Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Databases and Search Strategy
2.2. Selection Criteria
- Stroke patients (post-acute, subacute or chronic stroke);
- Randomized control trials (RCT);
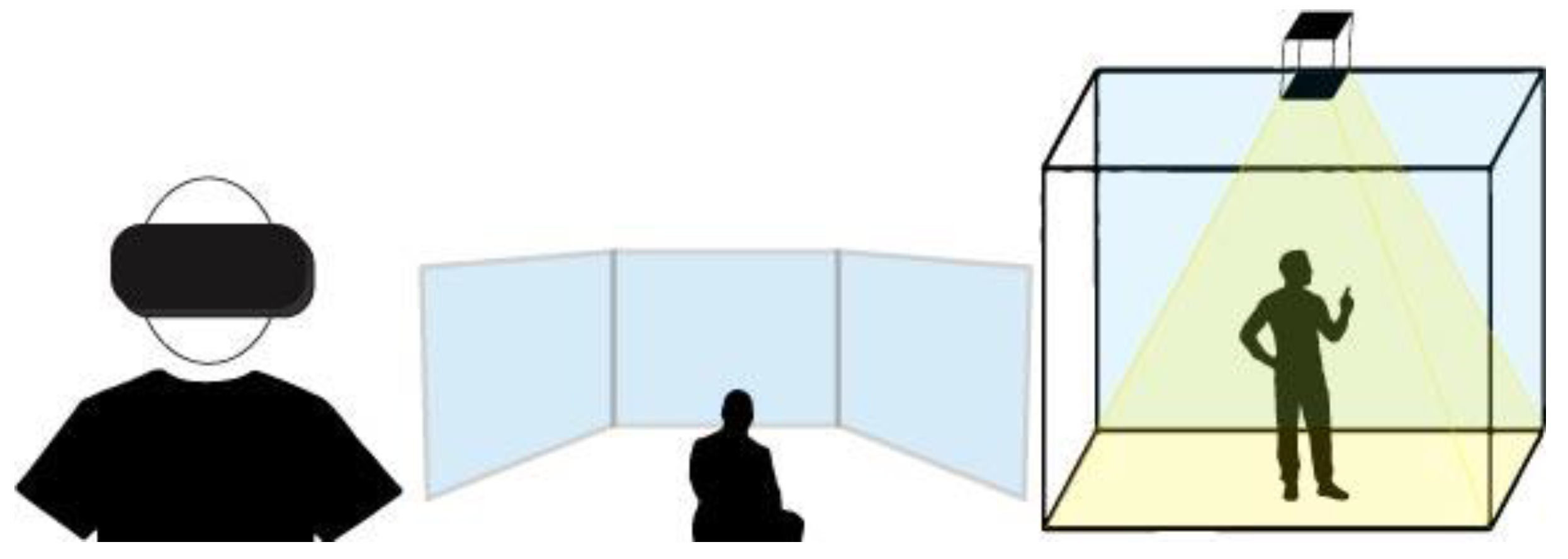
- FIVR (head-mounted display or large screen projection or CAVE);
- Concerning motor impairment recovery;
- Written in English.
- Observational, retrospective and cross-sectional studies, case reports, case series, case studies, reviews and meta-analysis;
- Studies involving only healthy subjects;
- Studies planned only for cognitive rehabilitation;
- Studies focused on cognitive disease (e.g., psychiatric disorder, dementia, mild cognitive impairment) or not regarding stroke (e.g., multiple sclerosis, Parkinson’s disease, spinal cord injury, traumatic brain injury, pain, cerebral palsy);
- Full-text not accessible through our institutional University Library System.
