A Parametric Model for the Analysis of the Impedance Spectra of Dielectric Sensors in Curing Epoxy Resins
Abstract
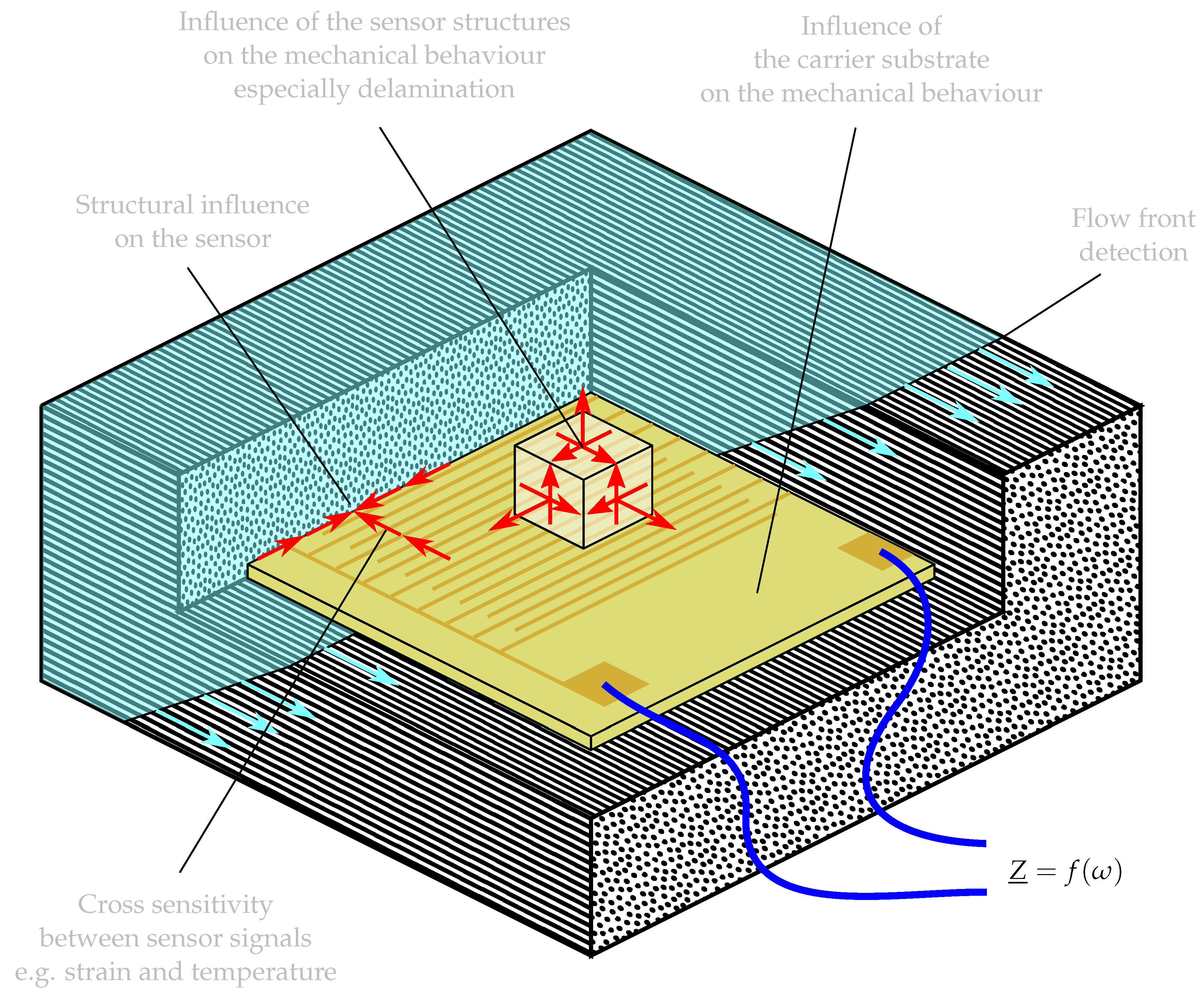
:1. Introduction
2. Materials and Methods
2.1. Modelling Electrode Polarisation and Ion Conduction
2.2. Dipole Relaxation Model
2.3. Fitting Algorithm
2.4. Cure Experiment
3. Results
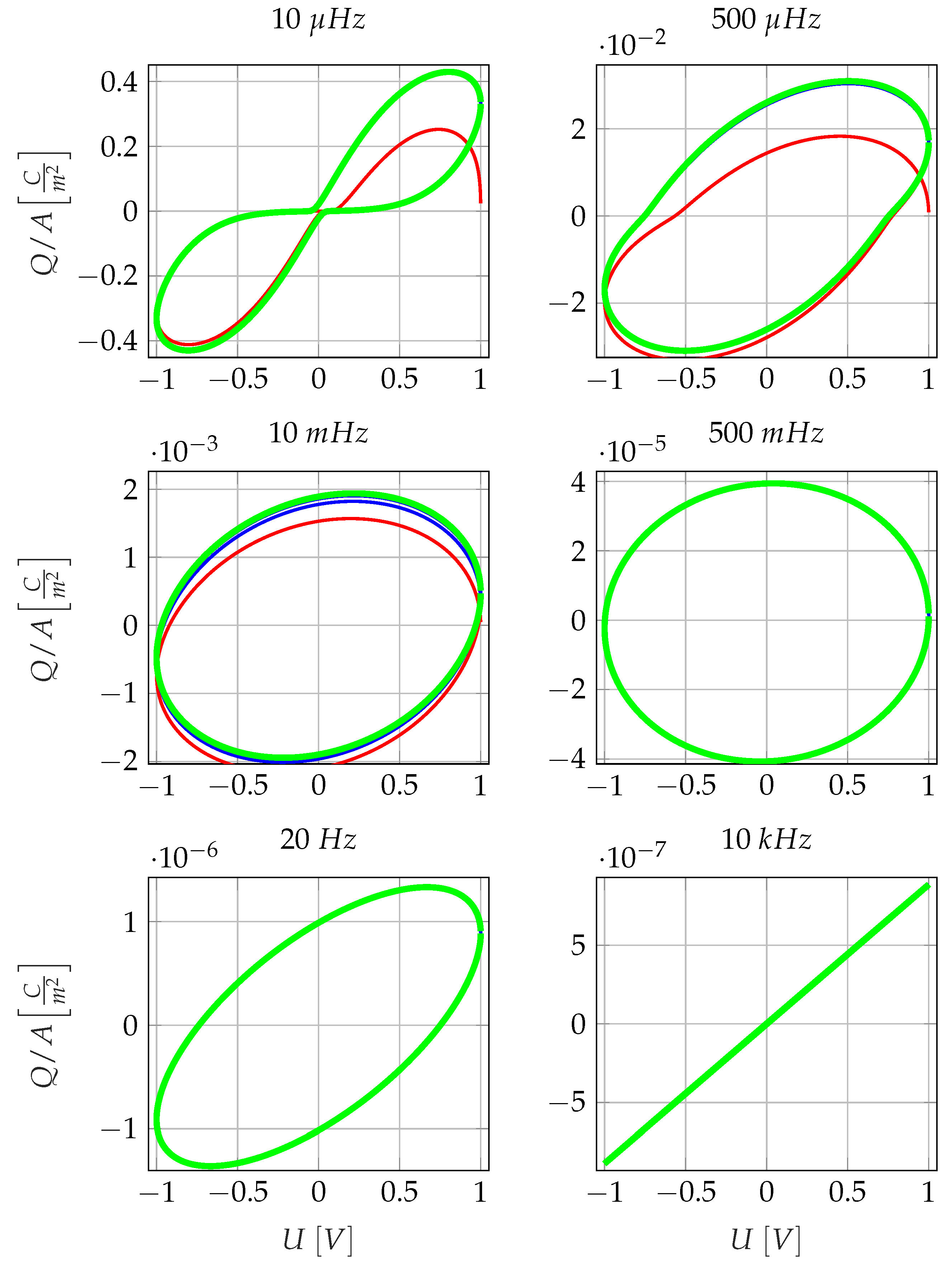
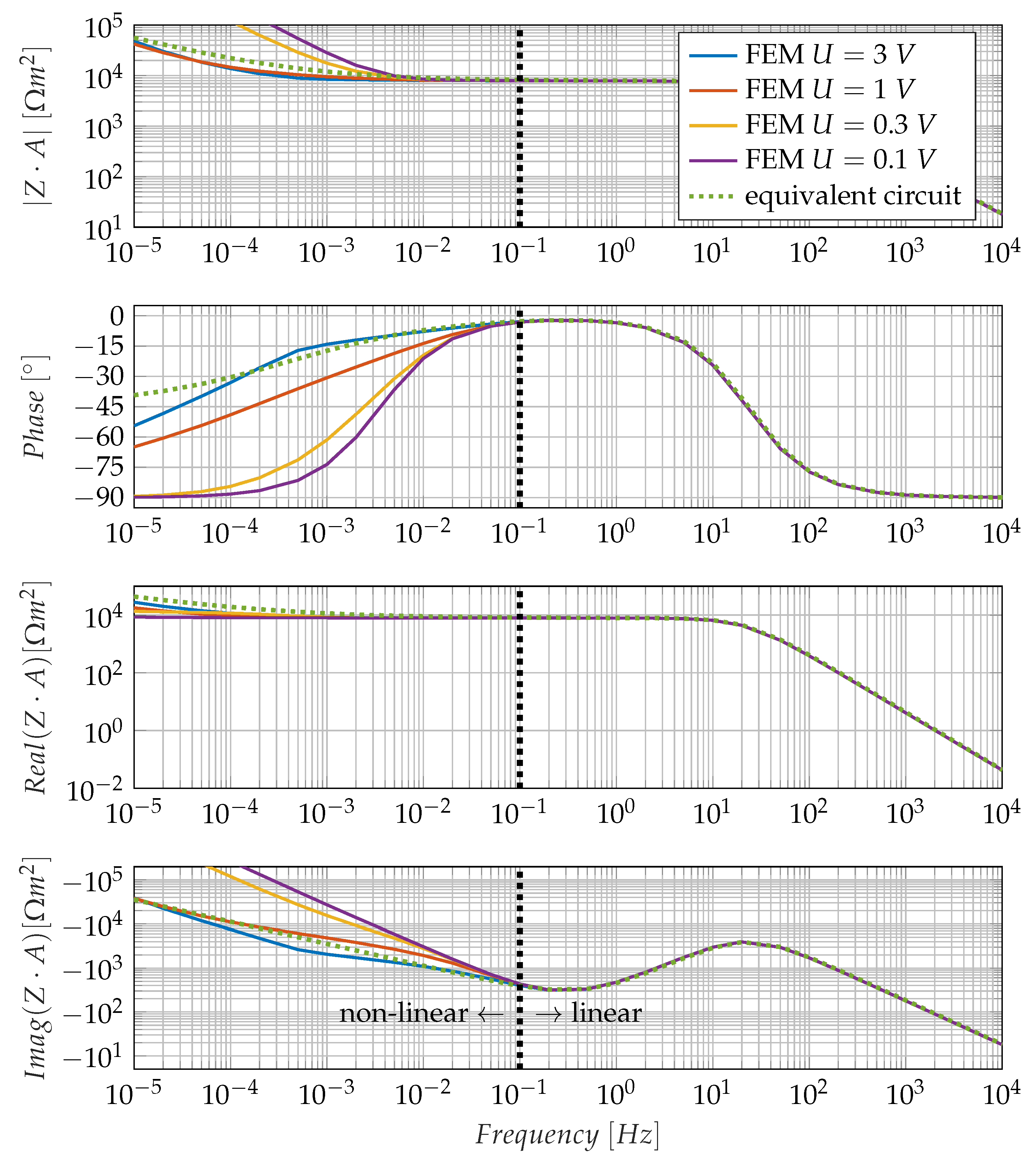
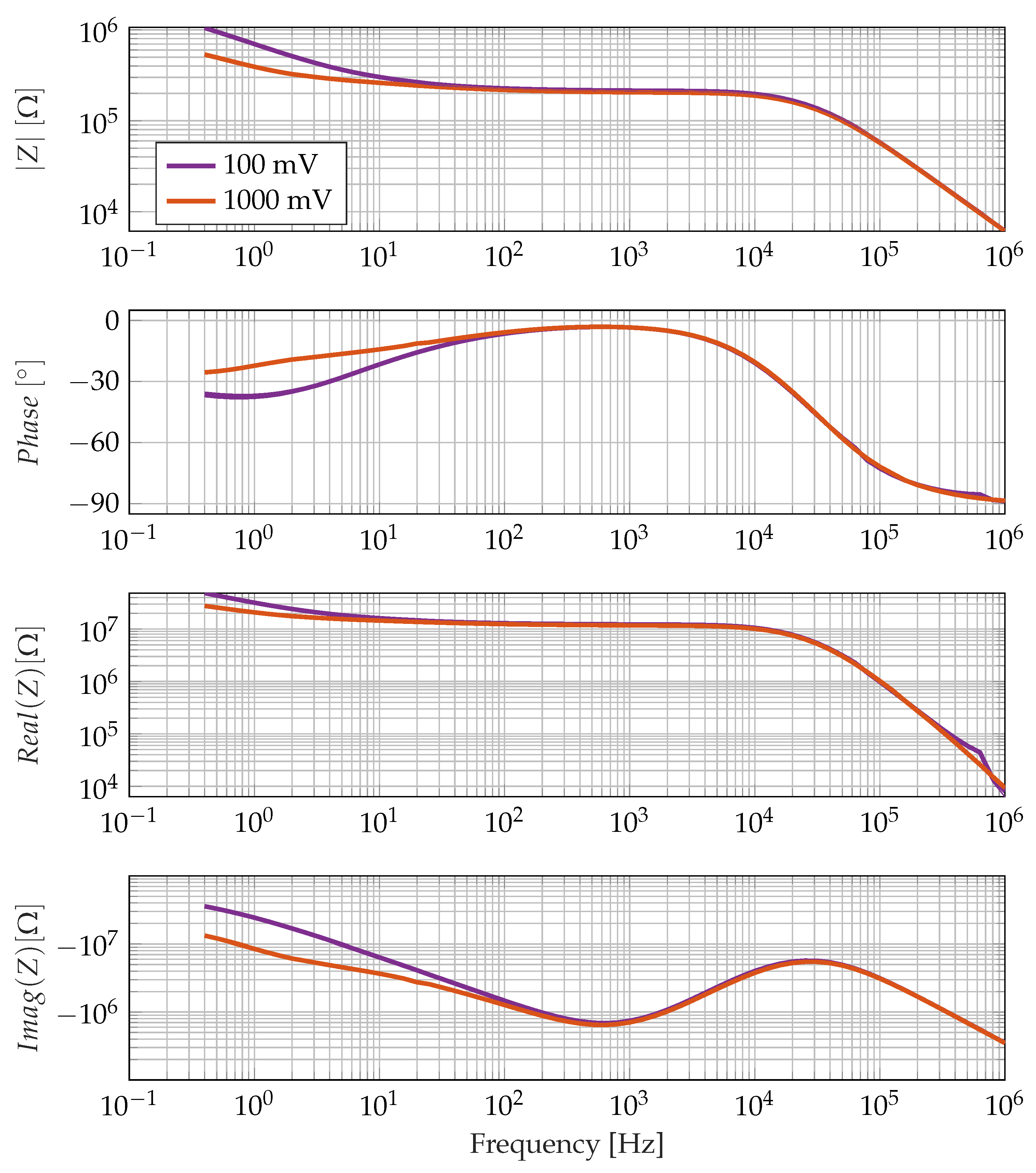
3.1. Non-Linearity of the Low-Frequency Electrode Polarisation Effect
3.2. Calorimetric Curing Process Analysis
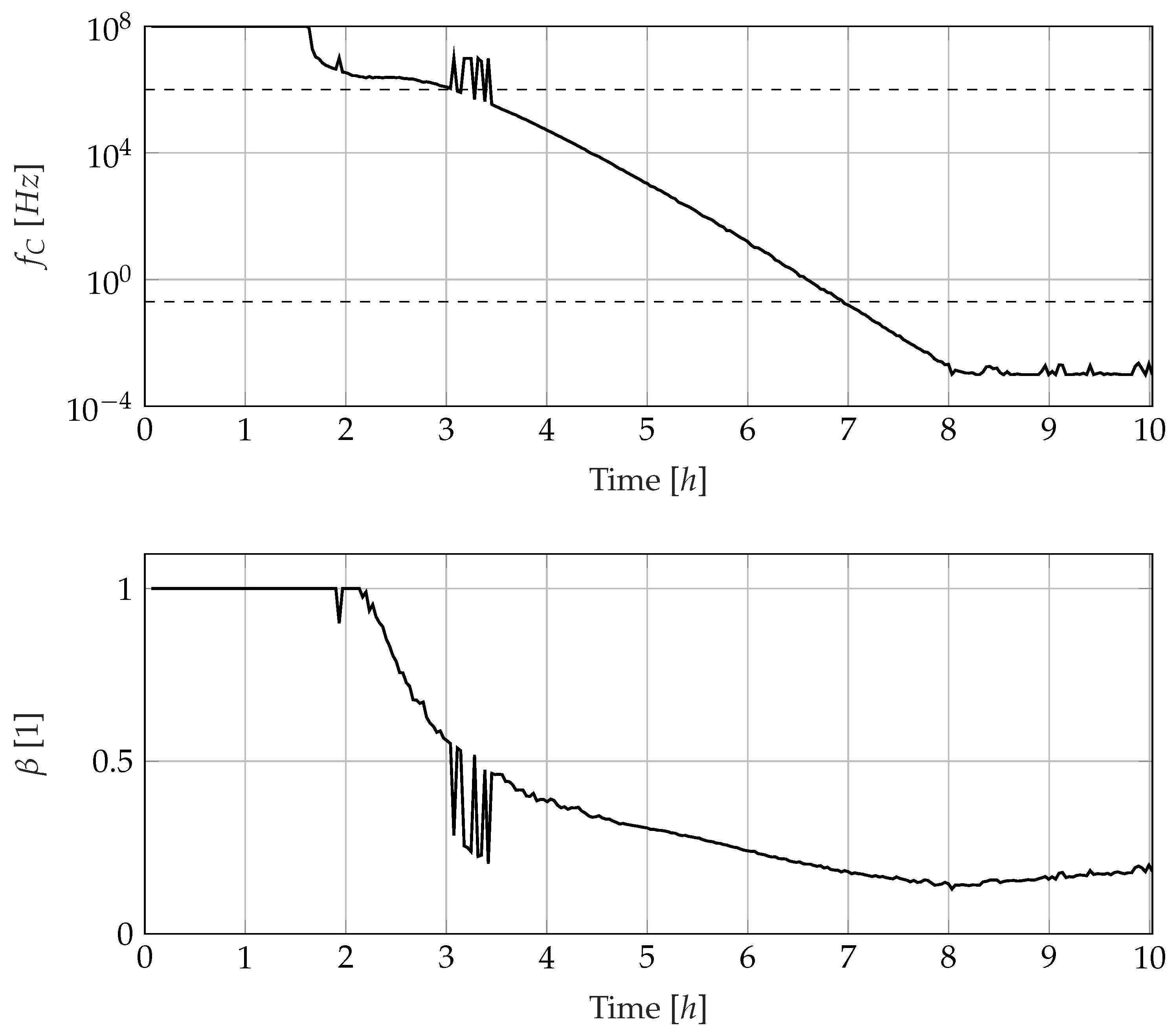
3.3. Development of Electrode Polarisation and Ion Conduction Behaviour
3.4. Development of the Dipole Relaxation Behaviour
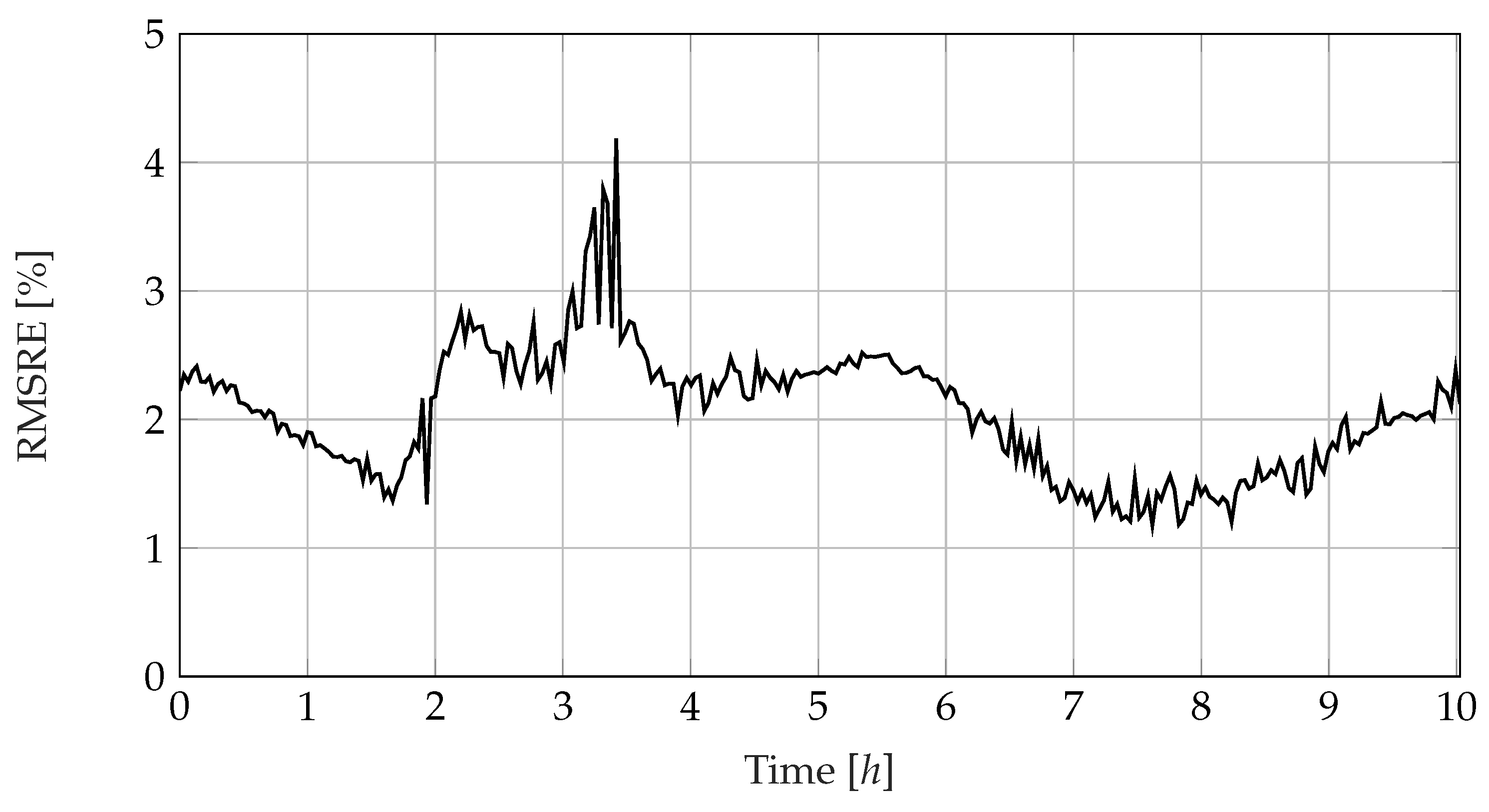
3.5. Considering the Fitting Error
4. Discussion
4.1. Confidence Regions of the Extracted Parameters
4.2. Influence of the Nonlinearity of Electrode Polarisation
4.3. Enabling and Disabling Parameters for Optimisation
4.4. Deducing Microscopic Behaviour from Macroscopic Parameters
4.5. Noise and Temperature Dependence
4.6. Minimum Knowledge Cure Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Symbols
| DSC | Differential Scanning Calorimetry |
| FEM | Finite Elements Method |
| IIMin | Imaginary Impedance Minimum |
| RMSRE | Root Mean Squared Relative Error |
| Complex quantity | |
| Vector quantity | |
| A | Area |
| Warburg impedance | |
| , | Shape parameters for dipole relaxation |
| Capacitance | |
| Capacitance for relaxed dipoles | |
| Capacitance for immobilised dipoles | |
| D | Diffusion constant |
| E | Electric field strength |
| e | Unit charge |
| Permittivity constant | |
| Relative permittivity | |
| Relaxed permittivity | |
| Permittivity for immobilised dipoles | |
| Fourier transformation | |
| f | Frequency |
| Characteristic frequency for dipole relaxation | |
| i | Amperage |
