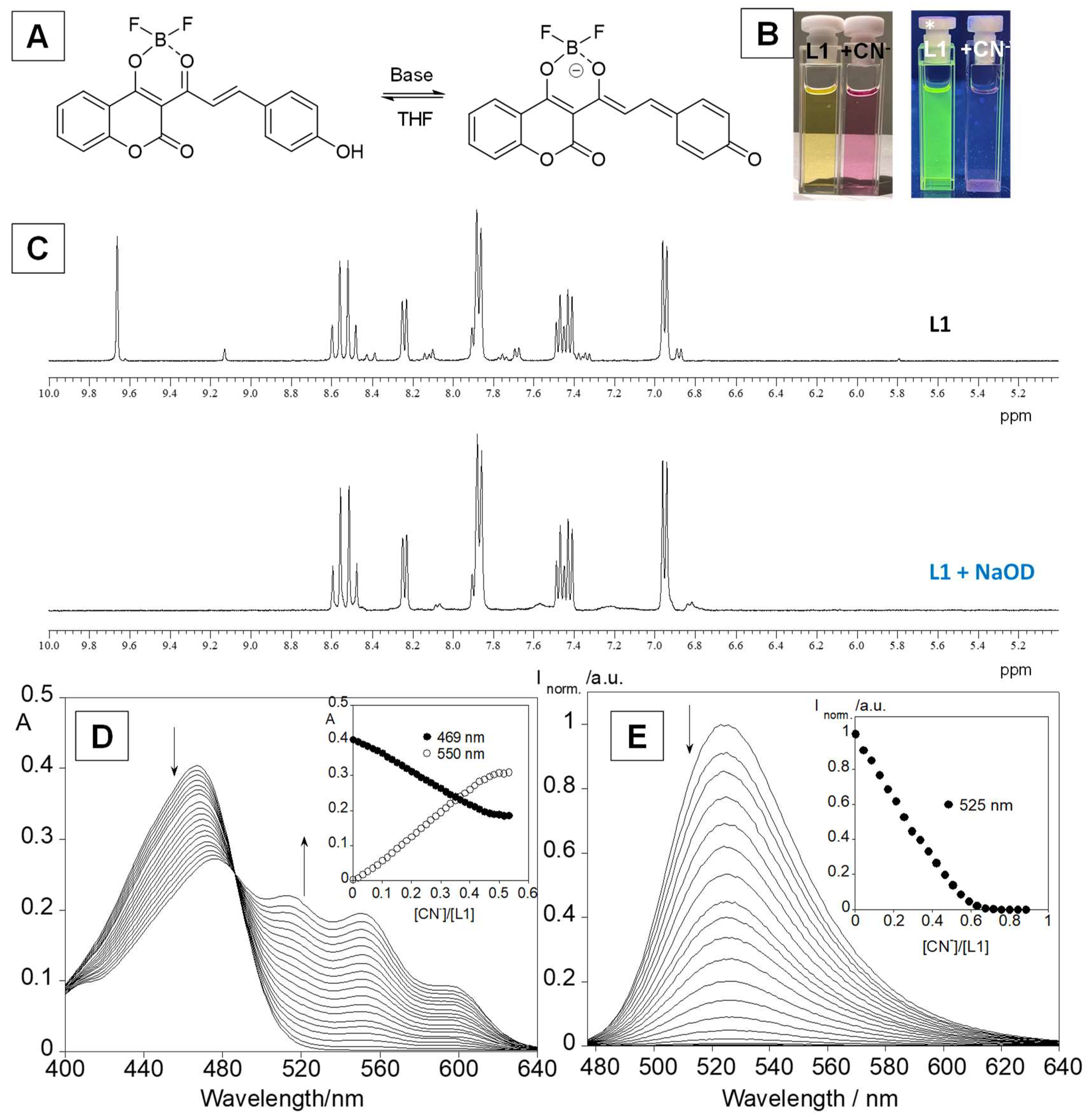
3.2. Anion Sensing
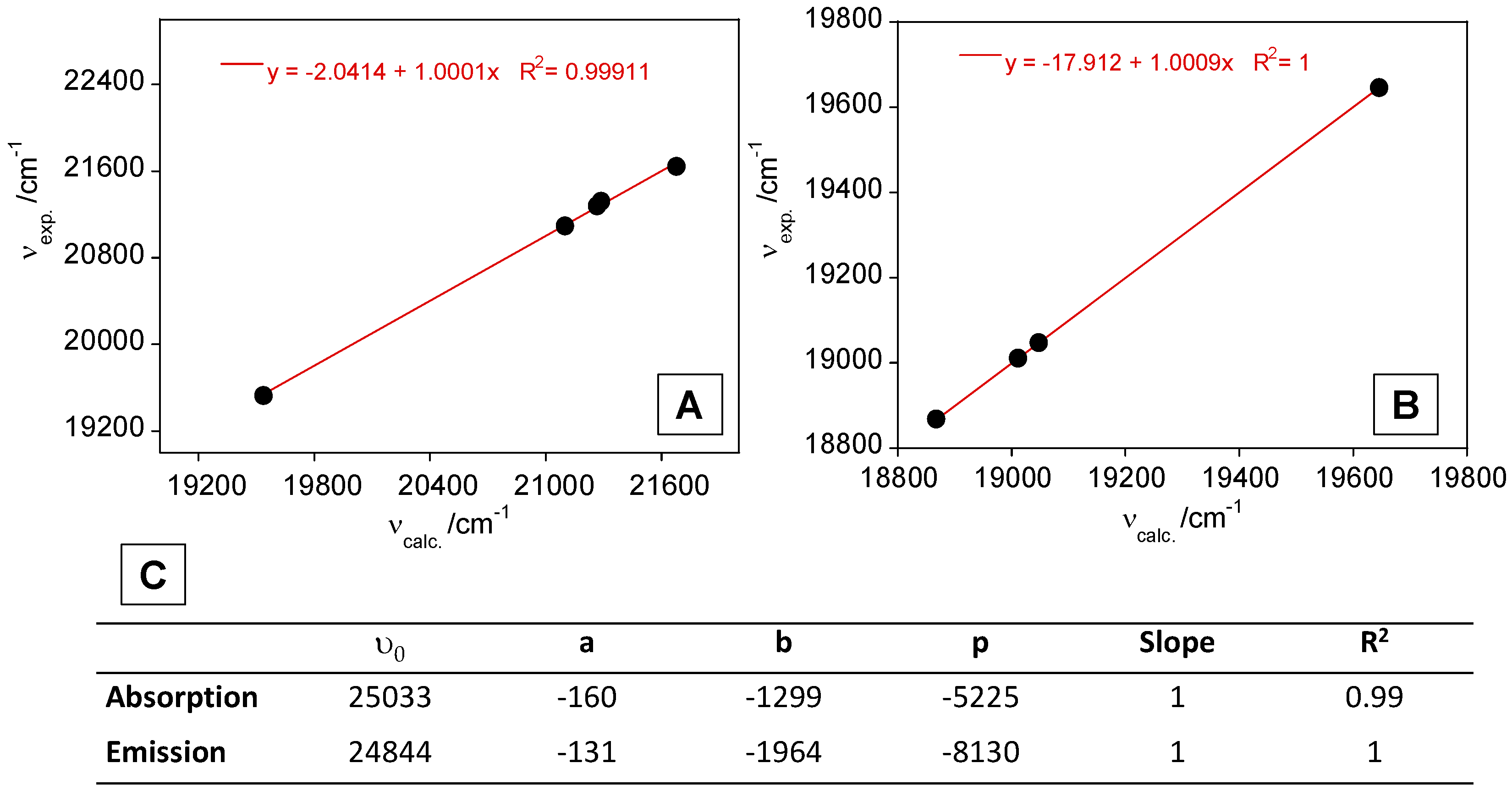
3.3. Theoretical Calculations and Structural Analysis of L1
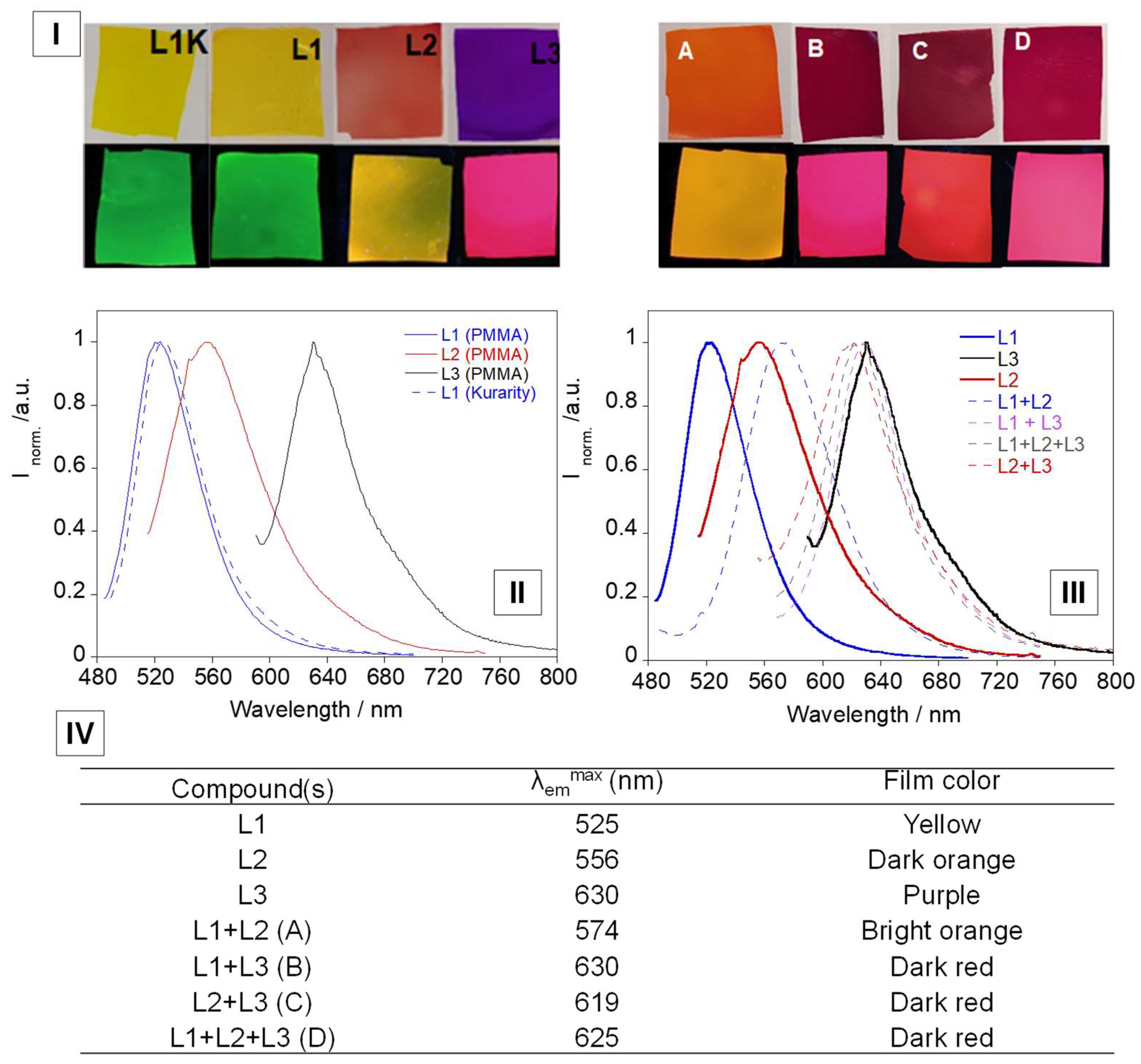
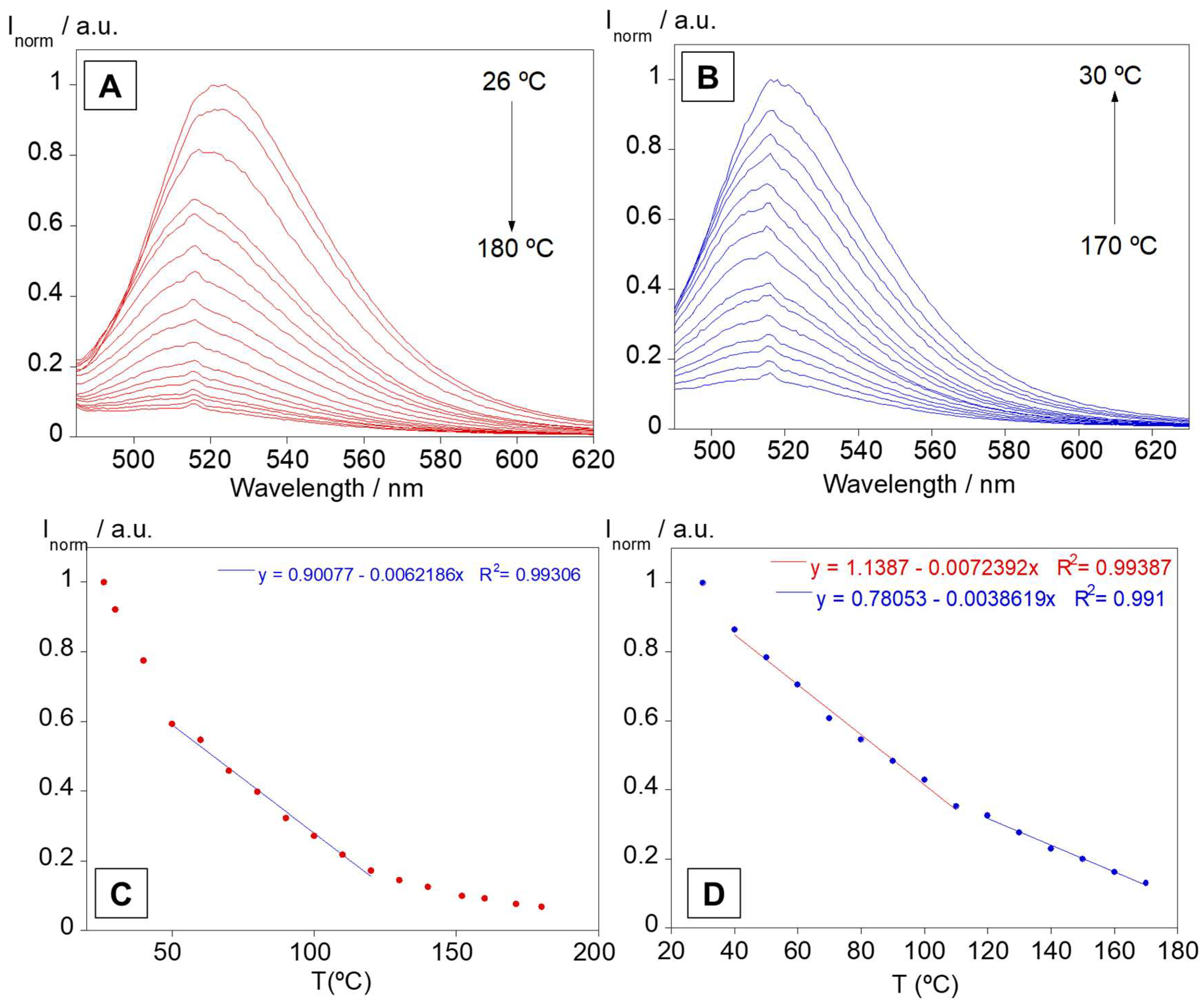
3.4. Solid Supports and Temperature Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, D.C.; Tasior, M.; Laurent, A.D.; Dobrzycki, Ł.; Cyrański, M.K.; Tkachenko, N.; Jacquemin, D.; Gryko, D.T. Photostable Orange-Red Fluorescent Unsymmetrical Diketopyrrolopyrrole–BF2 Hybrids. J. Mater. Chem. C Mater. 2020, 8, 7708–7717. [Google Scholar] [CrossRef]
- Delgado, D.; Abonia, R. Synthetic Approaches for BF2-Containing Adducts of Outstanding Biological Potential. A Review. Arab. J. Chem. 2022, 15, 103528. [Google Scholar] [CrossRef]
- Sangu, K.G.; Shinde, A.U.; Chopra, S.; Rode, H.B. Evading Antimicrobial Resistance Using Boron-Containing Therapeutics. Future Med. Chem. 2021, 13, 229–232. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Niu, G.; Xiao, H.; Wang, M.; Ge, J.; Wu, J.; Zhang, H.; Li, Y.; Wang, P. Coumarin-Based Boron Complexes with Aggregation-Induced Emission. J. Org. Chem. 2017, 82, 3456–3462. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Butler, T.; Morris, W.A.; Samonina-Kosicka, J.; Fraser, C.L. Mechanochromic Luminescence and Aggregation Induced Emission of Dinaphthoylmethane β-Diketones and Their Boronated Counterparts. ACS Appl. Mater. Interfaces 2016, 8, 1242–1251. [Google Scholar] [CrossRef]