2.3. Data Extraction and Analysis
2.4. Assessment of Risk of Bias
3. Results
3.1. Evidence Synthesis
Overview of the Trial Flow
3.2. Quality Assessment

3.3. Synthesis of the Results
3.4. Intervention Protocol for Experimental Group
3.5. Intervention Protocol for Control Group
3.6. Side Effects
3.7. Outcome Measure
| Author | Patient | Tools | Inclusion Criteria | Training | Intervention | Control Group | Assessment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Upper limb | ||||||||
| Mekbib et al., 2021 [67] | N = 23 EG 12; CG 11 | Mirroring neuron VR Rehabilitation (MNVR-Rehab): HMD, two HTC Vive tracking stations, Leap Motion and ALIENWARE laptop | (1) first ischemic or hemorrhagic stroke with moderate to severe upper limb dysfunction (2) stroke within 3 months (3) age > 18 years (4) neither hearing nor vision deficits (5) MMSE > 16 | 2 h per day, 4 days a week for 2 weeks | 60 min of virtual training (reach, grasp and release colored ball into a basket through MNVR-Rehab) plus 60 min of occupational training | Occupational therapy based on daily living activities, balance control, gait training, weight shift and upper limb functional training | Assessment at baseline and post-intervention (2 weeks). FM-UE; BI | MNVR-Rehab is an encouraging rehabilitation apparatus that may increase upper limb function in subacute stroke subjects compared to occupational therapy |
| Lin et al., 2021 [69] | N = 18 EG 9; CG 9 | Virtual reality mirror therapy (Oculus Rift and Leap Motion Controller and dedicated Software) | (1) 6 months post-unilateral infarction or hemorrhage stroke (2) FMUE between 23 and 60 (3) MMSE >24 | Sessions of 50 min, two days per week for 9 weeks | 30 min of virtual reality mirror therapy plus 20 min of traditional motor task specific exercises | 30 min of conventional mirror therapy plus 20 min of traditional motor task specific exercises | Assessment at baseline and post-intervention (9 weeks). FM-UE assessment | Adding virtual reality to mirror therapy can increase upper limb function in chronic stroke subjects. |
| Huang et al., 2020 [70] | N = 18 EG 20; CG 20 | HTC Vive | (1) first unilateral stroke (after 3 and 24 months) (2) hemiparesis with upper limb dysfunction after stroke (3) upper limb rehabilitation to convalescents levels of Brunnstrom stages III to V (4) able to sit and stand without help ford 2 min (BBS ≥ 3) | 20 sessions of 30 min, 3 times a week, over 8 weeks plus 1 h of upper limb conventional training | Upper limb conventional training plus immersive virtual reality gaming (shoot balloon, electric current stick and shooting game) | Upper limb conventional training plus physical training with climbing bar, ball bearing and pulley | Assessment at baseline and post-intervention (8 weeks). FM-UE; BBT; FIM self-care score | In stroke rehabilitation the use of an immersive virtual reality system improves upper limb function. |
| Ögün et al., 2019 [71] | N = 65 EG 33; CG 32 | HMD + Leap Motion | (1) MMSE score ≥ 25 (2) stroke between six and 24 months (3) Modified Ashworth Scale score < 3 (4) upper extremity and hand Brunnstrom score ≥ 4 | Sessions of 60 min of therapy, three days per week, for six weeks | immersive VR plus Leap motion training consists of four games (play task-oriented games that aimed at gripping and handling of objects with arm and at forearm motion and stability | 45 min of conventional upper extremity active exercises including the same used in the VR group plus 15 min of sham VR training | Assessment at baseline and post-intervention (six weeks). FM-UE; ARAT; FIM, PASS | Applying immersive VR with leap motion in stroke patients has a statistical significance on upper extremity function and daily life activities but not on independence. |
| Subramanian et al., 2015 [74] | N = 24 EG 12; CG 12 | Stereoscopic glasses + projector + screen + 3D virtual environment (CAREN) | (1) between 40 and 80 years (2) single ischemic or hemorrhagic stroke 6 to 60 months previously (3) scored 3 to 6/7 on the Chedoke-McMaster Stroke Assessment arm sub-scale (4) no other neurologic or neuromuscular/orthopedic problems affecting the upper limb and trunk | 12 sessions: 3 times per week over 4 weeks | A 3D virtual environment (CAREN system) simulated a supermarket scene. Subjects had to point 6 objects placed just beyond arm’s length, without physically touching them. | Participants had to point at targets in a physical environment | Arm and trunk kinematic assessment at baseline, after 4 weeks, and 3 months following intervention. Neuropsychological assessment only at baseline Neurocognitive assessment (Stroop test, RAVLT, TOL, ROCF copy) | An increase in kinematic data for upper limb motor recovery was related to milder neurocognitive deficits. |
