Deposition of Thick SiO2 Coatings to Carbonyl Iron Microparticles for Thermal Stability and Microwave Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe-SiO2 Powders
2.2. Scanning Electron Microscopy
2.3. Vibrating-Sample Magnetometer (VSM)
2.4. Thermal Analysis
2.5. Microwave Measurements
3. Results and Discussion
3.1. Theoretical Calculation of the Effective Permittivity
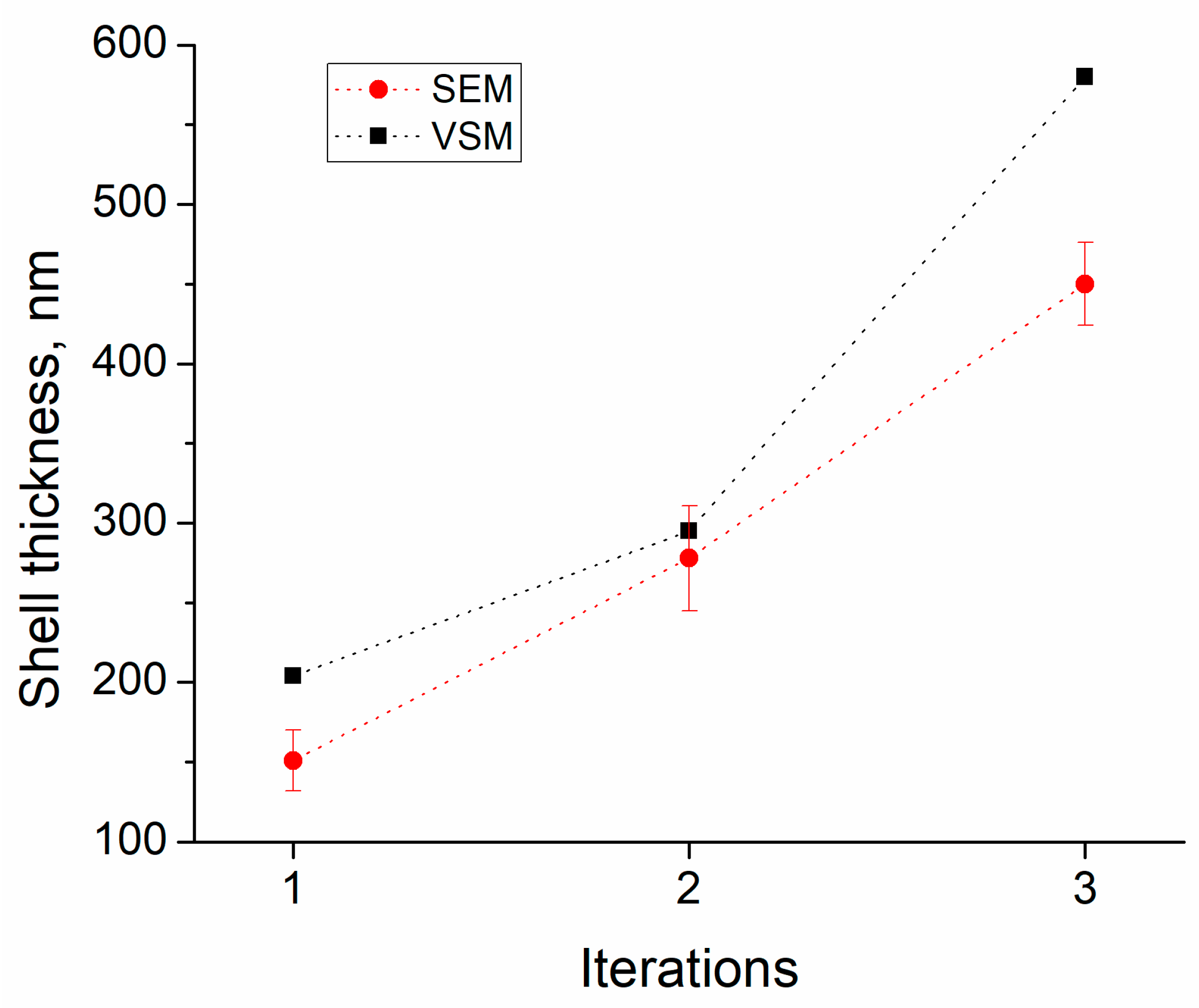
3.2. Scanning Electron Microscopy Analysis
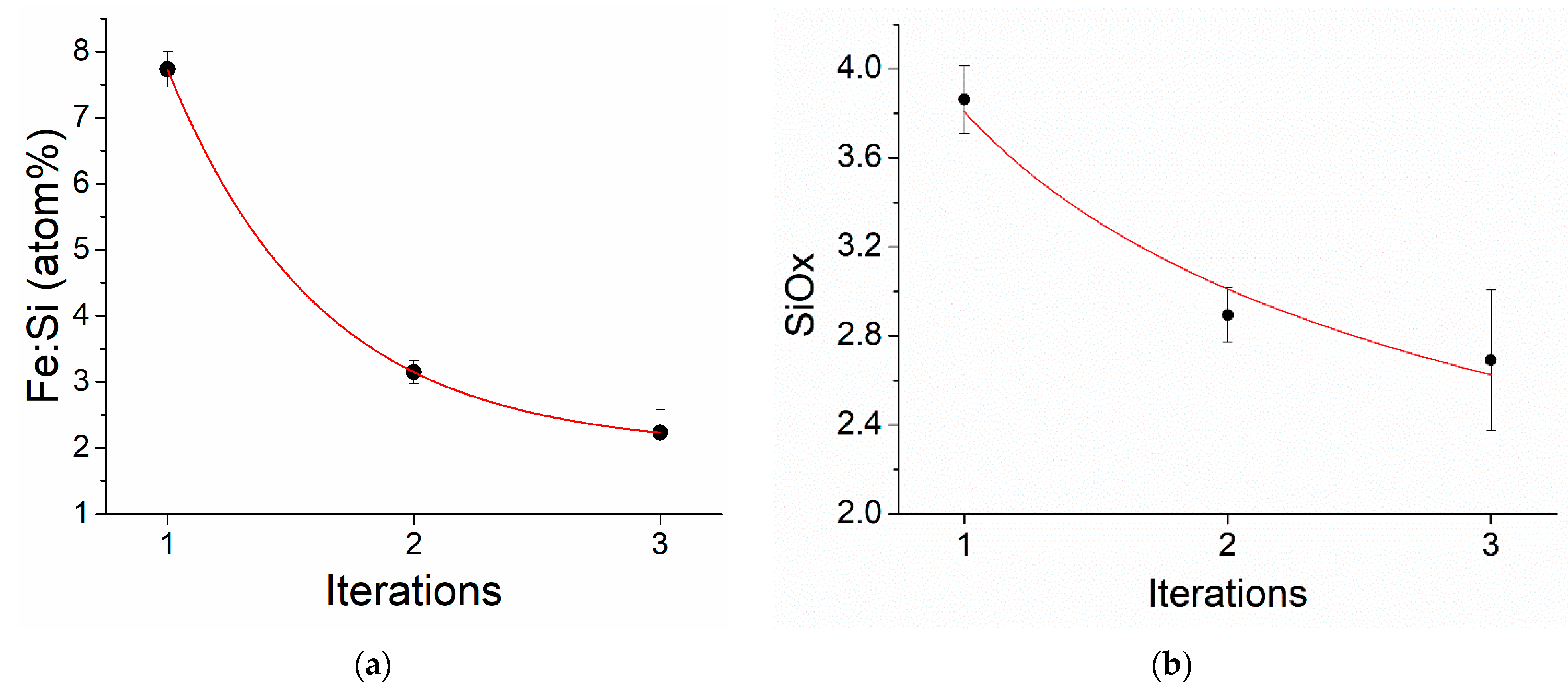
3.3. Elemental Analysis
3.4. Magneto Static (Vibrating Scanning Magnetometer Measurements)
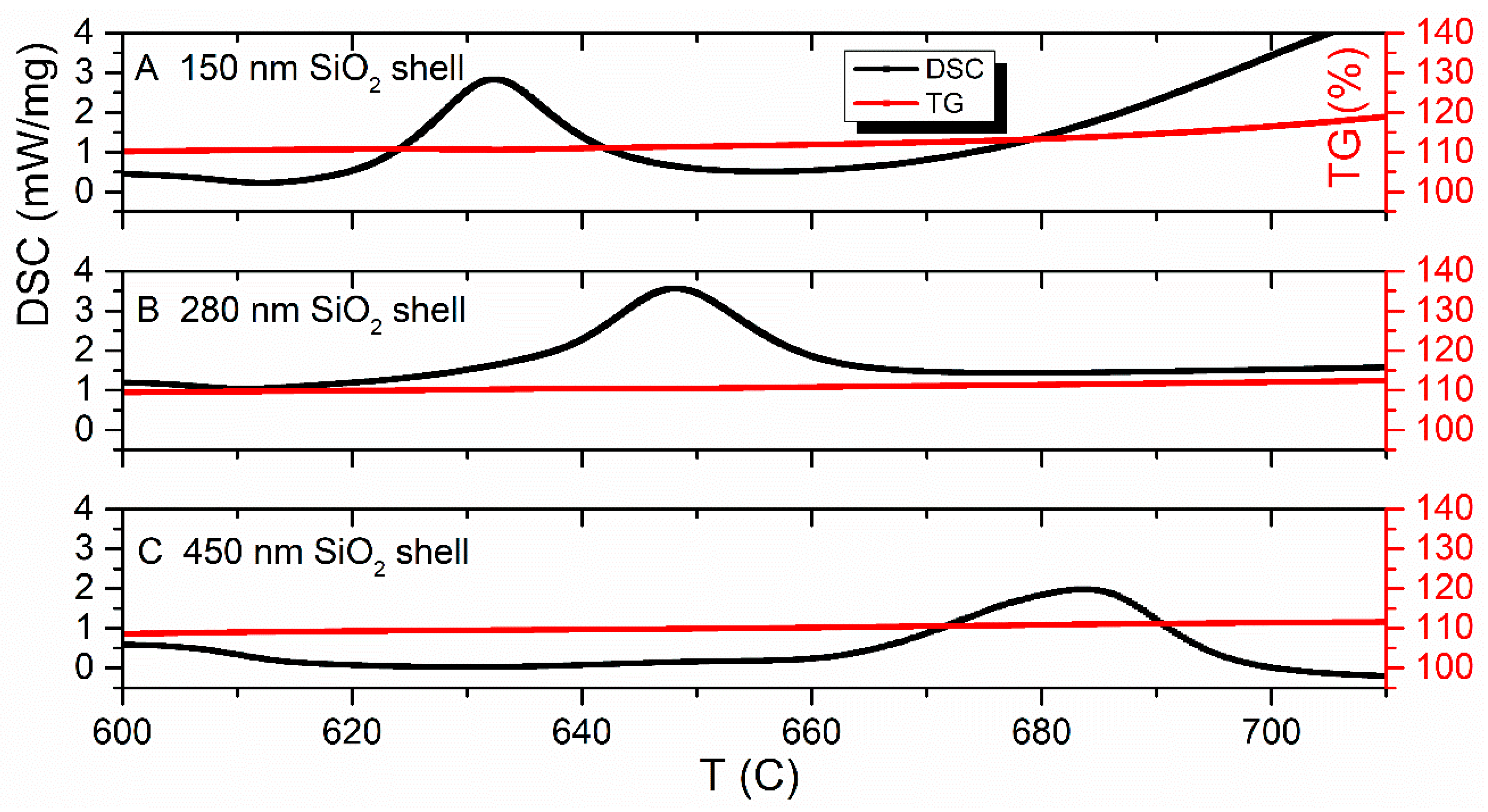
3.5. Synchronous Thermal Analysis (TGA & DSC)
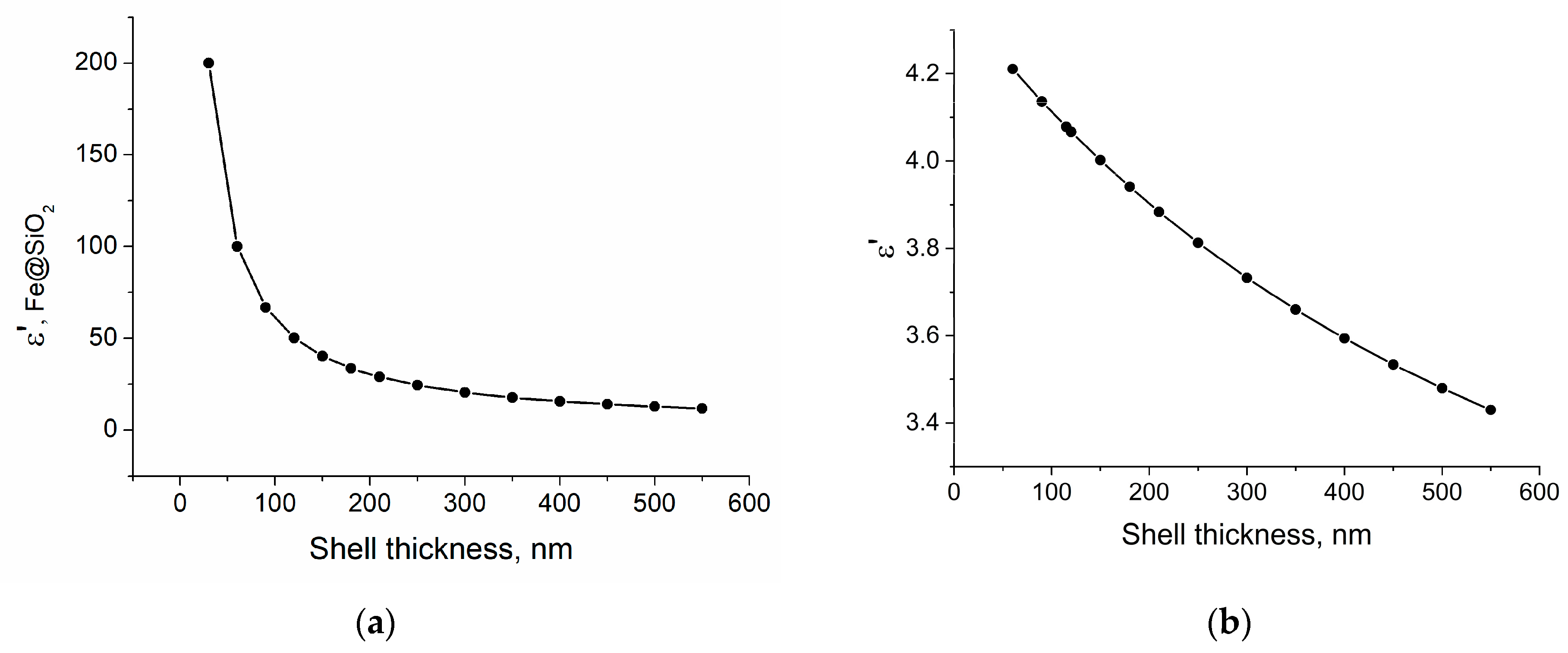
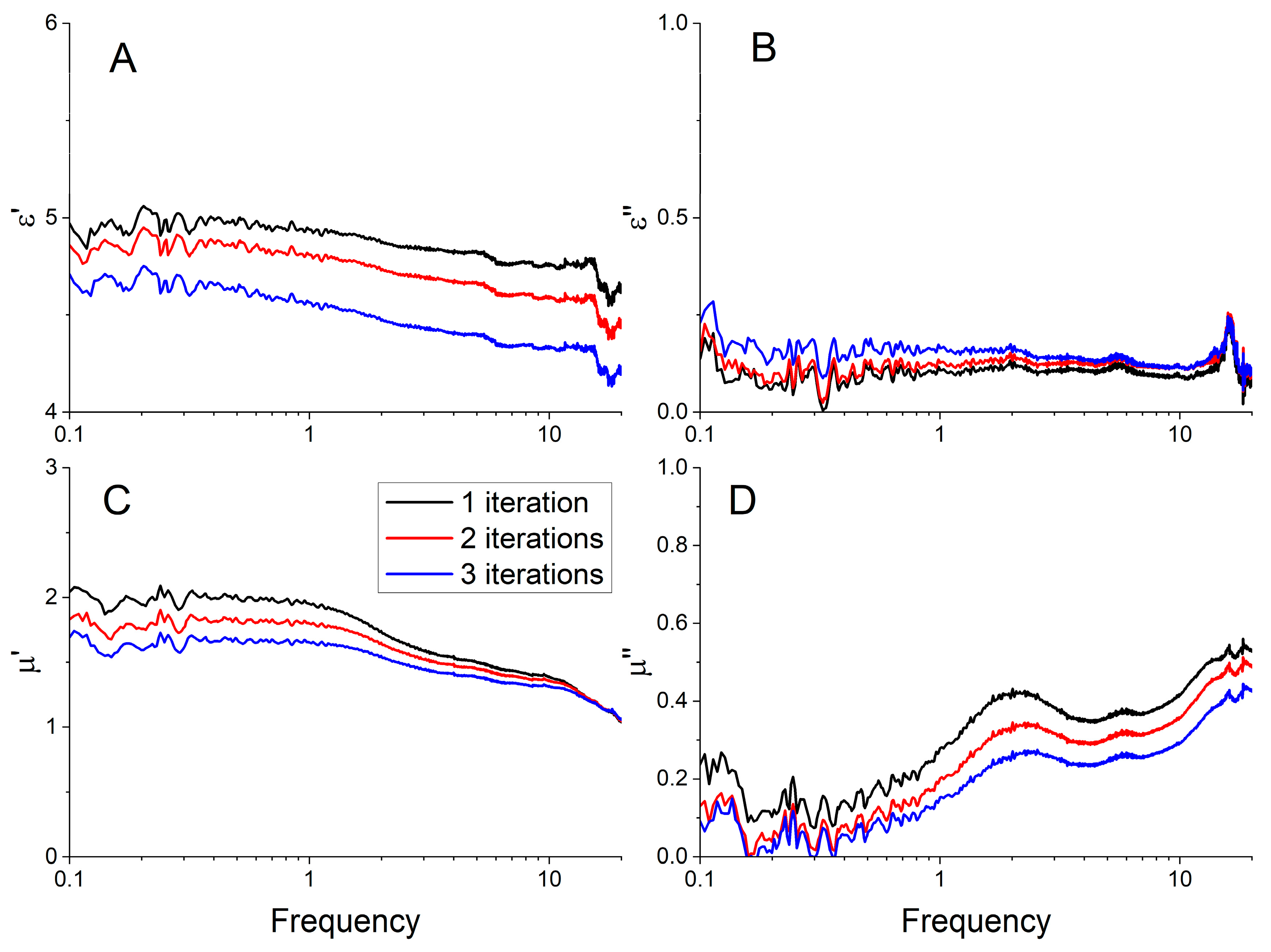