| j | Imaginary unit |
| Sensitivity of interdigitated electrodes sensor | |
| L | Electrode spacing |
| N | Number of measuring points |
| n | Run variable |
| Electric potential | |
| Q | Electric charge |
| Resistance | |
| Kation and anion concentration | |
| T | Temperature |
| Voltage | |
| Angular frequency | |
| Excitation angular frequency | |
| Z | Impedance |
References
- Yang, Y.; Plovie, B.; Vervust, T.; Vanfleteren, J. Capacitive sensor network for composites production monitoring. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Breede, A.; Kahali Moghaddam, M.; Brauner, C.; Lang, W.; Herrmann, A. Online process monitoring and control by dielectric sensors for a composite main spar. In Proceedings of the 20th International Conference on Composite Materials, Copenhagen, Denmark, 19–24 July 2011. [Google Scholar]
- Prussak, R.; Stefaniak, D.; Kappel, E.; Hühne, C.; Sinapius, M. Smart cure cycles for fiber metal laminates using embedded fiber Bragg grating sensors. Compos. Struct. 2019, 213, 252–260. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.W.; Lee, D.G. Smart cure cycle with cooling and reheating for co-cure bonded steel/carbon epoxy composite hybrid structures for reducing thermal residual stress. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1708–1721. [Google Scholar] [CrossRef]
- Kyriazis, A.; Pommer, C.; Lohuis, D.; Rager, K.; Dietzel, A.; Sinapius, M. Comparison of Different Cure Monitoring Techniques. Sensors 2022, 22, 7301. [Google Scholar] [CrossRef] [PubMed]
- Liebers, N.; Raddatz, F.; Schadow, F. Effective and flexible ultrasound sensors for cure monitoring for industrial composite production. In Proceedings of the DLRK 2012, Berlin, Germany, 10–12 September 2012. [Google Scholar]
- Pommer, C.; Sinapius, M. Proof of Concept for Pultrusion Control by Cure Monitoring Using Resonant Ultrasound Spectroscopy. J. Compos. Sci. 2020, 4, 115. [Google Scholar] [CrossRef]