| Subramanian et al., 2013 [76] | N = 32 EG 16; CG 16 | Stereoscopic glasses + projector + screen + 3D virtual environment (CAREN) | (1) between 40 and 80 years (2) single stroke 6 to 60 months previously (3) scored 3 to 6/7 on the Chedoke-McMaster Stroke Assessment arm sub-scale (4) no other neurologic or neuromuscular/orthopedic problems affecting the upper limb and trunk | 12 sessions of 45 min over 4 weeks | A 3D virtual environment (CAREN system) simulated a supermarket scene. Subjects had to point to 6 objects placed just beyond arm’s length, without physically touching them. | Participants had to point at targets in a physical environment | Assessment at baseline, after 4 weeks, and 3 months following intervention. FMA, RPSS, WMFT-FAS, MAL-AS mean scores, Motivation Task Evaluation Questionnaire, and kinematic analysis | Both groups improved arm motor impairment measures, clinical impairment scores and activity levels. Improvements can be attributed to practice intensity. VE training led to better results in arm motor recovery, especially in the moderate-to-severe group. |
| Crosbie et al., 2012 [77] | N = 18 EG 9; CG 9 | HMD + desktop computer + motion tracking system + sensors | (1) medically stable (2) 18–85 years (3) 6–24 months following a first stroke (4) able to follow a two-step command | Nine sessions of 30–45 min over three weeks | Virtual reality through an HMD for upper limb training. The virtual tasks simulated a range of upper limb tasks related to reach to target, reach and grasp and game tasks. | Conventional therapy based on muscle facilitation, stretching exercises, strengthening activities and functional tasks | Assessment at baseline, post-intervention, after three weeks, and 6 weeks following the intervention. ARAT and an exit questionnaire. | This pilot study has demonstrated the feasibility of a RCT in chronic stroke patients, if careful consideration is given to the recruitment methods and outcome measures. Larger trials are needed to offer high-quality evidence for the specific effect of virtual reality-mediated therapy in upper limb stroke rehabilitation. |
| Lower limb | ||||||||
| De Rooij et al., 2021 [68] | N = 52 EG 28; CG 24 | GRAIL | (1) WHO diagnosis of stroke (2) between 2 weeks and 6 months post-stroke (3) walking without help for balance and coordination (FAC > 3) (4) walking in daily life feeling self-limitation (5) community living (6) between 18 and 80 years | Sessions of 30 min, 2 times a week for 6 weeks | Training on GRAIL several virtual environment with different purpose and variable degree of complexity | 10–15 min of treadmill training and 15 min of functional gait training | Assessment at baseline, 6 weeks, and 3 months post-intervention. USER-P; TUG test; 6MWT Walking activity (total number of steps a day, duration of walking activity per day and step frequency); Mini-BESTest; FES-I; SIS-16; FSS, HADS anxiety and depression; SS-QOL | The effect of VRT was not statistically different from the effect of non-VRT on analyzed outcomes in community-living people after stroke, but virtual treadmill training was safe and well-tolerated by patients and therefore could be a useful supplement to stroke rehabilitation |
| Kim et al., 2015 [72] | N = 27 VRCA-G 10; CA-G 11; CG 7 | Treadmill + projector + screen + VR program | (1) 6 months post-stroke with hemiplegia (2) gait speed < 0.8 m/s (3) autonomous walking without device for more than 6 min (4) MMSE-K >24 | Sessions of 30 min, 3 times a week for 4 weeks (VRCA-G and CA-G) plus sessions of 30 min, 2 times a day for 4 weeks (all groups) | VRCA G trained with a treadmill in 4 different VR environments (sidewalk walking, overground walking, uphill walking and stepping over obstacles) and a progressive speed increase based on the patient’s condition. CA-G trained in the real world in 4 environments: overground walking, stair walking, slope walking and unstable surface walking. Both groups received also general physical therapy. | General physical training | Assessment at baseline and 4 weeks post-intervention. TUG test; ABC; 6MWT; GAITrite walking | In post-stroke subjects, VR treadmill training-based community ambulation and community ambulation training help enhancing dynamic balance ability, activities-specific balance confidence and temporal/spatial gait or gait endurance. These methods are both useful to increase functional skills but, due to VR effects on patient’s physical and psychological fields, it would be more beneficial using this therapy before the community ambulation training. |
| Cho et al., 2015 [73] | N = 22 EG 11; CG 11 | Treadmill + projector + screen + VR program | (1) single stroke, (2) 6 months post-stroke, (3) able to walk 10 m with and without the use of an assistive device, (4) able to understand and follow simple verbal instructions (Korean version of the MMSE score > 24) (5) no severe heart disease or uncontrolled hypertension. | Sessions of 30 min a day, 5 times a week for 4 weeks | VR training (treadmill training) with four cognitive load tasks (memory, arithmetic and two verbal tasks) | VR training (treadmill training) | Assessment at baseline, 3 days after the last experimental training. GAITRite walkway system for spatiotemporal gait parameters under single and dual task conditions. | Beneficial effect of VRTCL on walking function under single and dual task conditions in chronic stroke patients. In the VRTCL group there was a greater increase in walking function during the dual task condition than in the control group. |