3.6. Microwave Permittivity and Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Micheli, D.; Apollo, C.; Pastore, R.; Marchetti, M. X-Band microwave characterization of carbon-based nanocomposite material, absorption capability comparison and RAS design simulation. Compos. Sci. Technol. 2010, 70, 400–409. [Google Scholar] [CrossRef]
- Feyyaz, O.; Aysegul, K. Electromagnetic Waves and Human Health. In Electromagnetic Waves; Vitaliy, Z., Ed.; IntechOpen: Rijeka, Croatia, 2011; p. 22. [Google Scholar]
- Kaszuba-Zwoińska, J.; Gremba, J.; Gałdzińska-Calik, B.; Wójcik-Piotrowicz, K.; Thor, P.J. Electromagnetic field induced biological effects in humans. Prz. Lek. 2015, 72, 636–641. [Google Scholar]
- Li, Y.Z.; Chen, S.H.; Zhao, K.F.; Gui, Y.; Fang, S.X.; Xu, Y.; Ma, Z.J. Effects of electromagnetic radiation on health and immune function of operators. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi = Zhonghua Laodong Weisheng Zhiyebing Zazhi = Chin. J. Ind. Hyg. Occup. Dis. 2013, 31, 602–605. [Google Scholar]
- Xu, K.; Sharma, A.; Kang, J.; Hu, X.; Hao, Z.; Zhu, W. Heterogeneous Electronic and Photonic Devices Based on Monolayer Ternary Telluride Core/Shell Structures. Adv. Mater. 2020, 32, 2002548. [Google Scholar] [CrossRef] [PubMed]
- Zamani Kouhpanji, M.R.; Stadler, B.J.H. A Guideline for Effectively Synthesizing and Characterizing Magnetic Nanoparticles for Advancing Nanobiotechnology: A Review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef]
- Barrera, G.; Coisson, M.; Celegato, F.; Martino, L.; Tiwari, P.; Verma, R.; Kane, S.N.; Mazaleyrat, F.; Tiberto, P. Specific Loss Power of Co/Li/Zn-Mixed