- Lionetto, F.; Maffezzoli, A. Monitoring the cure state of thermosetting resins by ultrasound. Materials 2013, 6, 3783–3804. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, L.; Bellini, C.; Capriglione, D.; Ferrigno, L. Local monitoring of polymerization trend by an interdigital dielectric sensor. Int. J. Adv. Manuf. Technol. 2015, 79, 1007–1016. [Google Scholar] [CrossRef]
- Sorrentino, L.; Bellini, C. In-process monitoring of cure degree by coplanar plate sensors. Int. J. Adv. Manuf. Technol. 2016, 86, 2851–2859. [Google Scholar] [CrossRef]
- Delmonte, J. Electrical Properties of Epoxy Resins During Polymerization. J. Appl. Polym. Sci. 1959, 4, 108–113. [Google Scholar] [CrossRef]
- Maistros, G.M.; Bucknall, C.B. Modeling the Dielectric Behaviour of Epoxy Resin Blends During Curing. Polym. Eng. Sci. 1994, 34, 1517–1528. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Nishimura, A.; Wu, Z.; Zhang, H.; Han, Y.; Wang, T.; Wang, Y.; Huang, C.; Li, L.; et al. Monitoring Strain Response of Epoxy Resin during Curing and Cooling Using an Embedded Strain Gauge. Sensors 2020, 21, 172. [Google Scholar] [CrossRef]
- Crasto, A.S.; Kim, R.Y.; Russell, J.D. In situ monitoring of residual strain development during composite cure. Polym. Compos. 2002, 23, 454–463. [Google Scholar] [CrossRef]
- Dumstorff, G.; Paul, S.; Lang, W. Integration Without Disruption: The Basic Challenge of Sensor Integration. IEEE Sens. J. 2014, 14, 2102–2111. [Google Scholar] [CrossRef]
- Kyriazis, A.; Asali, K.; Sinapius, M.; Rager, K.; Dietzel, A. Adhesion of Multifunctional Substrates for Integrated Cure Monitoring Film Sensors to Carbon Fiber Reinforced Polymers. J. Compos. Sci. 2020, 4, 138. [Google Scholar] [CrossRef]