| Lee et al., 2014 [75] | N = 21 EG 10; CG 11 | HMD | (1) chronic stroke (2) no medications influencing balance (3) MMSE score < 24 (4) no pain or disability associated with acute musculoskeletal diseases (5) able to sit for over 10 s without help (6) able to stand without help for 1 min | 20 sessions of 30 min over 4 weeks for all participants + 20 sessions of 30 min over 4 weeks for EG | 16 exercises to train postural control organized on three degrees of difficulty (lying, sitting and standing) plus standard rehabilitation | Standard rehabilitation | Assessment at baseline and after 4 weeks post-intervention. TUG test; BBS; GAITrite walkway | Adding VR training to conventional rehabilitation leads an increase in a number of gait parameters (gait velocity, step length and stride length) compared with traditional therapy alone. |
| Kang et al. [78] | N = 30 TOF: 10; Treadmill G: 10; CG: 10 | HMD + treadmill | (1) hemiparetic stroke 6 months after diagnosis, (2) able to walk without help for more than 15 min, (3) no visual deficits or hemianopsia, (4) MMSE ≥ 21 (5) Brunnstrum stage > 4 | Sessions of 30 min, 3 times a week for 4 weeks plus conventional therapy 5 times a week for four weeks | Treadmill training with an HMD showing walking on a street with a progressive increase in speed plus conventional therapy. Treadmill group experienced treadmill without extra device and with a progressive rise of speed plus conventional therapy. | Stretching added range of motion training plus conventional therapy | Assessment at baseline and 4 weeks post-intervention. TUG test; FRT; 10 MWT; 6MWT | Treadmill training with optic flow can help improve balance and gait function in chronic post-stroke patients especially taking advantage of optic flow speed modulation. |
3.8. Upper Limb Function
3.9. Lower Limb Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Ammendolia, A.; Marinaro, C.; Demeco, A.; Moggio, L.; Costantino, C. International classification of functioning, disability and health (ICF) and correlation between disability and finance assets in chronic stroke patients. Acta Biomed. 2020, 91, e2020064. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Teasell, R.; Rice, D.; Richardson, M.; Campbell, N.; Madady, M.; Hussein, N.; Murie-Fernandez, M.; Page, S. The next revolution in stroke care. Expert Rev. Neurother. 2014, 14, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Moggio, L.; Demeco, A.; Fortunato, F.; Spanò, R.; Aiello, V.; Marotta, N.; Ammendolia, A. Efficacy of rehabilitative techniques in reducing hemiplegic shoulder pain in stroke: Systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101602. [Google Scholar] [CrossRef]
- Stewart, C.; Subbarayan, S.; Paton, P.; Gemmell, E.; Abraha, I.; Myint, P.K.; O’Mahony, D.; Cruz-Jentoft, A.J.; Cherubini, A.; Soiza, R.L. Non-pharmacological interventions for the improvement of post-stroke activities of daily living and disability amongst older stroke survivors: A systematic review. PLoS ONE 2018, 13, e0204774. [Google Scholar] [CrossRef]
- Background Concepts in Stroke Rehabilitation, EBRSR—Evidence-Based Review of Stroke Rehabilitation. Available online: http://www.ebrsr.com/evidence-review/3-background-concepts-stroke-rehabilitation (accessed on 7 December 2022).
- Cassidy, J.M.; Cramer, S.C. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl. Stroke Res. 2017, 8, 33–46. [Google Scholar] [CrossRef]
- Butler, A.J.; Page, S.J. Mental practice with motor imagery: Evidence for motor recovery and cortical reorganization after stroke. Arch. Phys. Med. Rehabil. 2006, 87, 2–11. [Google Scholar] [CrossRef]
- Rossi, F.; Savi, F.; Prestia, A.; Mongardi, A.; Demarchi, D.; Buccino, G. Combining action observation treatment with a brain–computer interface system: Perspectives on neurorehabilitation. Sensors 2021, 21, 8504. [Google Scholar] [CrossRef]
- You, S.H.; Jang, S.H.; Kim, Y.-H.; Hallett, M.; Ahn, S.H.; Kwon, Y.-H.; Kim, J.H.; Lee, M.Y. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Craig, A. Understanding Virtual Reality, 2nd ed.; Morgan Kaufmann: Burlington, MA, USA, 2018; Available online: https://www.elsevier.com/books/understanding-virtual-reality/sherman/978-0-12-800965-9 (accessed on 7 December 2022).
- Aly, A.A.I.; Abbasimoshaei, A.; Kern, T.A. Developing a VR training environment for fingers rehabilitation. In Proceedings of the 13th International Conference on Human Haptic Sensing and Touch Enabled Computer Applications, EuroHaptics, Hamburg, Germany, 22–25 May 2022. [Google Scholar]
- Goude, D.; Björk, S.; Rydmark, M. Game design in virtual reality systems for stroke rehabilitation. Stud. Health Technol. Inform. 2007, 125, 146–148. [Google Scholar] [PubMed]
- Shahmoradi, L.; Almasi, S.; Ahmadi, H.; Bashiri, A.; Azadi, T.; Mirbagherie, A.; Ansari, N.N.; Honarpishe, R. Virtual reality games for rehabilitation of upper extremities in stroke patients. J. Bodyw. Mov. Ther. 2021, 26, 113–122. [Google Scholar] [CrossRef]