Ferrite Powders for Magnetic Hyperthermia. Sensors 2020, 20, 2151. [Google Scholar] [CrossRef]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef]
- Khan, M.Z.; Hossain, M.; Khan, M.; Ali, M.; Aktar, S.; Moniruzzaman, M.; Khan, M. Influence of nanoparticle-based nano-nutrients on the growth performance and physiological parameters in tilapia (Oreochromis niloticus). RSC Adv. 2020, 10, 29918–29922. [Google Scholar] [CrossRef]
- Bombard, A.J.F.; Joekes, I.; Alcântara, M.R.; Knobel, M. Magnetic Susceptibility and Saturation Magnetization of some Carbonyl Iron Powders used in Magnetorheological Fluids. Mater. Sci. Forum 2003, 416–418, 753. [Google Scholar]
- Ge, C.; Wang, L.; Liu, G.; Wang, T.; Chen, H. Effects of particle size on electromagnetic properties of spherical carbonyl iron. J. Mater. Sci. Mater. Electron. 2019, 30, 8390–8398. [Google Scholar] [CrossRef]
- Weng, X.; Li, B.; Zhang, Y.; Lv, X.; Gu, G. Synthesis of flake shaped carbonyl iron/reduced graphene oxide/polyvinyl pyrrolidone ternary nanocomposites and their microwave absorbing properties. J. Alloys Compd. 2017, 695, 508–519. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Jacobo, S.E.; Lezama, L.; Kurlyandskaya, G.V.; Schegoleva, N.N. Microwave Resonant and Zero-Field Absorption Study of Doped Magnetite Prepared by a Co-Precipitation Method. Molecules 2014, 19, 8387–8401. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chou, M.-Y. Electronic Structure of Core-Shell Semiconductor Nanowires. 2006. Available online: https://ui.adsabs.harvard.edu/abs/2006APS..MARN31002Y/abstract (accessed on 6 December 2022).
