Solution-Processed Functionalized Graphene Film Prepared by Vacuum Filtration for Flexible NO2 Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene
2.2. Functionalization of Graphene Nanosheets
2.3. Characterization Methods
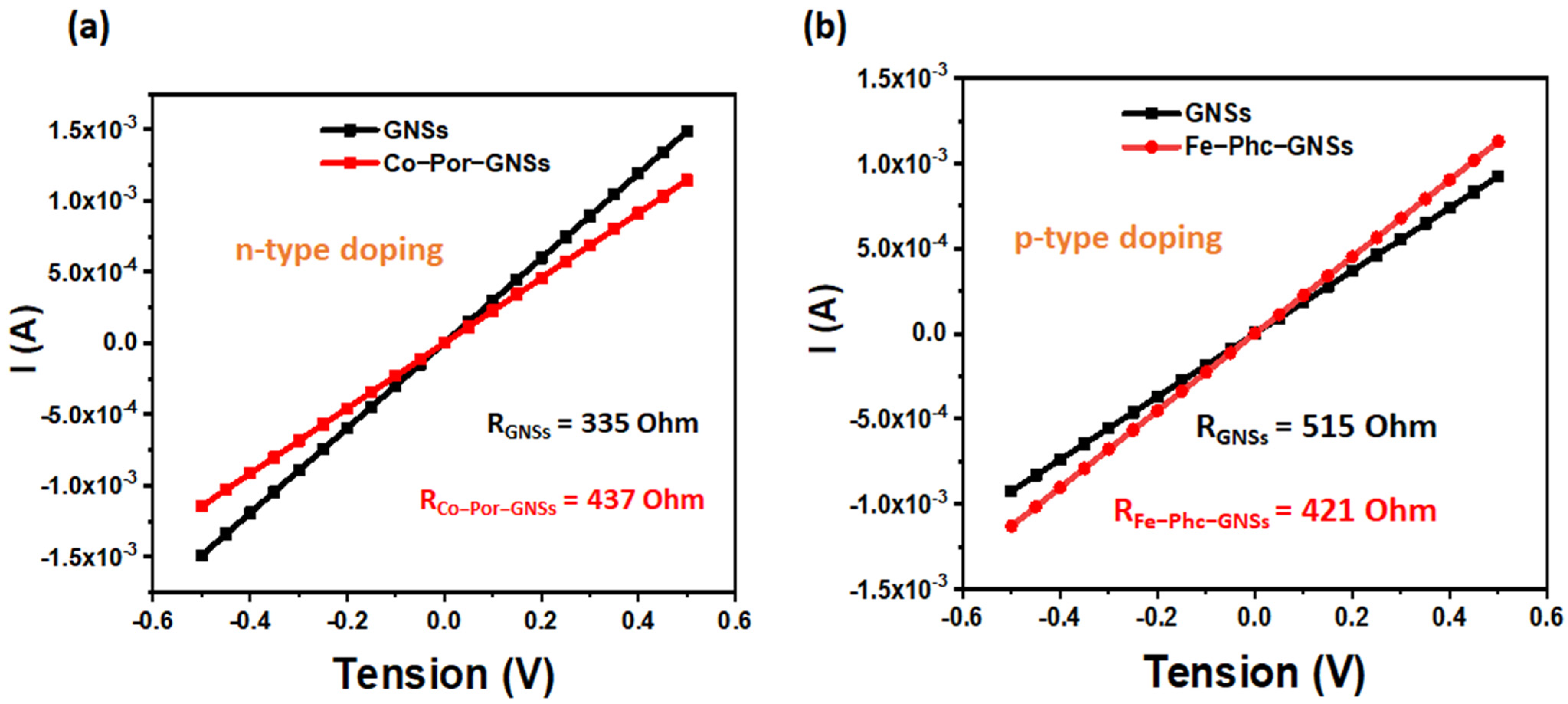
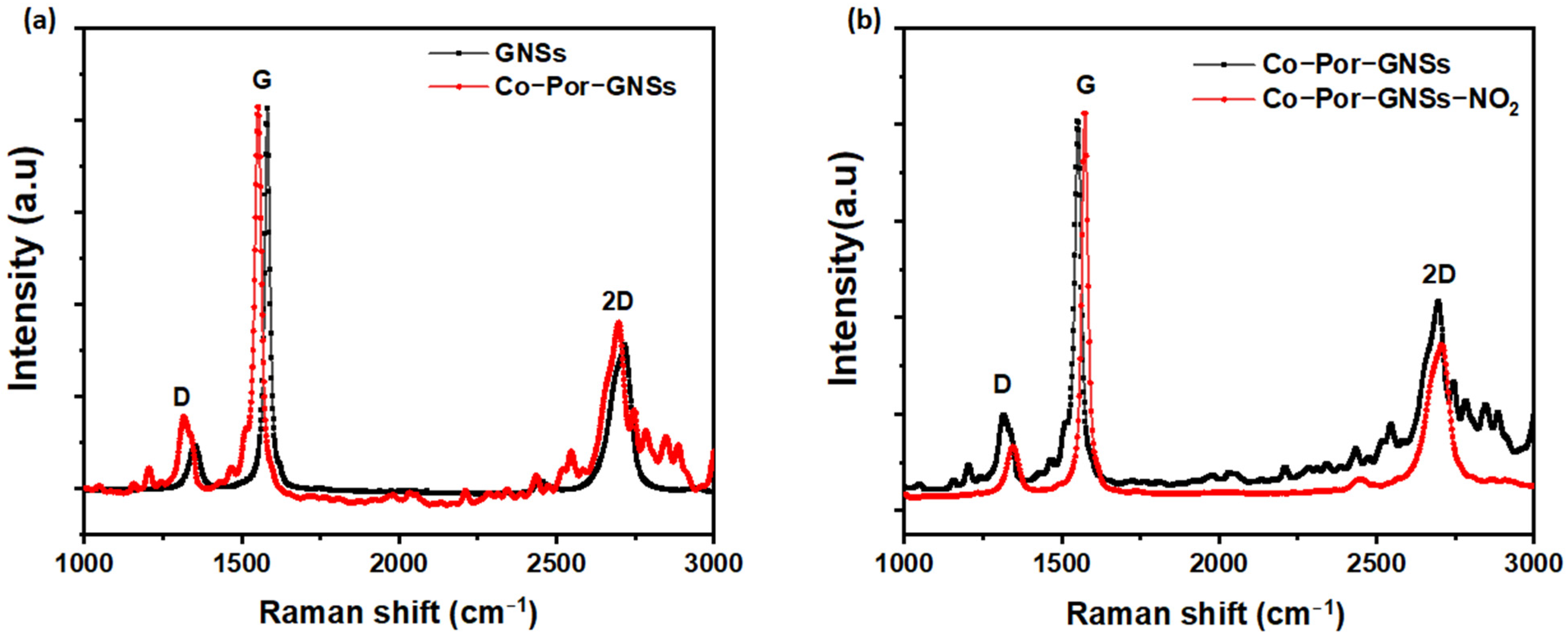
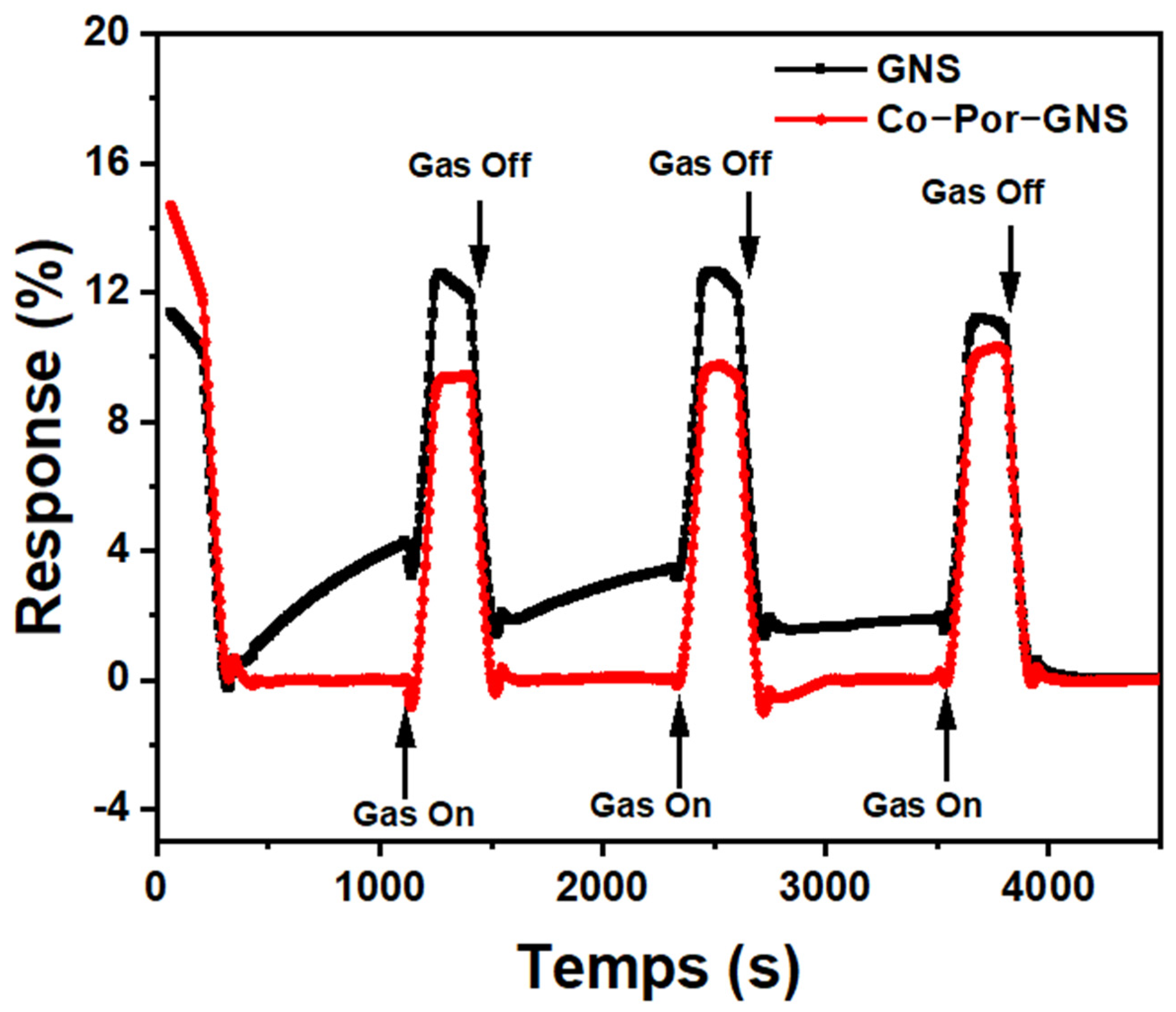
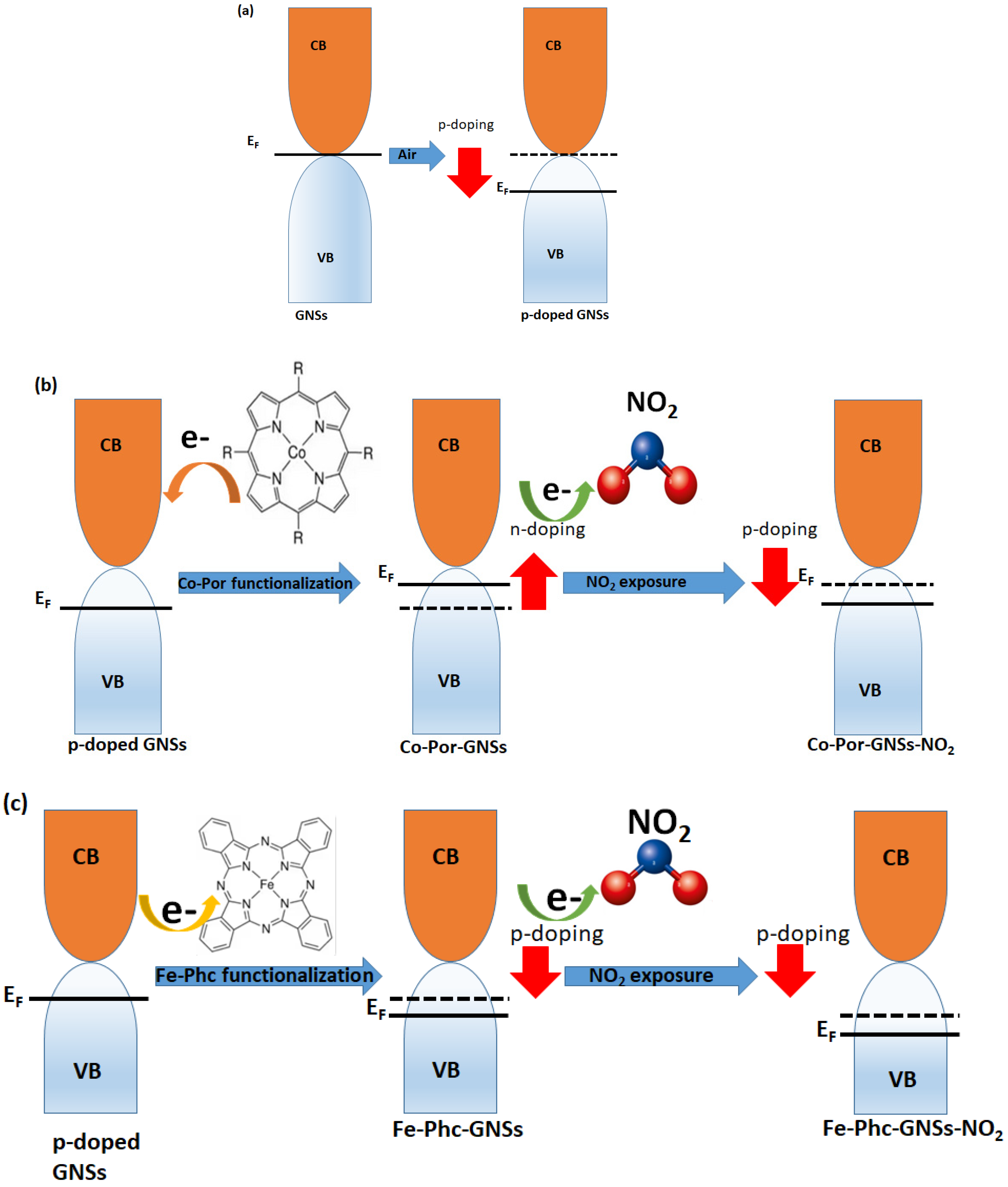
3. Results and Discussion
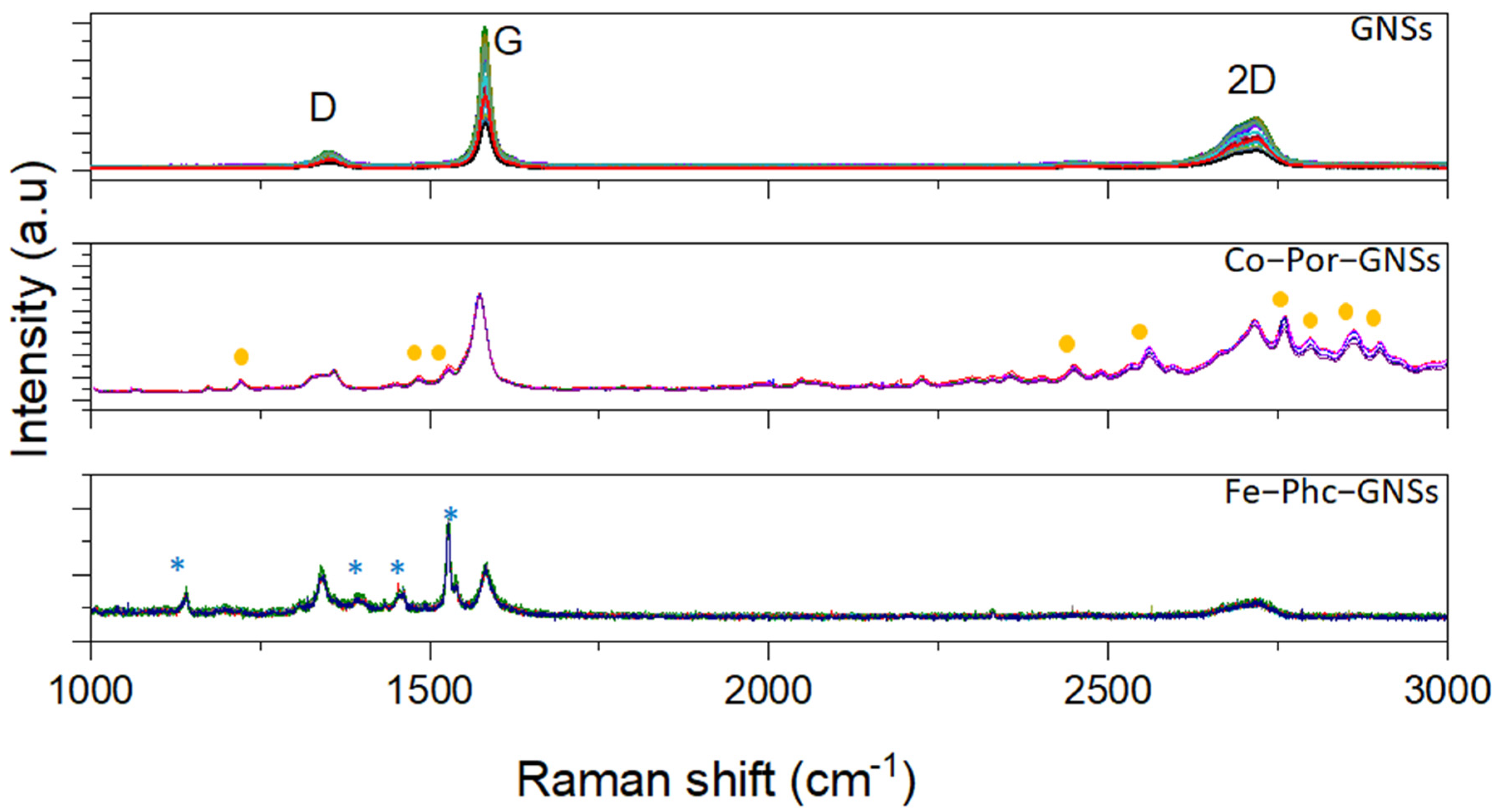
3.1. Raman Spectroscopy
3.2. X-ray Diffraction Analysis
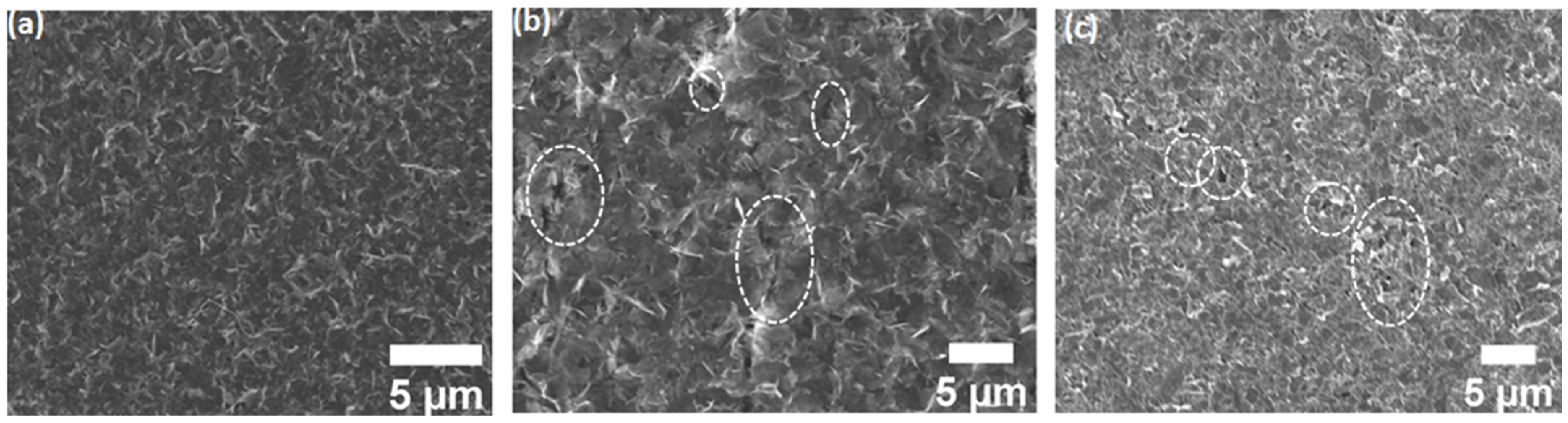
3.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
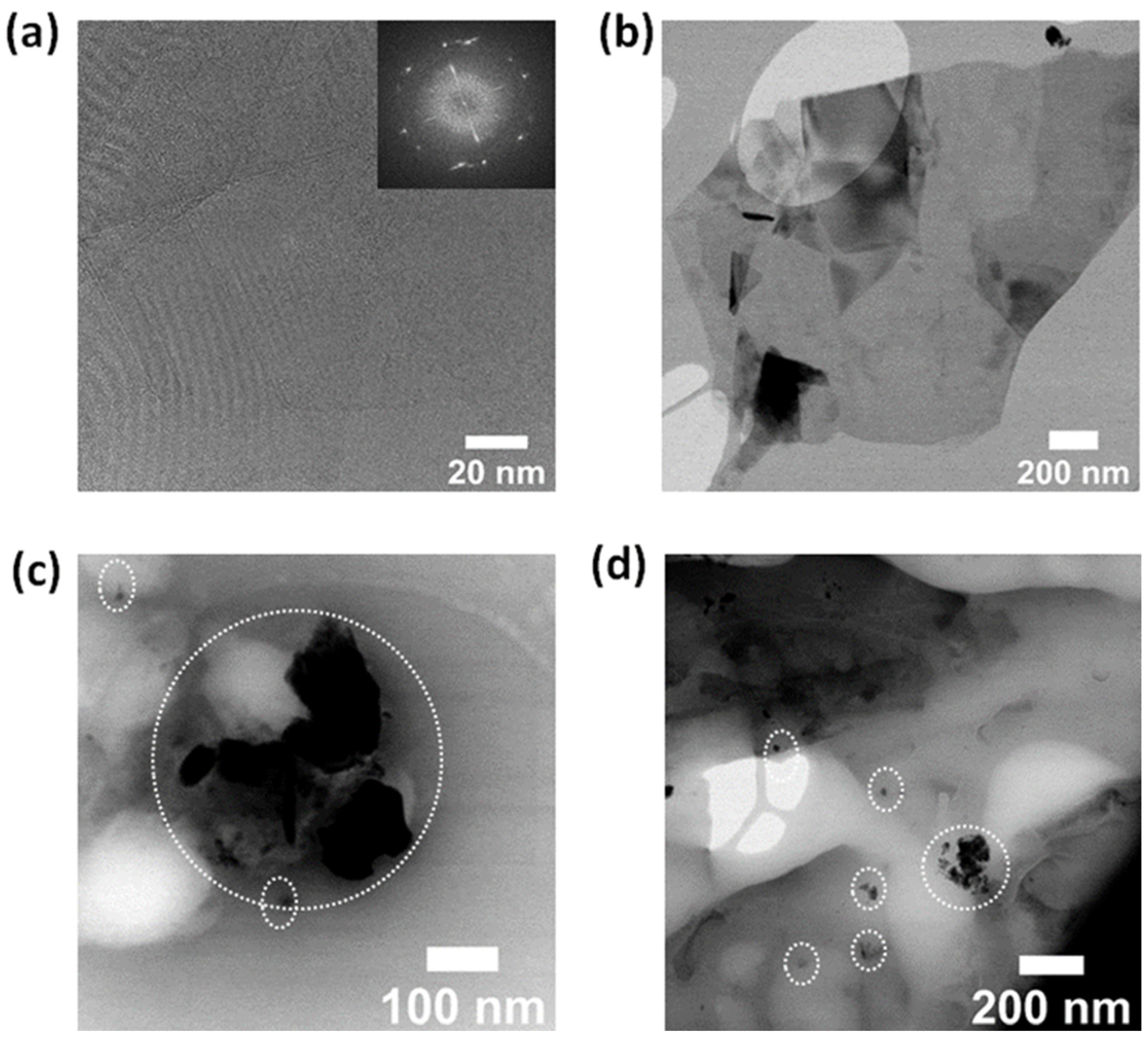
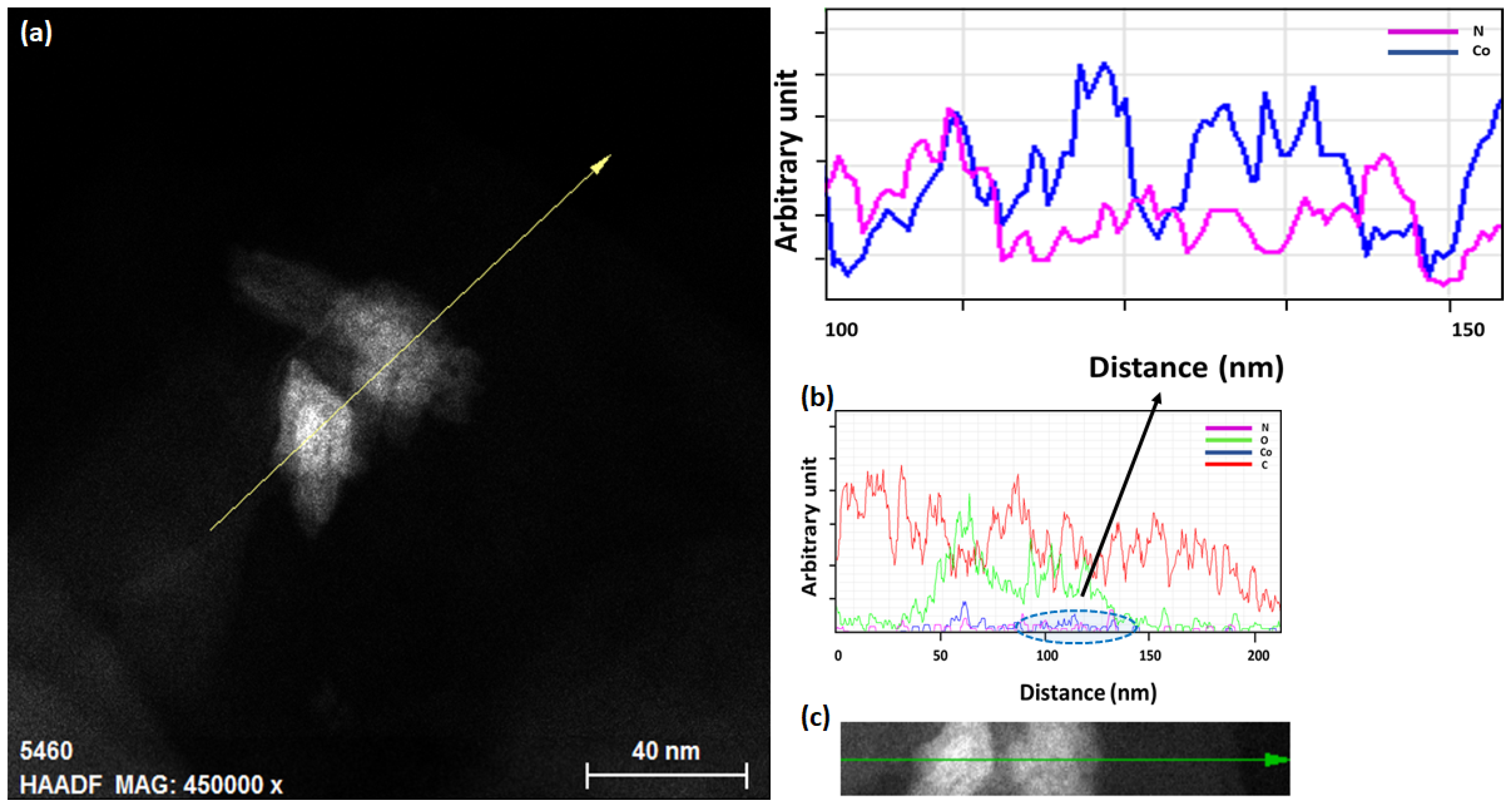
3.4. Transmission Electron Microscopy (TEM) and Energy-Dispersive Spectroscopy (EDS)
3.5. Atomic Force Microscopy (AFM)
3.6. Investigation of Sensing Capabilities of Pristine and Functionalized GNSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiruvengadathan, R.; Roy, S.C.; Sundriyal, P.; Bhattacharya, S. Carbon Nanostructures: Fundamentals to Applications; AIP Publishing LLC: Melville, NY, USA, 2021; p. 400. Available online: https://aip.scitation.org/doi/10.1063/9780735423114 (accessed on 5 January 2023).
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, R.; Hu, N.; Chai, J.; Cheng, Y.; Zhang, L.; Wei, H.; Kong, E.S.-W.; Zhang, Y. The Prospective Two-Dimensional Graphene Nanosheets: Preparation, Functionalization and Applications. Nano-Micro Lett. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Jaber-Ansari, L.; Hersam, M.C. Solution-processed graphene materials and composites. MRS Bull. 2012, 37, 1167–1175. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, J.; Xiang, W.; Zhao, J.; Guo, H.; Hu, J.; Han, X.; Hu, W. Fabrication of the Ni-NiCl2 Composite Cathode Material for Fast-Response Thermal Batteries. Front. Chem. 2021, 9, 679231. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotech. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—dependence on the reducing agent. J. Mater. Sci. 2020, 56, 3738–3754. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.S.; Jinesh, K.B.; Gopchandran, K.G.; Gopalan, G.K. Liquid phase exfoliated graphene for electronic applications. Mater. Res. Express 2017, 4, 095017. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Kairi, M.I.; Dayou, S.; Kairi, N.I.; Abu Bakar, S.; Vigolo, B.; Mohamed, A.R. Toward high production of graphene flakes—a review on recent developments in their synthesis methods and scalability. J. Mater. Chem. A 2018, 6, 15010–15026. [Google Scholar] [CrossRef]
- Mills, I.C.; Atkinson, R.W.; Kang, S.; Walton, H.; Anderson, H.R. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015, 5, e006946. [Google Scholar] [CrossRef]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.-H.; Ma, B.S.; Shin, E.; Kim, Y.; Kim, T.-S.; Kim, F.S.; Kim, I.-D.; Kim, B.J. High-Performance, Flexible NO2 Chemiresistors Achieved by Design of Imine-Incorporated n-Type Conjugated Polymers. Adv. Sci. 2022, 9, 2200270. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/Polyaniline Nanocomposite for Hydrogen Sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Zou, C.; Hu, J.; Su, Y.; Shao, F.; Tian, T.; Zhu, M.; Hu, N.; Yang, Z.; Zhang, Y. Conducting polymer-bridged three-dimensional heterojunctions of reduced graphene oxide/γ-Fe2O3 hybrids for high-performance NO2 gas sensing. Results Surf. Interfaces 2022, 7, 100057. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials—A Review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef]
- Tyagi, S.; Chaudhary, M.; Ambedkar, A.K.; Sharma, K.; Gautam, Y.K.; Singh, B.P. Metal oxide nanomaterial-based sensors for monitoring environmental NO2 and its impact on the plant ecosystem: A review. Sens. Diagn. 2022, 1, 106–129. [Google Scholar] [CrossRef]
- Rattan, S.; Kumar, S.; Goswamy, J. Gold nanoparticle decorated graphene for efficient sensing of NO2 gas. Sens. Int. 2022, 3, 100147. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Q.; Wei, C.; Li, X.; Yu, S.; Li, J.; Chen, Y. High-performance and wearable hazardous gases sensor based on n-n heterojunction film of NGO and tetrakis(1-pyrenyl)porphyrin. J. Hazard. Mater. 2021, 419, 126460. [Google Scholar] [CrossRef]