- Borrego, A.; Latorre, J.; Alcañiz, M.; Llorens, R. Embodiment and presence in virtual reality after stroke. A comparative study with healthy subjects. Front. Neurol. 2019, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vives, M.V.; Slater, M. From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 2005, 6, 332–339. [Google Scholar] [CrossRef]
- Slater, M. Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3549–3557. [Google Scholar] [CrossRef]
- Palumbo, A. Microsoft HoloLens 2 in medical and healthcare context: State of the art and future prospects. Sensors 2022, 22, 7709. [Google Scholar] [CrossRef]
- Sharar, S.R.; Alamdari, A.; Hoffer, C.; Hoffman, H.G.; Jensen, M.P.; Patterson, D.R. Circumplex model of affect: A measure of pleasure and arousal during virtual reality distraction analgesia. Games Health J. 2016, 5, 197–202. [Google Scholar] [CrossRef]
- Lange, B.; Koenig, S.; Chang, C.-Y.; McConnell, E.; Suma, E.; Bolas, M.; Rizzo, A. designing informed game-based rehabilitation tasks leveraging advances in virtual reality. Disabil. Rehabil. 2012, 34, 1863–1870. [Google Scholar] [CrossRef]
- Plechatá, A.; Sahula, V.; Fayette, D.; Fajnerová, I. Age-related differences with immersive and non-immersive virtual reality in memory assessment. Front. Psychol. 2019, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Korner-Bitensky, N.; Levin, M. Virtual reality in stroke rehabilitation: A systematic review of its effectiveness for upper limb motor recovery. Top. Stroke Rehabil. 2007, 14, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, S.V.; Fluet, G.G.; Tunik, E.; Merians, A.S. Sensorimotor training in virtual reality: A review. NeuroRehabilitation 2009, 25, 29–44. [Google Scholar] [CrossRef]
- Moro, S.B.; Carrieri, M.; Avola, D.; Brigadoi, S.; Lancia, S.; Petracca, A.; Spezialetti, M.; Ferrari, M.; Placidi, G.; Quaresima, V. A novel semi-immersive virtual reality visuo-motor task activates ventrolateral prefrontal cortex: A functional near-infrared spectroscopy study. J. Neural. Eng. 2016, 13, 036002. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Torrisi, M.; Piccolo, A.; Bonfiglio, G.; Tomasello, P.; Naro, A.; Calabrò, R.S. Improving post-stroke cognitive and behavioral abnormalities by using virtual reality: A case report on a novel use of nirvana. Appl. Neuropsychol. Adult 2018, 25, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, M.; Gurlitt, J.; Kozhevnikov, M. Learning relative motion concepts in immersive and non-immersive virtual environments. J. Sci. Educ.Technol. 2013, 22, 952–962. [Google Scholar] [CrossRef]
- de Araújo, A.V.L.; de Oliveira Neiva, J.F.; de Mello Monteiro, C.B.; Magalhães, F.H. Efficacy of virtual reality rehabilitation after spinal cord injury: A systematic review. Biomed. Res. Int. 2019, 2019, 7106951. [Google Scholar] [CrossRef]
- Triegaardt, J.; Han, T.S.; Sada, C.; Sharma, S.; Sharma, P. The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: Meta-analysis and systematic review in 1031 participants. Neurol. Sci. 2020, 41, 529–536. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Freitag, F.; Brucki, S.M.D.; Barbosa, A.F.; Chen, J.; de Oliveira Souza, C.; Valente, D.F.; Chien, H.F.; Bedeschi, C.; Voos, M.C. Is virtual reality beneficial for dual-task gait training in patients with Parkinson’s disease? A systematic review. Dement. Neuropsychol. 2019, 13, 259–267. [Google Scholar] [CrossRef]
- Wang, B.; Shen, M.; Wang, Y.-X.; He, Z.-W.; Chi, S.-Q.; Yang, Z.-H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Demeco, A.; Indino, A.; de Scorpio, G.; Moggio, L.; Ammendolia, A. Nintendo WiiTM versus Xbox KinectTM for functional locomotion in people with Parkinson’s disease: A systematic review and network meta-analysis. Disabil. Rehabil. 2022, 44, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Scaldaferri, G.; Santos, L.; Ribeiro, N.; Neto, M.; Melo, A. Effects of the Nintendo Wii training on balance rehabilitation and quality of life of patients with Parkinson’s disease: A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Bluett, B.; Bayram, E.; Litvan, I. The virtual reality of Parkinson’s disease freezing of gait: A systematic review. Parkinsonism Relat. Disord. 2019, 61, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Pi, Y.-L.; Chen, B.-L.; Wang, R.; Li, X.; Chen, P.-J. Cognitive motor intervention for gait and balance in Parkinson’s disease: Systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Virtual reality enhances gait in cerebral palsy: A training dose-response meta-analysis. Front. Neurol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Wu, J.; Loprinzi, P.D.; Ren, Z. The rehabilitative effects of virtual reality games on balance performance among children with cerebral palsy: A meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2019, 16, 4161. [Google Scholar] [CrossRef]
- Ren, Z.; Wu, J. The effect of virtual reality games on the gross motor skills of children with cerebral palsy: A meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2019, 16, 3885. [Google Scholar] [CrossRef]