- Nagarajan, S.; Yong, Z. Use of Core/Shell Structured Nanoparticles for Biomedical Applications. Recent Pat. Biomed. Eng. 2008, 1, 34–42. [Google Scholar]
- Kosevich, A.; Petrusevich, E.; Maklakov, S.; Naboko, A.; Kolesnikov, E.; Petrov, D.; Zezyulina, P.; Pokholok, K.; Filimonov, D.; Han, M. Low Weight Hollow Microspheres of Iron with Thin Dielectric Coating: Synthesis and Microwave Permeability. Coatings 2020, 10, 995. [Google Scholar] [CrossRef]
- Artemova, A.V.; Maklakov, S.S.; Osipov, A.V.; Petrov, D.A.; Shiryaev, A.O.; Rozanov, K.N.; Lagarkov, A.N. The Size Dependence of Microwave Permeability of Hollow Iron Particles. Sensors 2022, 22, 3086. [Google Scholar] [CrossRef]
- Yu, M.; Liang, C.; Liu, M.; Liu, X.; Yuan, K.; Cao, H.; Che, R. Yolk–shell Fe3O4-ZrO2 prepared by a tunable polymer surfactant assisted sol–gel method for high temperature stable microwave absorption. J. Mater. Chem. C 2014, 2, 7275–7283. [Google Scholar] [CrossRef]
- Maklakov, S.S.; Lagarkov, A.N.; Maklakov, S.A.; Adamovich, Y.A.; Petrov, D.A.; Rozanov, K.N.; Ryzhikov, I.A.; Zarubina, A.Y.; Pokholok, K.V.; Filimonov, D.S. Corrosion-resistive magnetic powder Fe-SiO2 for microwave applications. J. Alloys Compd. 2017, 706, 267–273. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Volodin, A.M.; Bespalko, Y.N. Effect of carbon shell on stabilization of single-phase lanthanum and praseodymium hexaaluminates prepared by a modified Pechini method. Ceram. Int. 2020, 46, 29150–29159. [Google Scholar] [CrossRef]
- Yakovlev, I.V.; Volodin, A.M.; Zaikovskii, V.I.; Stoyanovskii, V.O.; Lapina, O.B.; Vedyagin, A.A. Stabilizing effect of the carbon shell on phase transformation of the nanocrystalline alumina particles. Ceram. Int. 2018, 44, 4801–4806. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Shuvarakova, E.I.; Volodin, A.M. Silica-coated nanocrystalline TiO2 with improved thermal stability. Ceram. Int. 2019, 45, 3547–3553. [Google Scholar] [CrossRef]
- Chiriac, H.; Herea, D.-D.; Corodeanu, S. Microwire array for giant magneto-impedance detection of magnetic particles for biosensor prototype. J. Magn. Magn. Mater. 2007, 311, 425–428. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Feng, C.; Jin, C.; Li, W. Synthesis, magnetic and electromagnetic properties of Al2O3/Fe oxides composite-coated polyhedral Fe core–shell nanoparticles. Appl. Surf. Sci. 2013, 280, 132–137. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Liu, Z.; Liu, X.; Che, R. Hierarchical magnetic core-shell nanostructures for microwave absorption: Synthesis, microstructure and property studies. Sci. China Chem. 2014, 57, 3–12. [Google Scholar] [CrossRef]
- Zolfagharinia, S.; Kolvari, E.; Koukabi, N.; Hosseini, M.M. Core-shell zirconia-coated magnetic nanoparticles offering a strong option to prepare a novel and magnetized heteropolyacid based heterogeneous nanocatalyst for three- and four-component reactions. Arab. J. Chem. 2020, 13, 227–241. [Google Scholar] [CrossRef]
- Luo, H.; Gong, R.; Wang, X.; Song, K.; Chen, Y.; Harris, V.G. Enhanced Microwave Absorption of SiO2-Coated Fe0.65Co0.35 Flakes at a Wide Frequency Band (1–18 GHz). J. Electron. Mater. 2016, 45, 3640–3645. [Google Scholar] [CrossRef]
- Cao, C.; Ma, Z.; Ma, C.; Pan, W.; Liu, Q.; Wang, J. Synthesis and characterization of Fe/C core–shell nanoparticles. Mater. Lett. 2012, 88, 61–64. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Lu, X.; Zhu, Y.; Jiang, L. Self-healing superhydrophobic polyvinylidene fluoride/Fe3O4-polypyrrole fiber with core–sheath structures for superior microwave absorption. Nano Res. 2016, 9, 2034–2045. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, Z.; Wu, R. Synthesis of iron-based hexagonal microflakes for strong microwave attenuation. J. Alloys Compd. 2017, 718, 46–52. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, J.; Che, R.; Xu, J.; Liu, M.; Liu, Z. Synthesis and Microwave Absorption Properties of Yolk–Shell Microspheres with Magnetic Iron Oxide Cores and Hierarchical Copper Silicate Shells. ACS Appl. Mater. Interfaces 2013, 5, 2503–2509. [Google Scholar] [CrossRef]