- Pyo, S.; Choi, U.; Kim, O. Improved photo- and chemical-responses of graphene via porphyrin-functionalization for flexible, transparent, and sensitive sensors. Nanotechnology 2019, 30, 215501. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2016, 117, 2203–2256. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Iezhokin, I.; Den Boer, D.; Offermans, P.; Ridene, M.; Elemans, J.A.A.W.; Adriaans, G.P.; Flipse, C.F.J. Porphyrin molecules boost the sensitivity of epitaxial graphene for NH3detection. J. Phys. Condens. Matter 2016, 29, 065001. [Google Scholar] [CrossRef]
- Auwärter, W.; Ecija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef]
- Chaudhary, A.; Patra, R.; Rath, S.P. Binding of Catechols to Iron(III)–Octaethylporphyrin: An Experimental and DFT Investigation. Eur. J. Inorg. Chem. 2010, 33, 5211–5221. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Wang, B.; Chen, Z.; He, C.; Yiqun, W. Preparation, characterization and NH3-sensing properties of reduced graphene oxide/copper phthalocyanine hybrid material. Sens. Actuators B Chem. 2014, 193, 340–348. [Google Scholar] [CrossRef]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. Chemiresistive graphene sensors for ammonia detection. ACS Appl. Mater. Interfaces 2018, 10, 16169–16176. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Lei, Y.; Li, M.C. Porphyrin Functionalized Graphene for Sensitive Electrochemical Detection of Ultratrace Explosives. Electroanalysis 2011, 23, 885–893. [Google Scholar] [CrossRef]
- Ikuta, T.; Tamaki, T.; Masai, H.; Nakanishi, R.; Endo, K.; Terao, J.; Kenzo, M. Electrical detection of ppb region NO2 using Mg-porphyrin-modified graphene field-effect transistors. Nanoscale Adv. 2021, 3, 5793–5800. [Google Scholar] [CrossRef]