- Lai, C.; Zhao, Y.; Liang, Y.; Zou, X.; Lin, W. BF2 Group Chelated AIE Fluorescent Probe for Polarity Mapping of Lipid Droplets in Cells and in Vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120637. [Google Scholar] [CrossRef]
- The, A.B.; Sekar, N. NLOphoric Red Emitting Bis Coumarins with O-BF2-O Core—Synthesis, Photophysical Properties and DFT Studies. J. Fluoresc. 2016, 26, 471–486. [Google Scholar] [CrossRef]
- Rajeshirke, M.; The, A.B.; Sekar, N. Viscosity Sensitive Fluorescent Coumarin-Carbazole Chalcones and Their BF2 Complexes Containing Carboxylic Acid—Synthesis and Solvatochromism. J. Mol. Liq. 2018, 264, 358–366. [Google Scholar] [CrossRef]
- Balewski, Ł.; Szulta, S.; Jalińska, A.; Kornicka, A. A Mini-Review: Recent Advances in Coumarin-Metal Complexes With Biological Properties. Front. Chem. 2021, 9, 781779. [Google Scholar] [CrossRef]
- Suresh, V.M.; Bandyopadhyay, A.; Roy, S.; Pati, S.K.; Maji, T.K. Highly Luminescent Microporous Organic Polymer with Lewis Acidic Boron Sites on the Pore Surface: Ratiometric Sensing and Capture of F− Ions. Chem.