Home-Based Electrochemical Rapid Sensor (HERS): A Diagnostic Tool for Bacterial Vaginosis
Abstract
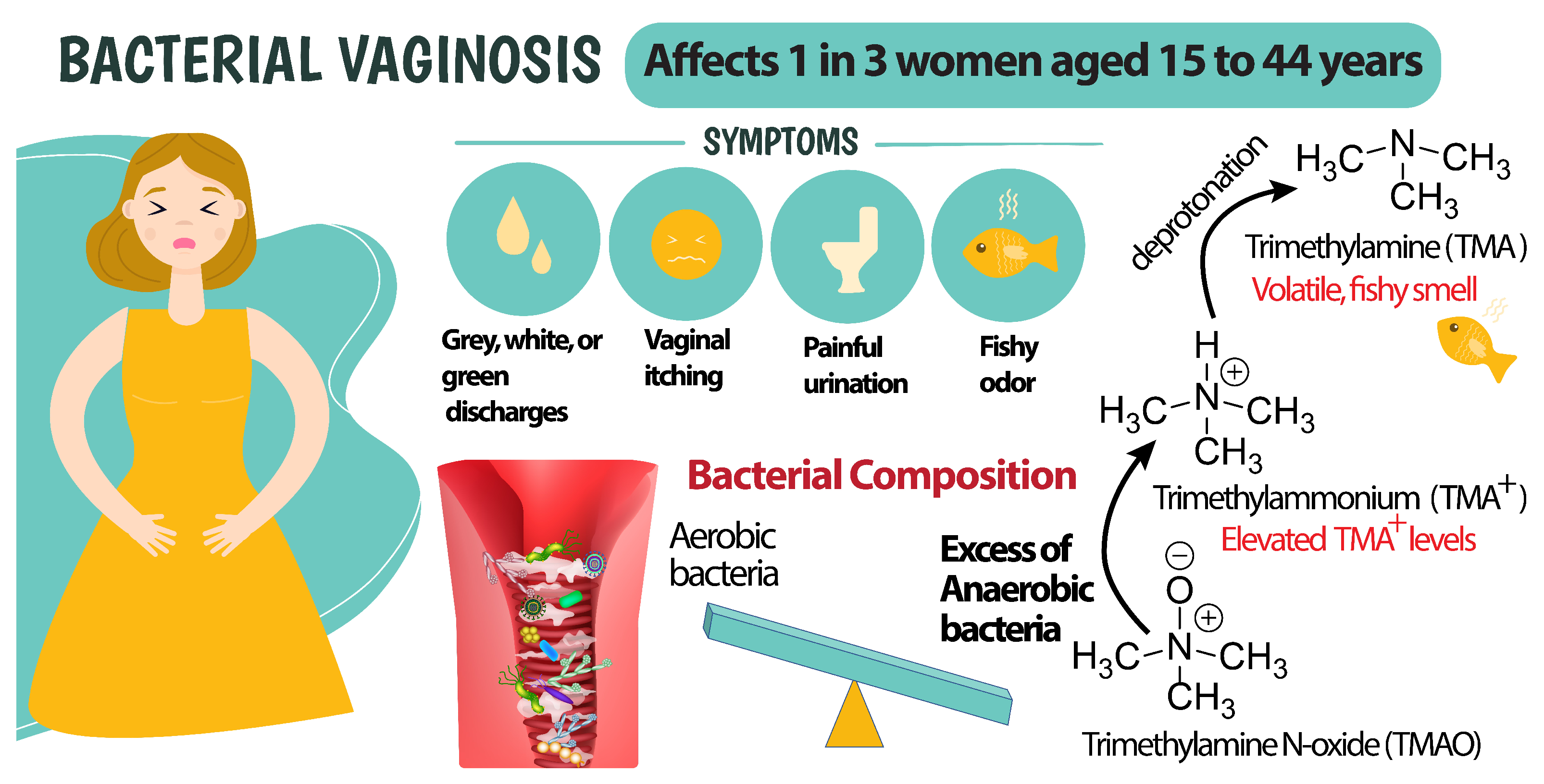