- Dieng, M.; Bensifia, M.; Borme, J.; Florea, I.; Abreu, C.M.; Jama, C.; Léonard, C.; Alpuim, P.; Pribat, D.; Yassar, A.; et al. Wet-Chemical Noncovalent Functionalization of CVD Graphene: Molecular Doping and Its Effect on Electrolyte-Gated Graphene Field-Effect Transistor Characteristics. J. Phys. Chem. C 2022, 126, 4522–4533. [Google Scholar] [CrossRef]
- Backes, C.; Paton, K.R.; Hanlon, D.; Yuan, S.; Katsnelson, M.I.; Houston, J.; Smith, R.J.; McCloskey, D.; Donegan, J.F.; Coleman, J.N. Spectroscopic metrics allow in situ measurement of mean size and thickness of liquid-exfoliated few-layer graphene nanosheets. Nanoscale 2016, 8, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman Spectroscopy of Graphene Edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, Y.; Jiang, S.; Song, L.; Hu, Y.; Gui, Z. A facile liquid phase exfoliation method to prepare graphene sheets with different sizes expandable graphite. Mater. Res. Bull. 2013, 48, 2985–2992. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Kim, B.S. Bio-functionalized few-layer graphene for in situ growth of gold nanoparticles, improvement of polymer properties, and dye removal. J. Clean. Prod. 2021, 310, 127515. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Pathak, M.; Tatrari, G.; Karakoti, M.; Pandey, S.; Sahu, P.S.; Saha, B.; Sahoo, N.G. Few layer graphene nanosheets from kinnow peel waste for high-performance supercapacitors: A comparative study with three different electrolytes. J. Energy Storage 2022, 55, 105729. [Google Scholar] [CrossRef]
