Light-Sensing Properties of Amorphous Vanadium Oxide Films Prepared by RF Sputtering
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Morpho-Structural Investigations
3.2. Absorption and Transmission Measurements on VxOy Films
3.3. Electrical I−V and C−V Characteristics
3.4. Current−Time Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Lee, S.; Yang, S.; Delaire, O.; Wu, J. Recent Progresses on Physics and Applications of Vanadium Dioxide. Mater. Today 2018, 21, 875–896. [Google Scholar] [CrossRef]
- Miyazaki, H.; Matsuura, T.; Ota, T. Vanadium Oxide-Based Photochromic Composite Film. RSC Adv. 2017, 7, 2388–2391. [Google Scholar] [CrossRef]
- Chang, T.; Cao, X.; Dedon, L.R.; Long, S.; Huang, A.; Shao, Z.; Li, N.; Luo, H.; Jin, P. Optical Design and Stability Study for Ultrahigh-Performance and Long-Lived Vanadium Dioxide-Based Thermochromic Coatings. Nano Energy 2018, 44, 256–264. [Google Scholar] [CrossRef]
- Mjejri, I.; Manceriu, L.M.; Gaudon, M.; Rougier, A.; Sediri, F. Nano-Vanadium Pentoxide Films for Electrochromic Displays. Solid State Ion. 2016, 292, 8–14. [Google Scholar] [CrossRef]
- Schneider, K. Optical Properties and Electronic Structure of V2O5, V2O3 and VO2. J. Mater. Sci. Mater. Electron. 2020, 31, 10478–10488. [Google Scholar] [CrossRef]
- McLeod, A.S.; Van Heumen, E.; Ramirez, J.G.; Wang, S.; Saerbeck, T.; Guenon, S.; Goldflam, M.; Anderegg, L.; Kelly, P.; Mueller, A.; et al. Nanotextured Phase Coexistence in the Correlated Insulator V2O3. Nat. Phys. 2017, 13, 80–86. [Google Scholar] [CrossRef]
- Lupi, S.; Baldassarre, L.; Mansart, B.; Perucchi, A.; Barinov, A.; Dudin, P.; Papalazarou, E.; Rodolakis, F.; Rueff, J.P.; Itié, J.P.; et al. A Microscopic View on the Mott Transition in Chromium-Doped V2O3. Nat. Commun. 2010, 1, 105. [Google Scholar] [CrossRef]