—A Eur. J. 2015, 21, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Novoa, S.; Gilroy, J.B. (Co)Polymers Containing Boron Difluoride 3-Cyanoformazanate Complexes: Emission Enhancement via Random Copolymerization. Polym. Chem. 2017, 8, 5388–5395. [Google Scholar] [CrossRef]
- Jiménez, R.; Duarte, F.; Nuti, S.; Campo, J.A.; Lodeiro, C.; Cano, M.; Cuerva, C. Thermochromic and Acidochromic Properties of Polymer Films Doped with Pyridyl-β-Diketonate Boron(III) Complexes. Dyes Pigment. 2020, 177, 108272. [Google Scholar] [CrossRef]
- Manaev, A.V.; Chibisova, T.A.; Traven, V.F. Boron Chelates in the Synthesis of α,β-Unsaturated Ketones of the Coumarin Series. Russ. Chem. Bull. 2006, 55, 2226–2232. [Google Scholar] [CrossRef]
- Traven, V.F.; Manaev, A.V.; Voevodina, I.V.; Okhrimenko, I.N. Synthesis and Structure of New 3-Pyrazolinylcoumarins and 3-Pyrazolinyl-2-Quinolones. Russ. Chem. Bull. 2008, 57, 1508–1515. [Google Scholar] [CrossRef]
- Manaev, A.V.; Chibisova, T.A.; Lyssenko, K.A.; Antipin, M.Y.; Traven’, V.F. Synthesis and Structures of Boron Complexes of Acyl Hydroxy Coumarins. Russ. Chem. Bull. 2006, 55, 2091–2094. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429115387. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, N.; Bhattacharyya, P.K.; Bania, K.K. Substituent and Solvent Effects on the Absorption Spectra of Cation−π Complexes of Benzene and Borazine: A Theoretical Study. J. Phys. Chem. A 2014, 118, 3760–3774. [Google Scholar] [CrossRef] [PubMed]