- Aladekomo, J.; Bragg, R. Structural transformations induced in graphite by grinding: Analysis of 002 X-ray diffraction line profiles. Carbon 1990, 28, 897–906. [Google Scholar] [CrossRef]
- Jafari, A.; Mortaheb, H.R.; Gallucci, F. Plasma treatment for enhanced functionalization of graphene nanosheets by octadecylamine. Chem. Eng. Res. Des. 2022. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, W.; Zhang, M.; Wang, Y.; Duan, Z.; Tan, C.; Liu, B.; Ouyan, F.; Yuan, Z.; Tai, H.; et al. Edge-Enriched Mo2TiC2Tx/MoS2 Heterostructure with Coupling Interface for Selective NO2 Monitoring. Adv. Funct. Mater. 2022, 32, 2203528. [Google Scholar] [CrossRef]
- Yavari, F.; Castillo, E.; Gullapalli, H.; Ajayan, P.M.; Koratkar, N. High sensitivity detection of NO2 and NH3 in air using chemical vapor deposition grown graphene. Appl. Phys. Lett. 2012, 100, 203120. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, A.; Huang, L.; Li, C.; Shi, G. High-Performance NO2Sensors Based on Chemically Modified Graphene. Adv. Mater. 2012, 25, 766–771. [Google Scholar] [CrossRef]
- Johnson, E.C.; Dolphin, D. The reactions of magnesium octaethylporphyrin and its Π-cations with nitrogen dioxide and nitrite. Tetrahedron Lett. 1976, 17, 2197–2200. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Kerdcharoen, T. A method for the detection of alcohol vapours based on optical sensing of magnesium 5,10,15,20-tetraphenyl porphyrin thin film by an optical spectrometer and principal component analysis. Anal. Chim. Acta 2012, 757, 75–82. [Google Scholar] [CrossRef]
- Zhu, P.; Li, S.; Zhao, C.; Zhang, Y.; Yu, J. 3D synergistical rGO/Eu(TPyP)(Pc) hybrid aerogel for high-performance NO2 gas sensor with enhanced immunity to humidity. J. Hazard. Mater. 2020, 384, 121426. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, L.; Zhou, Q.; Shi, G. Ultrasensitive and Selective Nitrogen Dioxide Sensor Based on Self-Assembled Graphene/Polymer Composite Nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 17003–17008. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pal, P.; Sharma, D.K.; Kumar, S.; Senguttuvan, T.D.; Gupta, B.K. Ultrasensitive Boron-Nitrogen-Codoped CVD Graphene-Derived NO2 Gas Sensor. ACS Mater. Au 2022, 2, 356–366. [Google Scholar] [CrossRef]
- Guo, L.; Li, T. Sub-ppb and ultra selective nitrogen dioxide sensor based on sulfur doped graphene. Sens. Actuators B Chem. 2018, 255, 2258–2263. [Google Scholar] [CrossRef]




| Sample | Response time (s) | Recovery time (s) |
|---|---|---|
| GNSs | 72 | 70 |
| Co-Por-GNSs | 63 | 54 |
| Fe-Phc-GNSs | 63 | 63 |
| Material Used | Type of Sensors | Operating Temperature | Detection Range | Sensitivity | LOD (ppb) | Res/Rec (Time) (s) | Ref. |
|---|---|---|---|---|---|---|---|
| Mo-Porphyrin modified graphene | FETs | RT | 2–800 ppb | Not reported | 0.3 | Not reported | [34] |