- Jeong, J.; Aetukuri, N.; Graf, T.; Schladt, T.D.; Samant, M.G.; Parkin, S.S.P. Suppression of Metal-Insulator Transition in VO2 by Electric Field-Induced Oxygen Vacancy Formation. Science 2013, 339, 1402–1405. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Ren, H.; Chen, Y.; Yan, W.; Wang, C.; Li, B.; Jiang, J.; Zou, C. Gate-Controlled VO 2 Phase Transition for High-Performance Smart Windows. Sci. Adv. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Chernova, N.A.; Roppolo, M.; Dillon, C.; Whittingham, M.S. Layered Vanadium and Molybdenum Oxides: Batteries and Electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Takahashi, K.; Wang, Y.; Cao, G.; Takahashi, K.; Wang, Y.; Cao, G. Growth and Electrochromic Properties of Single-Crystal V2O5 Nanorod Arrays. Appl. Phys. Lett. 2005, 86, 53102. [Google Scholar] [CrossRef]
- Tong, Z.; Lv, H.; Zhang, X.; Yang, H.; Tian, Y.; Li, N. Novel Morphology Changes from 3D Ordered Macroporous Structure to V2O5 Nanofiber Grassland and Its Application in Electrochromism. Sci. Rep. 2015, 5, 16864. [Google Scholar] [CrossRef] [PubMed]
- Romanitan, C.; Tudose, I.V.; Mouratis, K.; Popescu, M.; Pachiu, C.; Couris, S.; Koudoumas, E.; Suchea, M.P. Structural Investigations in Electrochromic Vanadium Pentoxide Thin Films. Phys. Status Solidi 2022, 219, 2100431. [Google Scholar] [CrossRef]
- Kikalov, D.O.; Malinenko, V.P.; Pergament, A.L.; Stefanovich, G.B. Optical Properties of Thin Films of Amorphous Vanadium Oxides. Tech. Phys. Lett. 1999, 25, 331–333. [Google Scholar] [CrossRef]