- Traven, V.F.; Cheptsov, D.A.; Svetlova, J.I.; Ivanov, I.V.; Cuerva, C.; Lodeiro, C.; Duarte, F.; Dunaev, S.F.; Chernyshev, V.V. The Role of the Intermolecular Π···π Interactions in the Luminescence Behavior of Novel Coumarin-Based Pyrazoline Materials. Dye. Pigment. 2021, 186, 108942. [Google Scholar] [CrossRef]
- Anandhan, K.; Cerón, M.; Perumal, V.; Ceballos, P.; Gordillo-Guerra, P.; Pérez-Gutiérrez, E.; Castillo, A.E.; Thamotharan, S.; Perkins, M.J. Solvatochromism and PH Effect on the Emission of a Triphenylimidazole-Phenylacrylonitrile Derivative: Experimental and DFT Studies. RSC Adv. 2019, 9, 12085–12096. [Google Scholar] [CrossRef]
- Oliveira, E.; Baptista, R.M.; Costa, S.P.; Raposo, M.M.; Lodeiro, C. Synthesis and Solvatochromism Studies of Novel Bis(Indolyl)Methanes Bearing Functionalized Arylthiophene Groups as New Colored Materials. Photochem. Photobiol. Sci. 2014, 13, 492–498. [Google Scholar] [CrossRef]