- Kyriazis, A.; Kilian, R.; Sinapius, M.; Rager, K.; Dietzel, A. Tensile Strength and Structure of the Interface between a Room-Curing Epoxy Resin and Thermoplastic Films for the Purpose of Sensor Integration. Polymers 2021, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, A.; Feder, J.; Rager, K.; von der Heide, C.; Dietzel, A.; Sinapius, M. Reducing the weakening effect in fibre-reinforced polymers caused by integrated film sensors. J. Compos. Sci. 2021, 5, 256. [Google Scholar] [CrossRef]
- Boll, D.; Schubert, K.; Brauner, C.; Lang, W. Miniaturized flexible interdigital sensor for in situ dielectric cure monitoring of composite materials. IEEE Sens. 2014, 14, 2193–2197. [Google Scholar] [CrossRef]
- Hübner, M.; Koerdt, M.; Hardi, E.; Herrmann, A.S.; Lang, W. Embedding miniaturized flexible sensors for online monitoring of fibre composite production. In Proceedings of the 18th European Conference on Composite Materials, Athens, Greece, 24–28 June 2018. [Google Scholar]
- Moghaddam, M.K.; Breede, A.; Chaloupka, A.; Bödecker, A.; Habben, C.; Meyer, E.M.; Brauner, C.; Lang, W. Design, fabrication and embedding of microscale interdigital sensors for real-time cure monitoring during composite manufacturing. Sens. Actuators A Phys. 2016, 243, 123–133. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Hübner, M.; Koerdt, M.; Brauner, C.; Lang, W. Sensors on a plasticized thermoset substrate for cure monitoring of CFRP production. Sens. Actuators A Phys. 2017, 267, 560–566. [Google Scholar] [CrossRef]
- Yang, Y.; Chiesura, G.; Vervust, T.; Degrieck, J.; Vanfleteren, J. Design and fabrication of a shielded interdigital sensor for noninvasive In situ real-time production monitoring of polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2028–2037. [Google Scholar] [CrossRef]