- Lee, S.H.; Cheong, H.M.; Seong, M.J.; Liu, P.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman Spectroscopic Studies of Amorphous Vanadium Oxide Thin Films. Solid State Ionics 2003, 165, 111–116. [Google Scholar] [CrossRef]
- Uchaker, E.; Zheng, Y.Z.; Li, S.; Candelaria, S.L.; Hu, S.; Cao, G.Z. Better than Crystalline: Amorphous Vanadium Oxide for Sodium-Ion Batteries. J. Mater. Chem. A 2014, 2, 18208–18214. [Google Scholar] [CrossRef]
- Petnikota, S.; Chua, R.; Zhou, Y.; Edison, E.; Srinivasan, M. Amorphous Vanadium Oxide Thin Films as Stable Performing Cathodes of Lithium and Sodium-Ion Batteries. Nanoscale Res. Lett. 2018, 13, 363. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, Z.; Li, Y. Enhanced Lithium Storage Performance of V2O5. RSC Adv. 2018, 8, 39371–39376. [Google Scholar] [CrossRef]
- Chiku, M.; Takeda, H.; Matsumura, S.; Higuchi, E.; Inoue, H. Amorphous Vanadium Oxide/Carbon Composite Positive Electrode for Rechargeable Aluminum Battery. ACS Appl. Mater. Interfaces 2015, 7, 24385–24389. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, W.; Chen, W.; Zheng, Y. Recent Progress on Vanadium Dioxide Nanostructures and Devices: Fabrication, Properties, Applications and Perspectives. Nanomaterials 2021, 11, 388. [Google Scholar] [CrossRef]
- Mazur, M.; Lubańska, A.; Domaradzki, J.; Wojcieszak, D. Complex Research on Amorphous Vanadium Oxide Thin Films Deposited by Gas Impulse Magnetron Sputtering. Appl. Sci. 2022, 12, 8966. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, J.; Feng, H.; Cui, J. Study on Mixed Vanadium Oxide Thin Film Deposited by RF Magnetron Sputtering and Its Application. Phys. Procedia 2011, 18, 73–76. [Google Scholar] [CrossRef]
- Kim, H.S.; Chauhan, K.R.; Kim, J.; Choi, E.H. Flexible Vanadium Oxide Film for Broadband Transparent Photodetector. Appl. Phys. Lett. 2017, 110, 101909. [Google Scholar] [CrossRef]