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
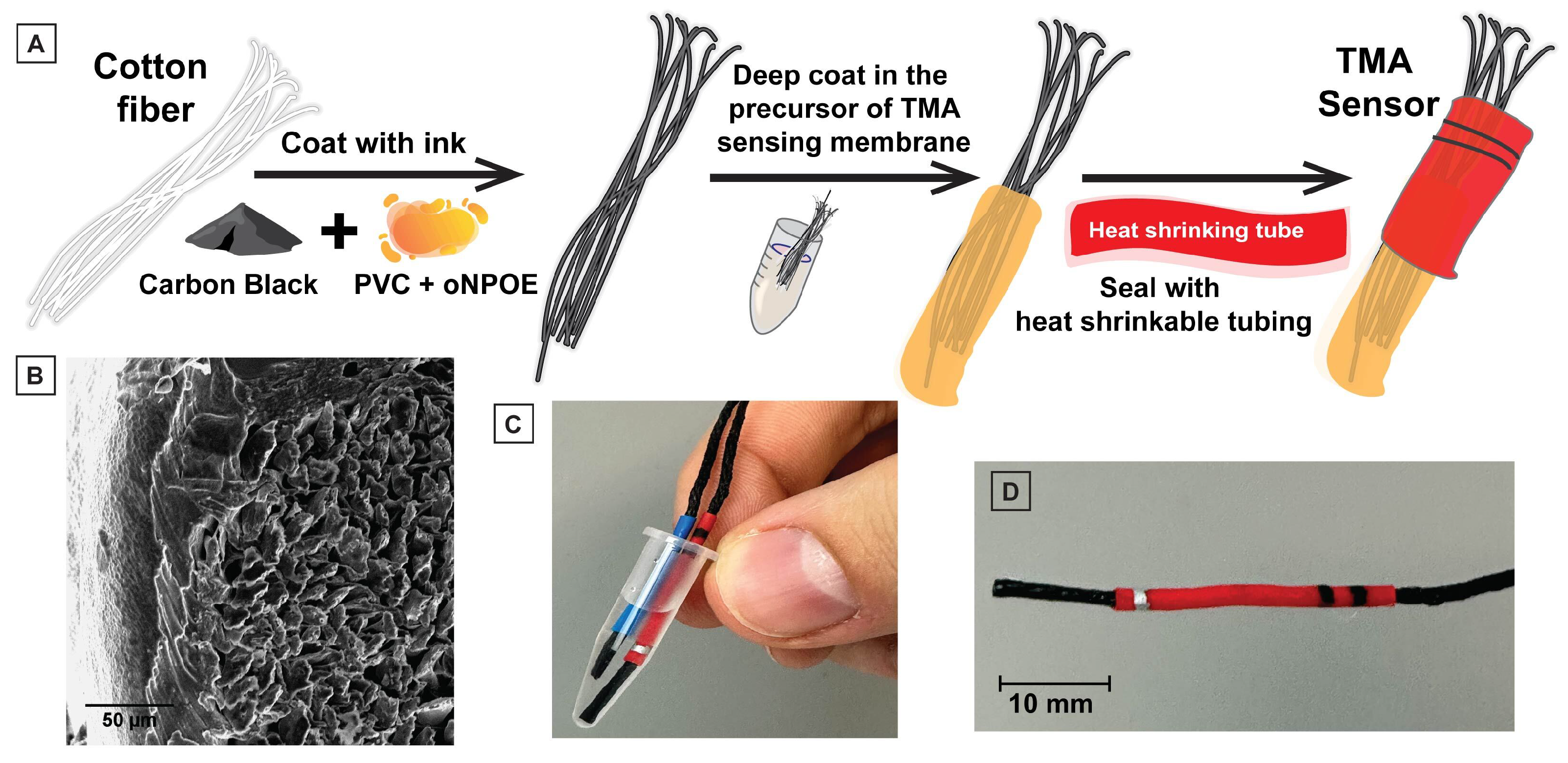
2.2. Sensor Fabrication