- Yang, Y.; Chiesura, G.; Vervust, T.; Bossuyt, F.; Luyckx, G.; Degrieck, J.; Vanfleteren, J. Design and fabrication of a flexible dielectric sensor system for in situ and real-time production monitoring of glass fibre reinforced composites. Sens. Actuators A Phys. 2016, 243, 103–110. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, K.; Vervust, T.; Vanfleteren, J. Multifunctional and miniaturized flexible sensor patch: Design and application for in situ monitoring of epoxy polymerization. Sens. Actuators B Chem. 2018, 261, 144–152. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.G. Non-Isothermal in Situ Dielectric Cure Monitoring for Thermosetting Matrix Composites. J. Compos. Mater. 2004, 38, 977–993. [Google Scholar] [CrossRef]
- Day, D.R.; Lewis, T.J.; Lee, H.L.; Senturia, S.D. The Role of Boundary Layer Capacitance at Blocking Electrodes in the Interpretation of Dielectric Cure Data in Adhesives. J. Adhes. 1985, 18, 73–90. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Cirkel, P.A.; van der Ploeg, J.P.M.; Koper, G.J.M. Electrode effects in dielectric spectroscopy of colloidal suspensions. Phys. A 1997, 235, 269–278. [Google Scholar] [CrossRef]
- Kim, H.; Char, K. Dielectric changes during the curing of epoxy resin based on the diglycidyl ether of bisphenol A (DGEBA) with diamine. Bull. Korean Chem. Soc. 1999, 20, 1329–1334. [Google Scholar]
- Yang, Y.; Plovie, B.; Chiesura, G.; Vervust, T.; Daelemans, L.; Mogosanu, D.-E.; Wuytens, P.; De Clerck, K.; Vanfleteren, J. Fully Integrated Flexible Dielectric Monitoring Sensor System for Real-Time In Situ Prediction of the Degree of Cure and Glass Transition Temperature of an Epoxy Resin. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Casalini, R.; Corezzi, S.; Livi, A.; Levita, G.; Rolla, P.A. Dielectric parameters to monitor the crosslink of epoxy resins. J. Appl. Polym. Sci. 1997, 65, 17–25. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling electrode polarization in dielectric spectroscopy: Ion mobility and mobile ion concentration of single-ion polymer electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef]