- Sun, X.; Ma, G.; Lv, X.; Sui, M.; Li, H.; Wu, F.; Wang, J. Controllable Fabrication of Fe3O4/ZnO Core–Shell Nanocomposites and Their Electromagnetic Wave Absorption Performance in the 2–18 GHz Frequency Range. Materials 2018, 11, 780. [Google Scholar] [CrossRef]
- Liu, X.G.; Geng, D.Y.; Meng, H.; Shang, P.J.; Zhang, Z.D. Microwave-absorption properties of ZnO-coated iron nanocapsules. Appl. Phys. Lett. 2008, 92, 173117. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Shi, Z.C.; Fan, R.H.; Gao, M.; Guo, J.Y.; Qi, X.G.; Sun, K.N. Microwave absorption properties of Fe-Al2O3 nanoembedments prepared by mechanosynthesis. Mater. Chem. Phys. 2011, 130, 615–618. [Google Scholar] [CrossRef]
- Xi, L.; Wang, Z.; Zuo, Y.; Shi, X. The enhanced microwave absorption property of CoFe2O4nanoparticles coated with a Co3Fe7–Co nanoshell by thermal reduction. Nanotechnology 2010, 22, 045707. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Mrlík, M.; Ilčíková, M.; Mosnáček, J.; Münster, L.; Pavlínek, V. Synthesis of Silicone Elastomers Containing Silyl-Based Polymer-Grafted Carbonyl Iron Particles: An Efficient Way To Improve Magnetorheological, Damping, and Sensing Performances. Macromolecules 2017, 50, 2189–2200. [Google Scholar] [CrossRef]
- Xu, J.; Pei, L.; Li, J.; Pang, H.; Li, Z.; Li, B.; Xuan, S.; Gong, X. Flexible, self-powered, magnetism/pressure dual-mode sensor based on magnetorheological plastomer. Compos. Sci. Technol. 2019, 183, 107820. [Google Scholar] [CrossRef]
- Wright, R.F.; Lu, P.; Ziomek-Moroz, M.; Ohodnicki, P.R. Optical Fiber-Based Corrosion Sensor with Fe/SiO2 Coating. ECS Meet. Abstr. 2019. [Google Scholar] [CrossRef]
- Sung, H.K.; Oh, S.Y.; Park, C.; Kim, Y. Colorimetric Detection of Co2+ Ion Using Silver Nanoparticles with Spherical, Plate, and Rod Shapes. Langmuir 2013, 29, 8978–8982. [Google Scholar] [CrossRef]
- Jeong, U.; Shin, H.H.; Kim, Y. Functionalized magnetic core–shell Fe-SiO2 nanoparticles as recoverable colorimetric sensor for Co2+ ion. Chem. Eng. J. 2015, 281, 428–433. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Dolmatov, A.V.; Maklakov, S.S.; Zezyulina, P.A.; Osipov, A.V.; Petrov, D.A.; Naboko, A.S.; Polozov, V.I.; Maklakov, S.A.; Starostenko, S.N.; Lagarkov, A.N. Deposition of a SiO2 Shell of Variable Thickness and Chemical Composition to Carbonyl Iron: Synthesis and Microwave Measurements. Sensors 2021, 21, 4624. [Google Scholar] [CrossRef]
- Engen, G.F.; Hoer, C.A. Thru-Reflect-Line: An Improved Technique for Calibrating the Dual Six-Port Automatic Network Analyzer. IEEE Trans. Microw. Theory Tech. 1979, 27, 987–993. [Google Scholar] [CrossRef]
- Nicolson, A.M.; Ross, G.F. Measurement of the Intrinsic Properties of Materials by Time-Domain Techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Weir, W.B. Automatic measurement of complex dielectric constant and permeability at microwave frequencies. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Petrov, D.A.; Rozanov, K.N.; Koledintseva, M.Y. Influence of Higher-order Modes in Coaxial Waveguide on Measurements of Material Parameters. In Proceedings of the 2018 IEEE Symposium on Electromagnetic Compatibility, Signal Integrity and Power Integrity (EMC, SI & PI), Long Beach, CA, USA, 30 July –3 August 2018; pp. 66–70. [Google Scholar]