- Umar, Z.A.; Ahmed, R.; Asghar, H.; Liaqat, U.; Fayyaz, A.; Baig, M.A. VO2 Thin Film Based Highly Responsive and Fast VIS/IR Photodetector. Mater. Chem. Phys. 2022, 290, 126655. [Google Scholar] [CrossRef]
- Wang, S.; Wu, L.; Zhang, H.; Wang, Z.; Qin, Q.; Wang, X.; Lu, Y.; Li, L.; Li, M. Facile Synthesis of Two Dimensional (2D) V2O5 Nanosheets Film towards Photodetectors. Materials 2022, 15, 8313. [Google Scholar] [CrossRef]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Julien, C.M. Growth and Surface Characterization of V2O5 Thin Films Made by Pulsed-Laser Deposition. J. Vac. Sci. Technol. Vac. Surf. Film. 2004, 22, 2453–2458. [Google Scholar] [CrossRef]
- Meng, L.J.; Silva, R.A.; Cui, H.N.; Teixeira, V.; dos Santos, M.P.; Xu, Z. Optical and Structural Properties of Vanadium Pentoxide Films Prepared by d.c. Reactive Magnetron Sputtering. Thin Solid Films 2006, 515, 195–200. [Google Scholar] [CrossRef]
- Choi, S.G.; Seok, H.J.; Rhee, S.; Hahm, D.; Bae, W.K.; Kim, H.K. Magnetron-Sputtered Amorphous V2O5 Hole Injection Layer for High Performance Quantum Dot Light-Emitting Diode. J. Alloys Compd. 2021, 878, 160303. [Google Scholar] [CrossRef]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Julien, C.M. On the Growth Mechanism of Pulsed-Laser Deposited Vanadium Oxide Thin Films. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 218–225. [Google Scholar] [CrossRef]
- Silversmit, G.; Poelman, H.; De Gryse, R. Influence of Magnetron Deposition Parameters on the Stoichiometry of Sputtered V2O5 Films. Surf. Interface Anal. 2004, 36, 1163–1166. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS Studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectros. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Özer, N. Electrochemical Properties of Sol-Gel Deposited Vanadium Pentoxide Films. Thin Solid Films 1997, 305, 80–87. [Google Scholar] [CrossRef]