- Serghei, A.; Tress, M.; Sangoro, J.R.; Kremer, F. Electrode polarization and charge transport at solid interfaces. Phys. Rev. B 2009, 80, 184301. [Google Scholar] [CrossRef]
- Tian, F.; Ohki, Y. Charge transport and electrode polarization in epoxy resin at high temperatures. J. Phys. D Appl. Phys. 2014, 47, 045311. [Google Scholar] [CrossRef]
- Skordos, A.; Partridge, I. Impedance cure and flow monitoring in the processing of advanced composites. In Proceedings of the International Conference for Manufacturing of Advanced Composites, Belfast, UK, 27–28 September 2001. [Google Scholar]
- Mijovic, J.; Yee, C.F.W. Use of Complex Impedance To Monitor the Progress of Reactions in Epoxy/Amine Model Systems. Macromolecules 1994, 27, 7287–7293. [Google Scholar] [CrossRef]
- Boukamp, B. Interpretation of the Gerischer impedance in solid state ionics. Solid State Ionics 2003, 157, 29–33. [Google Scholar] [CrossRef]
- Pototskaya, V.V.; Gichan, O.I. The Gerischer finite length impedance: A case of unequal diffusion coefficients. J. Electroanal. Chem. 2019, 852, 113511. [Google Scholar] [CrossRef]
- Boukamp, B. Electrochemical impedance spectroscopy in solid state ionics: Recent advances. Solid State Ionics 2004, 169, 65–73. [Google Scholar] [CrossRef]
- Ciucci, F.; Chen, C. Analysis of Electrochemical Impedance Spectroscopy Data Using the Distribution of Relaxation Times: A Bayesian and Hierarchical Bayesian Approach. Electrochim. Acta 2015, 167, 439–454. [Google Scholar] [CrossRef]
- Song, J.; Bazant, M.Z. Electrochemical Impedance Imaging via the Distribution of Diffusion Times. Phys. Rev. Lett. 2018, 120, 116001. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.W.; Cole, R.H. Dielectric Relaxation in Glycerol, Propylene Glycol, and n-Propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Part C 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Takenaga, M.; Kozuka, S.; Hayashi, M.; Endo, H. Determination of Ultratrace Amounts of Metallic and Chloride Ion Impurities in Organic Materials for Microelectronics Devices After a Microwave Digestion Method. Analyst 1997, 122, 129–132. [Google Scholar] [CrossRef]