- Manivannan, S.; Al-Amri, M.; Postans, M.; Westacott, L.J.; Gray, W.; Zaben, M. The effectiveness of virtual reality interventions for improvement of neurocognitive performance after traumatic brain injury: A systematic review. J. Head Trauma Rehabil. 2019, 34, E52–E65. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Padua, E.; Romagnoli, C.; Mercuri, N.B. Cognitive rehabilitation post traumatic brain injury: A systematic review for emerging use of virtual reality technology. J. Clin. Neurosci. 2019, 66, 209–219. [Google Scholar] [CrossRef]
- Ahn, S.; Hwang, S. Virtual rehabilitation of upper extremity function and independence for stoke: A meta-analysis. J. Exerc. Rehabil. 2019, 15, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Karamians, R.; Proffitt, R.; Kline, D.; Gauthier, L.V. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: A meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.J.; Park, S.W. The effects of virtual reality training on function in chronic stroke patients: A systematic review and meta-analysis. Biomed. Res. Int. 2019, 2019, 7595639. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P.F.M.J. Effect of specific over nonspecific vr-based rehabilitation on poststroke motor recovery: A systematic meta-analysis. Neurorehabil. Neural. Repair. 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Schröder, J.; van Criekinge, T.; Embrechts, E.; Celis, X.; Van Schuppen, J.; Truijen, S.; Saeys, W. Combining the benefits of tele-rehabilitation and virtual reality-based balance training: A systematic review on feasibility and effectiveness. Disabil. Rehabil. Assist. Technol. 2019, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.C.D.S.; Freitas, T.B.; Bonuzzi, G.M.G.; Soares, M.A.A.; Leite, P.H.W.; Mazzini, N.A.; Almeida, M.R.G.; Pompeu, J.E.; Torriani-Pasin, C. Effects of virtual reality for stroke individuals based on the international classification of functioning and health: A systematic review. Top. Stroke Rehabil. 2017, 24, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ogourtsova, T.; Souza Silva, W.; Archambault, P.S.; Lamontagne, A. Virtual reality treatment and assessments for post-stroke unilateral spatial neglect: A systematic literature review. Neuropsychol. Rehabil. 2017, 27, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The use of virtual reality for balance among individuals with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef]
- Gibbons, E.M.; Thomson, A.N.; de Noronha, M.; Joseph, S. Are virtual reality technologies effective in improving lower limb outcomes for patients following stroke—A systematic review with meta-analysis. Top. Stroke Rehabil. 2016, 23, 440–457. [Google Scholar] [CrossRef]
- de Rooij, I.J.M.; van de Port, I.G.L.; Meijer, J.-W.G. Effect of virtual reality training on balance and gait ability in patients with stroke: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 1905–1918. [Google Scholar] [CrossRef]
- Chen, J.; Jin, W.; Zhang, X.-X.; Xu, W.; Liu, X.-N.; Ren, C.-C. Telerehabilitation approaches for stroke patients: Systematic review and meta-analysis of randomized controlled trials. J. Stroke Cerebrovasc. Dis. 2015, 24, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.R.A.; Carregosa, A.A.; Masruha, M.R.; Dos Santos, P.A.; Da Silveira Coêlho, M.L.; Ferraz, D.D.; Da Silva Ribeiro, N.M. The use of Nintendo Wii in the rehabilitation of poststroke patients: A systematic review. J. Stroke Cerebrovasc. Dis. 2015, 24, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Luque-Moreno, C.; Ferragut-Garcías, A.; Rodríguez-Blanco, C.; Heredia-Rizo, A.M.; Oliva-Pascual-Vaca, J.; Kiper, P.; Oliva-Pascual-Vaca, Á. A decade of progress using virtual reality for poststroke lower extremity rehabilitation: Systematic review of the intervention methods. Biomed. Res. Int. 2015, 2015, 342529. [Google Scholar] [CrossRef]
- Pedroli, E.; Serino, S.; Cipresso, P.; Pallavicini, F.; Riva, G. Assessment and rehabilitation of neglect using virtual reality: A systematic review. Front. Behav. Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.R.; Whittinghill, D.M. Intrinsic or extrinsic? Using videogames to motivate stroke survivors: A systematic review. Games Health J. 2015, 4, 253–258. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 2014, CD010820. [Google Scholar] [CrossRef]
- Rodrigues-Baroni, J.M.; Nascimento, L.R.; Ada, L.; Teixeira-Salmela, L.F. Walking training associated with virtual reality-based training increases walking speed of individuals with chronic stroke: Systematic review with meta-analysis. Braz. J. Phys. Ther. 2014, 18, 502–512. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef]
- Massetti, T.; Trevizan, I.L.; Arab, C.; Favero, F.M.; Ribeiro-Papa, D.C.; de Mello Monteiro, C.B. Virtual reality in multiple sclerosis—A systematic review. Mult. Scler. Relat. Disord. 2016, 8, 107–112. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 9 December 2022).
- CASP Checklists-Critical Appraisal Skills Programme. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 9 December 2022).