- Baltakesmez, A.; Aykaç, C.; ·Güzeldir, B. Phase transition and changing properties of nanostructured V2O5 thin films deposited by spray pyrolysis technique, as a function of tungsten dopant. Appl. Phys. A 2019, 125, 441. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Q.; Koughia, C.; Ye, F.; Sanayei, M.; Wen, S.-J.; Kasap, S. Characterization of vanadium oxide thin films with different stoichiometry using Raman spectroscopy. Thin Solid Films 2016, 620, 64–69. [Google Scholar] [CrossRef]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides, J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc Method for Optical Absorption Edge Determination: ZnO Thin Films as a Model System. Phys. Status Solidi Basic Res. 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Meyn, J.P.; Danger, T.; Petermann, K.; Huber, G. Spectroscopic Characterization of V4+ -Doped Al2O3 and YAlO3. J. Lumin. 1993, 55, 55–62. [Google Scholar] [CrossRef]
- Nasim, F.; Ali, A.; Bhatti, A.S.; Naseem, S. Temperature Dependent Tuning of the Flat Band Voltages of TiO2/Si Interfaces. J. Appl. Phys. 2011, 110, 114517. [Google Scholar] [CrossRef]
- Wang, Q.; Brier, M.; Joshi, S.; Puntambekar, A.; Chakrapani, V. Defect-Induced Burstein-Moss Shift in Reduced V2O5 Nanostructures. Phys. Rev. B 2016, 94, 45305. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Ahmed, N.; Hassan, Z.; Bououdina, M. High-Performance p–n Heterojunction Photodetectors Based on V2O5 Nanorods by Spray Pyrolysis. Appl. Phys. A 2016, 122, 817. [Google Scholar] [CrossRef]