- Wright, W. Charakterisation of a Bisphenol A Resin. A collaborative study carried out by technical panel PTP3 of the technical cooperation programme. Brit. Poly. J. 1983, 15, 224–242. [Google Scholar] [CrossRef]
- Zhang, L.; Kojima, H. An Advanced Study on Ion Impurities on Solder Resist and the Solutions for High-Temperature Reliability Performance. In Proceedings of the IEEE 66th Electronic Components and Technology Conference, Las Vegas, NV, USA, 31 May–3 June 2016. [Google Scholar]
- Bogner, G.; Debray, A.; Hoehn, K. High Performance Epoxy Casting Resins for SMD-LED Packaging. In Proceedings of the Symposium on Integrated Optoelectronics, San Jose, CA, USA, 20–26 January 2000. [Google Scholar]
- Coleman, T.F.; Li, Y. On the convergence of interior-reflective Newton methods for nonlinear minimization subject to bounds. Math. Program. 1994, 67, 189–224. [Google Scholar] [CrossRef]
- Coleman, T.F.; Li, Y. An interior trust region approach for nonlinear minimization subject to bounds. SIAM J. Optim. 1996, 6, 418–445. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Mech. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for least-squares estimation of nonlinear parameters. J. Soc. Industr. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- More, J.J. The Levenberg-Marquardt Algorithm: Implementation and Theory. In Proceedings of the Conference on Numerical Analysis, Dundee, Scotland, 28 June–1 July 1977. [Google Scholar]