- Mayoral, M.J.; Cano, M.; Campo, J.A.; Heras, J.V.; Pinilla, E.; Torres, M.R. Reactivity of Bis(Long Chain Substituted β-Diketonato)Palladium(II) [Pd(OOR2)2] towards HBF4: Formation of Luminescent [BF2(OOR2)] Derivatives. X-Ray Structure of [1,3-Di(4-n-Butoxyphenyl)Propane-1,3-Dionato]Difluoroboron(III). Inorg. Chem. Commun. 2004, 7, 974–978. [Google Scholar] [CrossRef]


| Solvent | α | β | π* | εr | η |
|---|---|---|---|---|---|
| DMF | 0 | 0.69 | 0.88 | 36.7 | 1.4305 |
| Ethanol | 0.86 | 0.75 | 0.54 | 24.3 | 1.3610 |
| Chloroform | 0.2 | 0.1 | 0.69 | 9.08 | 1.4459 |
| THF | 0 | 0.55 | 0.58 | 7.52 | 1.4072 |
| Dioxane | 0 | 0.37 | 0.55 | 2.25 | 1.4224 |
| λabsmax (nm) | λemmax (nm) | Δλ (nm) | Stokes Shift (cm−1) | λexcmax (nm) | Φf (%) | τf (ns) | |
|---|---|---|---|---|---|---|---|
| L1 | 470 | 526 | 56 | 178571 | 472 | 33 | 0.32 |
| L2 | 495 | 560 | 65 | 153846 | 492 | 4 | 0.11 |
| L3 | 574 | 613 | 39 | 256410 | 572 | 1 | 0.03 |
| L1 | λabsmax (nm) | λemmax (nm) | Δλ (nm) | λexcmax (nm) | Color | Φf (%) | τf (ns) |
|---|---|---|---|---|---|---|---|
| Chloroform | 470 | 526 | 56 | 472 | Yellow | 33 | 0.32 |
| THF | 469 | 525 | 56 | 471 | Yellow | 32 | 0.45 |
| Dioxane | 462 | 509 | 47 | 464 | Yellow | 22 | 0.34 |
| Ethanol | 474 | 530 | 56 | 472 | Yellow | 15 | 0.37 |