- Mekbib, D.B.; Debeli, D.K.; Zhang, L.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; Jiang, H.; Zhu, J.; Zhao, Z.; et al. A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Ann. N. Y. Acad. Sci. 2021, 1493, 75–89. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, I.J.M.; van de Port, I.G.L.; Punt, M.; Abbink-van Moorsel, P.J.M.; Kortsmit, M.; van Eijk, R.P.A.; Visser-Meily, J.M.A.; Meijer, J.-W.G. Effect of virtual reality gait training on participation in survivors of subacute stroke: A randomized controlled trial. Phys. Ther. 2021, 101, pzab051. [Google Scholar] [CrossRef]
- Lin, C.W.; Kuo, L.C.; Lin, Y.C.; Su, F.C.; Lin, Y.A.; Hsu, H.Y. Development and testing of a virtual reality mirror therapy system for the sensorimotor performance of upper extremity: A pilot randomized controlled trial. IEEE Access 2021, 9, 14725–14734. [Google Scholar] [CrossRef]
- Huang, L.-L.; Chen, M.-H. Effectiveness of the immersive virtual reality in upper extremity rehabilitation. In Proceedings of the Cross-Cultural Design, Applications in Health, Learning, Communication, and Creativity: 12th International Conference, CCD 2020, Part of the 22nd HCI International Conference, HCII 2020, Copenhagen, Denmark, 19–24 July 2020; pp. 89–98. [Google Scholar]
- Ögün, M.N.; Kurul, R.; Yaşar, M.F.; Turkoglu, S.A.; Avci, Ş.; Yildiz, N. Effect of leap motion-based 3d immersive virtual reality usage on upper extremity function in ischemic stroke patients. Arq. Neuropsiquiatr. 2019, 77, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Park, Y.; Lee, B.-H. Effects of community-based virtual reality treadmill training on balance ability in patients with chronic stroke. J. Phys. Ther. Sci. 2015, 27, 655. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, M.K.; Lee, H.-J.; Lee, W.H. Virtual reality training with cognitive load improves walking function in chronic stroke patients. Tohoku J. Exp. Med. 2015, 236, 273–280. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Chilingaryan, G.; Levin, M.F.; Sveistrup, H. Influence of training environment and cognitive deficits on use of feedback for motor learning in chronic stroke. In Proceedings of the 2015 International Conference on Virtual Rehabilitation, ICVR, Valencia, Spain, 9–12 June 2015; pp. 38–43. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, Y.; Lee, B.-H. Augmented reality-based postural control training improves gait function in patients with stroke: Randomized controlled trial. Hong Kong Physiother. J. 2014, 32, 51–57. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Lourenço, C.B.; Chilingaryan, G.; Sveistrup, H.; Levin, M.F. Arm motor recovery using a virtual reality intervention in chronic stroke: Randomized control trial. Neurorehabil. Neural. Repair 2013, 27, 13–23. [Google Scholar] [CrossRef]
- Crosbie, J.H.; Lennon, S.; McGoldrick, M.C.; McNeill, M.D.J.; McDonough, S.M. Virtual reality in the rehabilitation of the arm after hemiplegic stroke: A randomized controlled pilot study. Clin. Rehabil. 2012, 26, 798–806. [Google Scholar] [CrossRef]
- Kang, H.-K.; Kim, Y.; Chung, Y.; Hwang, S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: Randomized controlled trials. Clin. Rehabil. 2012, 26, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Faria-Fortini, I.; Michaelsen, S.M.; Cassiano, J.G.; Teixeira-Salmela, L.F. Upper extremity function in stroke subjects: Relationships between the international classification of functioning, disability, and health domains. J. Hand Ther. 2011, 24, 257–265. [Google Scholar] [CrossRef]
- Bowden, M.G.; Behrman, A.L.; Neptune, R.R.; Gregory, C.M.; Kautz, S.A. Locomotor rehabilitation of individuals with chronic stroke: Difference between responders and nonresponders. Arch. Phys. Med. Rehabil. 2013, 94, 856–862. [Google Scholar] [CrossRef]
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e0219781. [Google Scholar] [CrossRef]
- Cano Porras, D.; Sharon, H.; Inzelberg, R.; Ziv-Ner, Y.; Zeilig, G.; Plotnik, M. Advanced virtual reality-based rehabilitation of balance and gait in clinical practice. Ther. Adv. Chronic Dis. 2019, 10, 2040622319868379. [Google Scholar] [CrossRef]
- Yates, M.; Kelemen, A.; Sik Lanyi, C. Virtual reality gaming in the rehabilitation of the upper extremities post-stroke. Brain Inj. 2016, 30, 855–863. [Google Scholar] [CrossRef]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The effectiveness of immersive virtual reality in physical recovery of stroke patients: A systematic review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef]
- Demeco, A.; Marotta, N.; Moggio, L.; Pino, I.; Marinaro, C.; Barletta, M.; Petraroli, A.; Palumbo, A.; Ammendolia, A. quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J. Electromyogr. Kinesiol. 2021, 56, 102485. [Google Scholar] [CrossRef]
- Carozzo, S.; Vatrano, M.; Coschignano, F.; Battaglia, R.; Calabrò, R.S.; Pignolo, L.; Contrada, M.; Tonin, P.; Cerasa, A.; Demeco, A. Efficacy of visual feedback training for motor recovery in post-operative subjects with knee replacement: A randomized controlled trial. J. Clin. Med. 2022, 11, 7355. [Google Scholar] [CrossRef]