- Rager, K.; Jaworski, D.; von der Heide, C.; Kyriazis, A.; Sinapius, M.; Constantinou, I.; Dietzel, A. Space-filling curve resistor on ultra-thin polyetherimide foil for strain impervious temperature sensing. Sensors 2021, 21, 6479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chiesura, G.; Luyckx, G.; Vervust, T.; Bossuyt, F.; Kaufmann, M.; Vanfleteren, J.; Degrieck, J. In-situ on-line cure monitoring of composites by embedded interdigital sensor. In Proceedings of the 16th European Conference on Composite Materials, Seville, Spain, 22–26 June 2014. [Google Scholar]
- Igreja, R.; Dias, C.J. Analytical evaluation of the interdigital electrodes capacitance for a multi-layered structure. Sens. Actuators A Phys. 2004, 112, 291–301. [Google Scholar] [CrossRef]
- Kim, D.; Centea, T.; Nutt, S.R. In-situ cure monitoring of an out-of-autoclave prepreg: Effects of out-time on viscosity, gelation and vitrification. Compos. Sci. Technol. 2014, 102, 132–138. [Google Scholar] [CrossRef]
- Pethrick, R.; Hayward, D. Real time dielectric relaxation studies of dynamic polymeric systems. Prog. Polym. Sci. 2002, 27, 1983–2017. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriazis, A.; Charif, S.; Rager, K.; Dietzel, A.; Sinapius, M. A Parametric Model for the Analysis of the Impedance Spectra of Dielectric Sensors in Curing Epoxy Resins. Sensors 2023, 23, 1825. https://doi.org/10.3390/s23041825
Kyriazis A, Charif S, Rager K, Dietzel A, Sinapius M. A Parametric Model for the Analysis of the Impedance Spectra of Dielectric Sensors in Curing Epoxy Resins. Sensors. 2023; 23(4):1825. https://doi.org/10.3390/s23041825
Chicago/Turabian StyleKyriazis, Alexander, Samir Charif, Korbinian Rager, Andreas Dietzel, and Michael Sinapius. 2023. "A Parametric Model for the Analysis of the Impedance Spectra of Dielectric Sensors in Curing Epoxy Resins" Sensors 23, no. 4: 1825. https://doi.org/10.3390/s23041825
APA StyleKyriazis, A., Charif, S., Rager, K., Dietzel, A., & Sinapius, M. (2023). A Parametric Model for the Analysis of the Impedance Spectra of Dielectric Sensors in Curing Epoxy Resins. Sensors, 23(4), 1825. https://doi.org/10.3390/s23041825