- Guo, X.; Tan, Y.; Hu, Y.; Zafar, Z.; Liu, J.; Zou, J. High quality VO2 thin films synthesized from V2O5 powder for sensitive near-infrared detection. Sci. Rep. 2021, 11, 21749. [Google Scholar] [CrossRef] [PubMed]
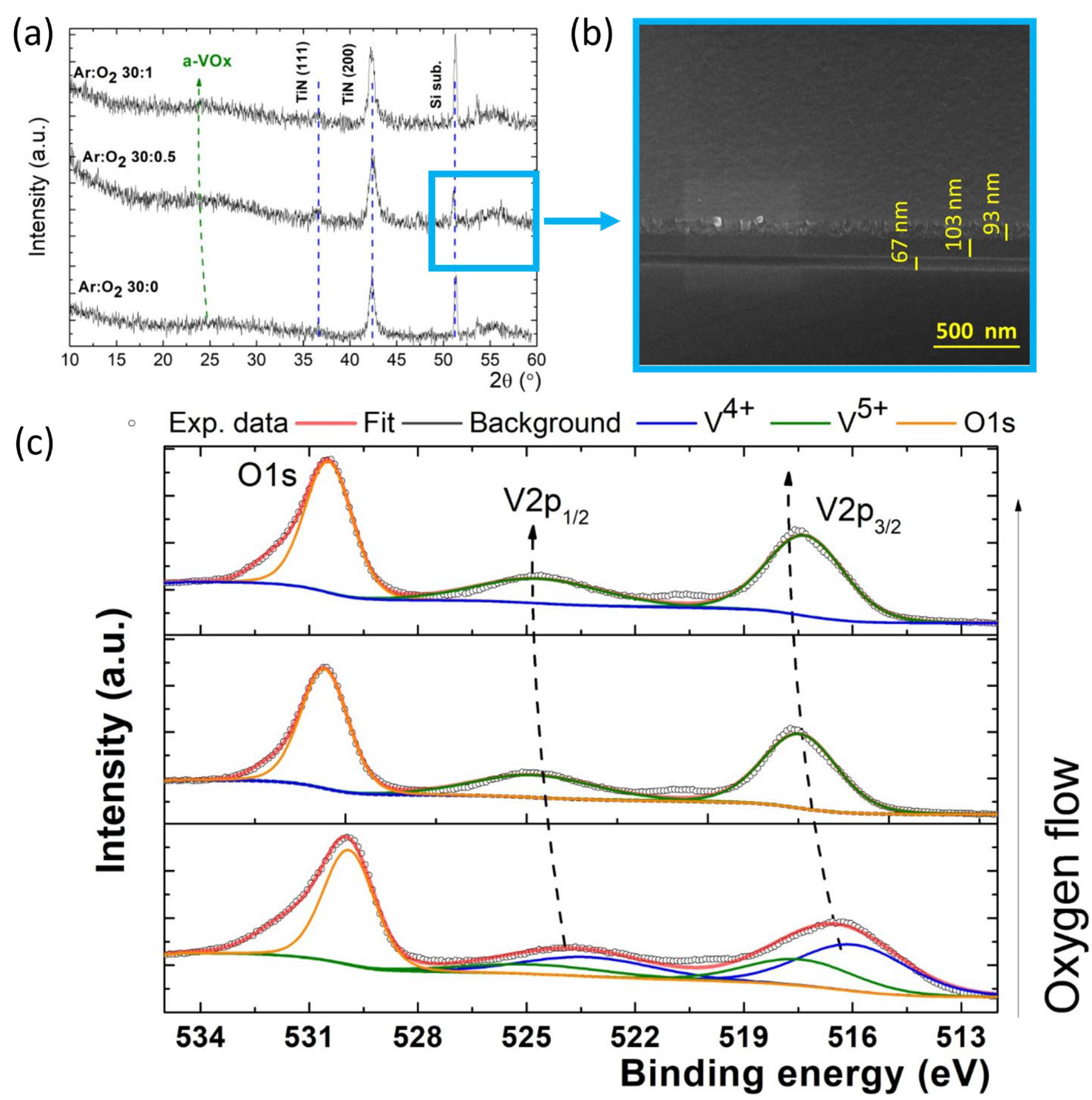
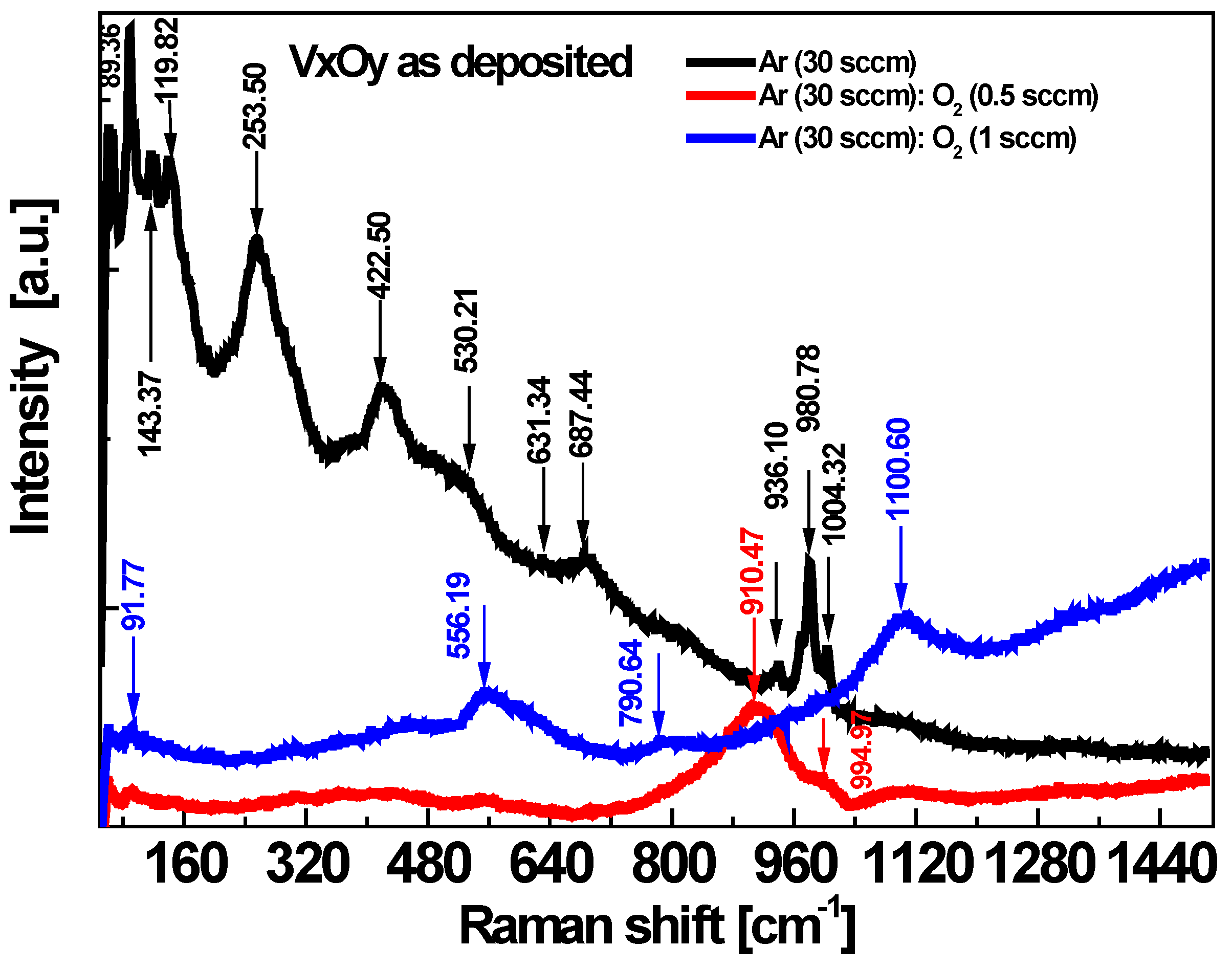
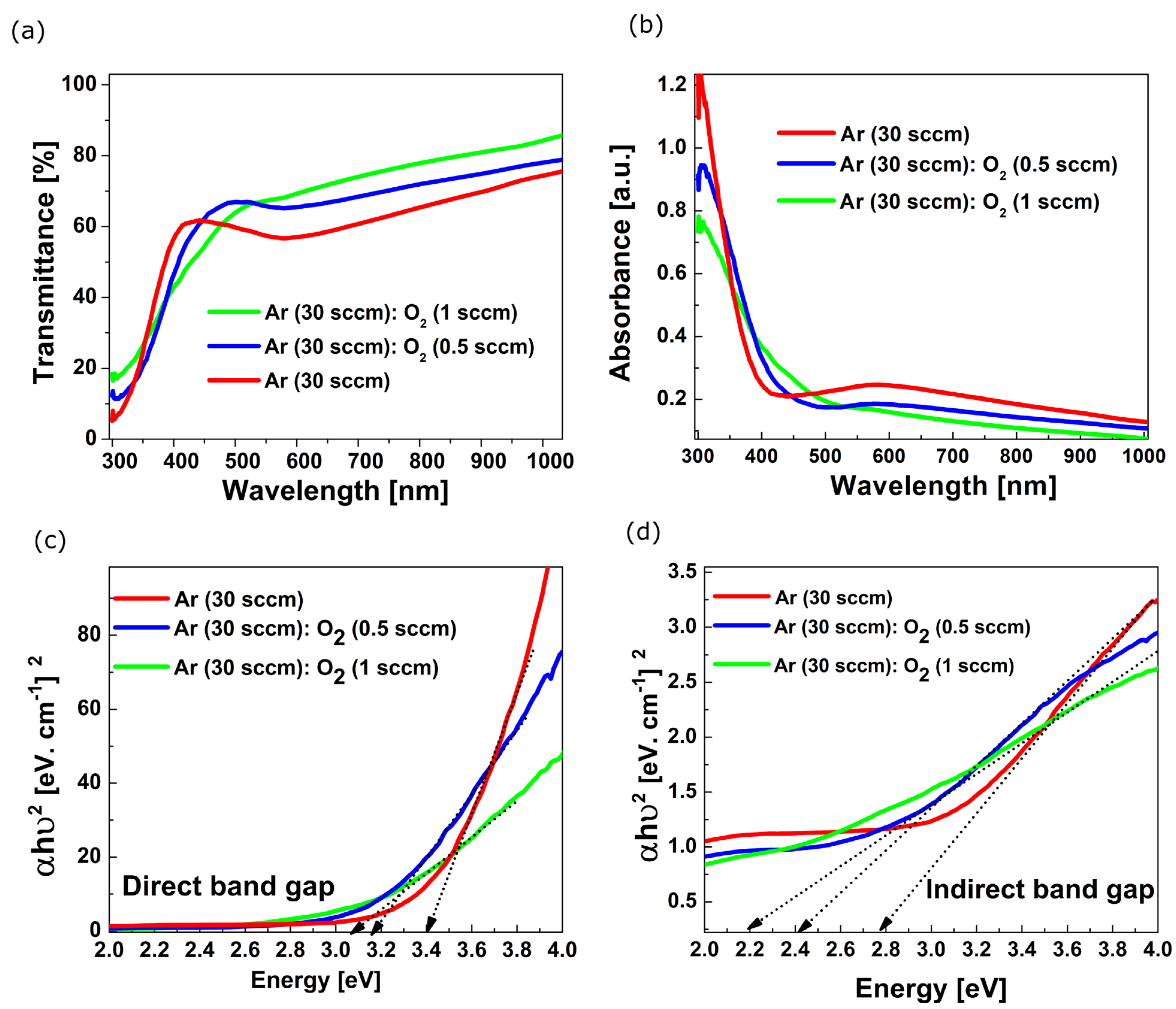

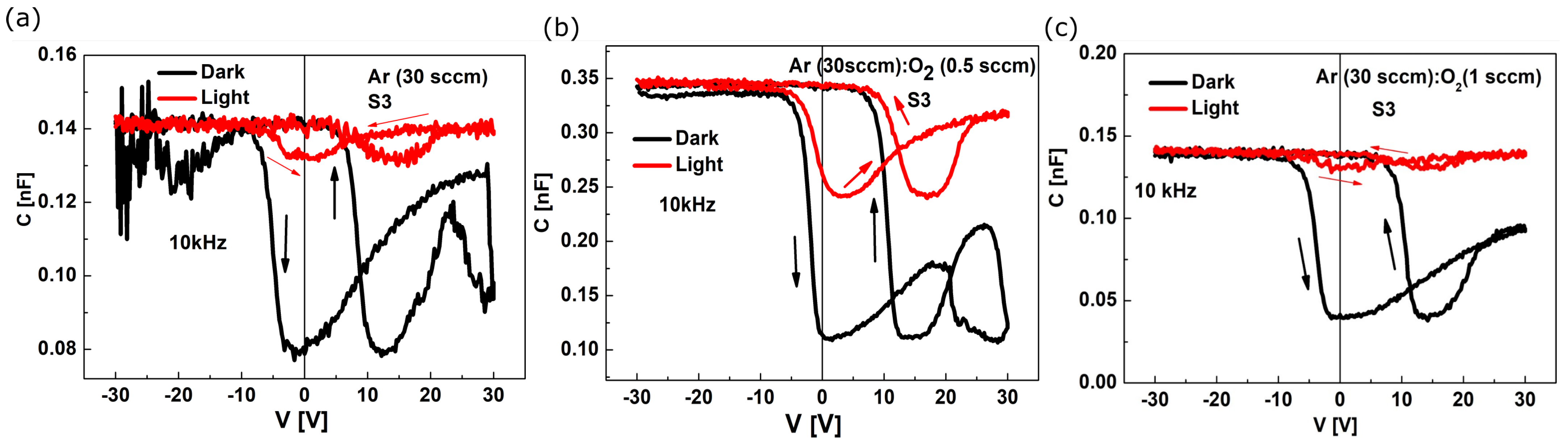


| Film Deposition Process | Egopt Direct [eV] | Egopt Indirect [eV] |
|---|---|---|
| (A) Ar (30 sccm) | 3.37 | 2.76 |
| (B) Ar (30 sccm):O2 (0.5 sccm) | 3.16 | 2.39 |
| (C) Ar (30 sccm):O2 (1 sccm) | 3.08 | 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plugaru, R.; Mihalache, I.; Romaniţan, C.; Comanescu, F.; Vulpe, S.; Craciun, G.; Plugaru, N.; Djourelov, N. Light-Sensing Properties of Amorphous Vanadium Oxide Films Prepared by RF Sputtering. Sensors 2023, 23, 1759. https://doi.org/10.3390/s23041759
Plugaru R, Mihalache I, Romaniţan C, Comanescu F, Vulpe S, Craciun G, Plugaru N, Djourelov N. Light-Sensing Properties of Amorphous Vanadium Oxide Films Prepared by RF Sputtering. Sensors. 2023; 23(4):1759. https://doi.org/10.3390/s23041759
Chicago/Turabian StylePlugaru, Rodica, Iuliana Mihalache, Cosmin Romaniţan, Florin Comanescu, Silviu Vulpe, Gabriel Craciun, Neculai Plugaru, and Nikolay Djourelov. 2023. "Light-Sensing Properties of Amorphous Vanadium Oxide Films Prepared by RF Sputtering" Sensors 23, no. 4: 1759. https://doi.org/10.3390/s23041759
APA StylePlugaru, R., Mihalache, I., Romaniţan, C., Comanescu, F., Vulpe, S., Craciun, G., Plugaru, N., & Djourelov, N. (2023). Light-Sensing Properties of Amorphous Vanadium Oxide Films Prepared by RF Sputtering. Sensors, 23(4), 1759. https://doi.org/10.3390/s23041759