- Buccino, G. Action observation treatment: A novel tool in neurorehabilitation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130185. [Google Scholar] [CrossRef]
- Thieme, H.; Morkisch, N.; Mehrholz, J.; Pohl, M.; Behrens, J.; Borgetto, B.; Dohle, C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018, 7, CD008449. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S.; Altschuler, E.L. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 2009, 132, 1693–1710. [Google Scholar] [CrossRef] [PubMed]
- Miclaus, R.S.; Roman, N.; Henter, R.; Caloian, S. Lower extremity rehabilitation in patients with post-stroke sequelae through virtual reality associated with mirror therapy. Int. J. Environ. Res. Public Health 2021, 18, 2654. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 2001, 14, S103–S109. [Google Scholar] [CrossRef]
- Buccino, G.; Colagè, I.; Gobbi, N.; Bonaccorso, G. Grounding meaning in experience: A broad perspective on embodied language. Neurosci. Biobehav. Rev. 2016, 69, 69–78. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fabbri-Destro, M.; Nuara, A.; Gatti, R.; Avanzini, P. The role of mirror mechanism in the recovery, maintenance, and acquisition of motor abilities. Neurosci. Biobehav. Rev. 2021, 127, 404–423. [Google Scholar] [CrossRef]
- Whitty, E.; Mansour, H.; Aguirre, E.; Palomo, M.; Charlesworth, G.; Ramjee, S.; Poppe, M.; Brodaty, H.; Kales, H.C.; Morgan-Trimmer, S.; et al. Efficacy of lifestyle and psychosocial interventions in reducing cognitive decline in older people: Systematic review. Ageing Res. Rev. 2020, 62, 101113. [Google Scholar] [CrossRef]
- Weiss, T.; Kizony, R.; Feintuch, U.; Rand, D.; Katz, N. Virtual reality applications in neurorehabilitation, Chapter 19. In Textbook of Neural Repair and Rehabilitation; Cambridge University Press: Cambrige, UK, 2014; Available online: https://www.cambridge.org/core/books/abs/textbook-of-neural-repair-and-rehabilitation/virtual-reality-applications-in-neurorehabilitation/DFDDECA59C7113FAA09A87FA39A34E33 (accessed on 16 December 2022).
- Arcuri, F.; Porcaro, C.; Ciancarelli, I.; Tonin, P.; Cerasa, A. Electrophysiological correlates of virtual-reality applications in the rehabilitation setting: New perspectives for stroke patients. Electronics 2021, 10, 836. [Google Scholar] [CrossRef]
- Kiper, P.; Przysiężna, E.; Cieślik, B.; Broniec-Siekaniec, K.; Kucińska, A.; Szczygieł, J.; Turek, K.; Gajda, R.; Szczepańska-Gieracha, J. Effects of immersive virtual therapy as a method supporting recovery of depressive symptoms in post-stroke rehabilitation: Randomized controlled trial. Clin. Interv. Aging 2022, 17, 1673–1685. [Google Scholar] [CrossRef]
- Pons, J.S.; Raya, R.; González, J. Emerging Therapies in Neurorehabilitation II; Springer International Publishing: Manhattan, NY, USA, 2016; Available online: https://www.springerprofessional.de/emerging-therapies-in-neurorehabilitation-ii/6653434 (accessed on 16 December 2022).
- Chatterjee, K.; Buchanan, A.; Cottrell, K.; Hughes, S.; Day, T.W.; John, N.W. Immersive virtual reality for the cognitive rehabilitation of stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 719–728. [Google Scholar] [CrossRef]
| Eligibility Criteria | Random Allocation | Concealed Allocation | Baseline Comparability | Subjects Blinding | Therapist Blinding | Assessor Blinding | Adequate Follow-Up (>85%) | Intention-to-Treat Analysis | Between-Group Comparisons | Points Estimates and Measure of Variability Provided | Total PEDro Score | Sample Size ≥ 50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mekbib et al. [67] | x | x | x | x | x | x | x | 6/10 | no | ||||
| De Rooij et al. [68] | x | x | x | x | x | x | x | x | x | 8/10 | no | ||
| Lin et al. [69] | x | x | x | x | x | x | x | x | 7/10 | no | |||
| Huang et al. [70] | x | x | x | x | x | x | x | x | 7/10 | no | |||
| Ögün et al. [71] | x | x | x | x | x | x | x | x | x | 8/10 | no | ||
| Kim et al. [72] | x | x | x | x | x | x | x | 7/10 | no | ||||
| Cho et al. [73] | x | x | x | x | x | x | x | 7/10 | no | ||||
| Subramanian et al. [74] | x | x | x | x | x | x | x | 7/10 | no | ||||
| Lee et al. [75] | x | x | x | x | x | x | x | 7/10 | no | ||||
| Subramanian et al. [76] | x | x | x | x | x | x | x | 7/10 | no | ||||
| Crosbie et al. [77] | x | x | x | x | x | x | x | x | x | 8/10 | no | ||
| Kang et al. [78] | x | x | x | x | x | x | x | x | 7/10 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. https://doi.org/10.3390/s23031712
Demeco A, Zola L, Frizziero A, Martini C, Palumbo A, Foresti R, Buccino G, Costantino C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors. 2023; 23(3):1712. https://doi.org/10.3390/s23031712
Chicago/Turabian StyleDemeco, Andrea, Laura Zola, Antonio Frizziero, Chiara Martini, Arrigo Palumbo, Ruben Foresti, Giovanni Buccino, and Cosimo Costantino. 2023. "Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review" Sensors 23, no. 3: 1712. https://doi.org/10.3390/s23031712