| DMF | 512/548 | No emission | Orange | No emission | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, G.; Duarte, F.; Cheptsov, D.A.; Volodin, N.Y.; Ivanov, I.V.; Santos, H.M.; Capelo-Martinez, J.L.; Cuerva, C.; Oliveira, E.; Traven, V.F.; et al. Exploring Coumarin-Based Boron Emissive Complexes as Temperature Thermometers in Polymer-Supported Materials. Sensors 2023, 23, 1689. https://doi.org/10.3390/s23031689
Pedro G, Duarte F, Cheptsov DA, Volodin NY, Ivanov IV, Santos HM, Capelo-Martinez JL, Cuerva C, Oliveira E, Traven VF, et al. Exploring Coumarin-Based Boron Emissive Complexes as Temperature Thermometers in Polymer-Supported Materials. Sensors. 2023; 23(3):1689. https://doi.org/10.3390/s23031689
Chicago/Turabian StylePedro, Gonçalo, Frederico Duarte, Dmitrii A. Cheptsov, Nikita Yu. Volodin, Ivan V. Ivanov, Hugo M. Santos, Jose Luis Capelo-Martinez, Cristián Cuerva, Elisabete Oliveira, Valerii F. Traven, and et al. 2023. "Exploring Coumarin-Based Boron Emissive Complexes as Temperature Thermometers in Polymer-Supported Materials" Sensors 23, no. 3: 1689. https://doi.org/10.3390/s23031689
APA StylePedro, G., Duarte, F., Cheptsov, D. A., Volodin, N. Y., Ivanov, I. V., Santos, H. M., Capelo-Martinez, J. L., Cuerva, C., Oliveira, E., Traven, V. F., & Lodeiro, C. (2023). Exploring Coumarin-Based Boron Emissive Complexes as Temperature Thermometers in Polymer-Supported Materials. Sensors, 23(3), 1689. https://doi.org/10.3390/s23031689