| Eu(TPyP)(PC)/rGO | Chemiresistive | RT | 0.5–100 ppm | Not reported | 80 | 172/828 (20 ppm) | [51] |
| Polymer/rGO | Chemiresistive | RT | 0.15–5 ppm | 1.03 ppm−1 | 150 | 180/360 (0.5 ppm) | [52] |
| BNG | Chemiresistive | RT | 1–80 ppb | 0.05% | Not measured | 177/392 (1 ppb) | [53] |
| Sulphur doped graphene | MEMS | RT | 500 ppt–100 ppm | 1.8% | 0.5 | - | [54] |
| Co-Por-GNSs | Chemiresistive | RT | 0.5–2 ppm | 13.1% | Not measured | 63/54 (0.5 ppm) | This work |
| Fe-Phc-GNSs | Chemiresistive | RT | 0.5–2 ppm | 8.3% | Not measured | 63/63 (0.5 ppm) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieng, M.; Sankar, S.; Ni, P.; Florea, I.; Alpuim, P.; Capasso, A.; Yassar, A.; Bouanis, F.Z. Solution-Processed Functionalized Graphene Film Prepared by Vacuum Filtration for Flexible NO2 Sensors. Sensors 2023, 23, 1831. https://doi.org/10.3390/s23041831
Dieng M, Sankar S, Ni P, Florea I, Alpuim P, Capasso A, Yassar A, Bouanis FZ. Solution-Processed Functionalized Graphene Film Prepared by Vacuum Filtration for Flexible NO2 Sensors. Sensors. 2023; 23(4):1831. https://doi.org/10.3390/s23041831
Chicago/Turabian StyleDieng, Mbaye, Siva Sankar, Pingping Ni, Ileana Florea, Pedro Alpuim, Andrea Capasso, Abderrahim Yassar, and Fatima Zahra Bouanis. 2023. "Solution-Processed Functionalized Graphene Film Prepared by Vacuum Filtration for Flexible NO2 Sensors" Sensors 23, no. 4: 1831. https://doi.org/10.3390/s23041831