- LeFrancois, S.; Pasquet, D.; Maze-Merceur, G. A new model for microwave characterization of composite materials in guided-wave medium. IEEE Trans. Microw. Theory Tech. 1996, 44, 1557–1562. [Google Scholar] [CrossRef]
- Karkkainen, K.; Sihvola, A.; Nikoskinen, K. Analysis of a three-dimensional dielectric mixture with finite difference method. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1013–1018. [Google Scholar] [CrossRef]
- Chettiar, U.K.; Engheta, N. Internal homogenization: Effective permittivity of a coated sphere. Opt. Express 2012, 20, 22976–22986. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Ortiz, D.; Alcaraz de la Osa, R.; Saiz, J.M.; González, F.; Moreno, F. Electromagnetic Effective Medium Modelling of Composites with Metal-Semiconductor Core-Shell Type Inclusions. Catalysts 2019, 9, 626. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant oxides. Eur. Phys. J. Appl. Phys. 2004, 28, 265–291. [Google Scholar] [CrossRef]
- Wang, C.; Baer, D.R.; Amonette, J.E.; Engelhard, M.H.; Antony, J.; Qiang, Y. Morphology and Electronic Structure of the Oxide Shell on the Surface of Iron Nanoparticles. J. Am. Chem. Soc. 2009, 131, 8824–8832. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Surzhikov, A.P.; Zhuravkov, S.P.; Vlasov, V.A.; Pustovalov, A.V.; Yavorovsky, N.A. The oxidation kinetics study of ultrafine iron powders by thermogravimetric analysis. J. Therm. Anal. Calorim. 2014, 115, 1447–1452. [Google Scholar] [CrossRef]
- James Abraham, J.; Nisar, U.; Monawwar, H.; Abdul Quddus, A.; Shakoor, R.A.; Saleh, M.I.; Kahraman, R.; Al-Qaradawi, S.; Aljaber, A.S. Improved electrochemical performance of SiO2-coated Li-rich layered oxides-Li1.2Ni0.13Mn0.54Co0.13O2. J. Mater. Sci. Mater. Electron. 2020, 31, 19475–19486. [Google Scholar] [CrossRef]
- Jäger, N.; Meindlhumer, M.; Zitek, M.; Spor, S.; Hruby, H.; Nahif, F.; Julin, J.; Rosenthal, M.; Keckes, J.; Mitterer, C.; et al. Impact of Si on the high-temperature oxidation of AlCr(Si)N coatings. J. Mater. Sci. Technol. 2022, 100, 91–100. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. A novel coating material that uses nano-sized SiO2 particles to intensify hydrophobicity and corrosion protection properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]







| Number of Iterations | 1 | 2 | 3 |
|---|---|---|---|
| Thickness of SiO2 | 151 ± 19 nm | 278 ± 30 nm | 450 ± 26 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolmatov, A.V.; Maklakov, S.S.; Artemova, A.V.; Petrov, D.A.; Shiryaev, A.O.; Lagarkov, A.N. Deposition of Thick SiO2 Coatings to Carbonyl Iron Microparticles for Thermal Stability and Microwave Performance. Sensors 2023, 23, 1727. https://doi.org/10.3390/s23031727
Dolmatov AV, Maklakov SS, Artemova AV, Petrov DA, Shiryaev AO, Lagarkov AN. Deposition of Thick SiO2 Coatings to Carbonyl Iron Microparticles for Thermal Stability and Microwave Performance. Sensors. 2023; 23(3):1727. https://doi.org/10.3390/s23031727
Chicago/Turabian StyleDolmatov, Arthur V., Sergey S. Maklakov, Anastasia V. Artemova, Dmitry A. Petrov, Artem O. Shiryaev, and Andrey N. Lagarkov. 2023. "Deposition of Thick SiO2 Coatings to Carbonyl Iron Microparticles for Thermal Stability and Microwave Performance" Sensors 23, no. 3: 1727. https://doi.org/10.3390/s23031727
APA StyleDolmatov, A. V., Maklakov, S. S., Artemova, A. V., Petrov, D. A., Shiryaev, A. O., & Lagarkov, A. N. (2023). Deposition of Thick SiO2 Coatings to Carbonyl Iron Microparticles for Thermal Stability and Microwave Performance. Sensors, 23(3), 1727. https://doi.org/10.3390/s23031727