2.3. Measurement Protocols
3. Results and Discussion
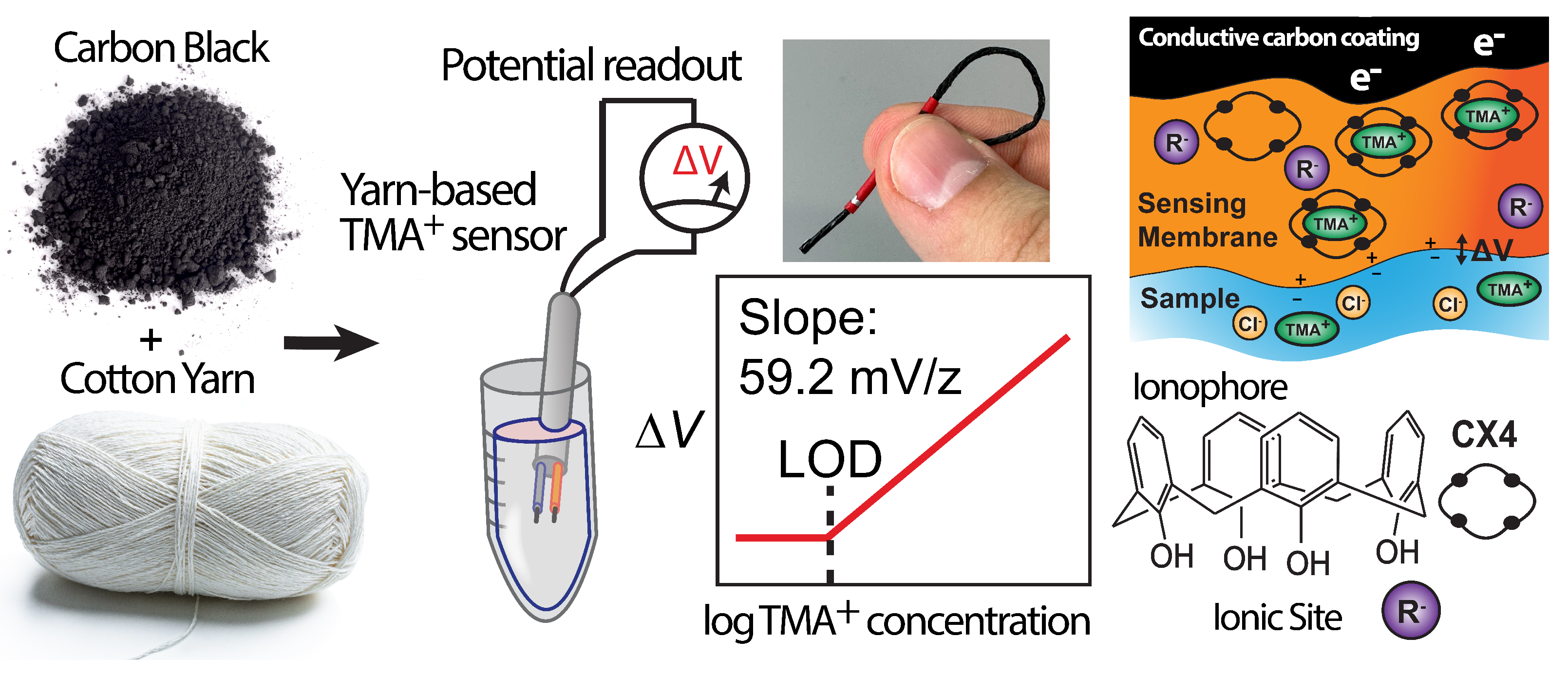
3.1. Design and Fabrication of the HERS
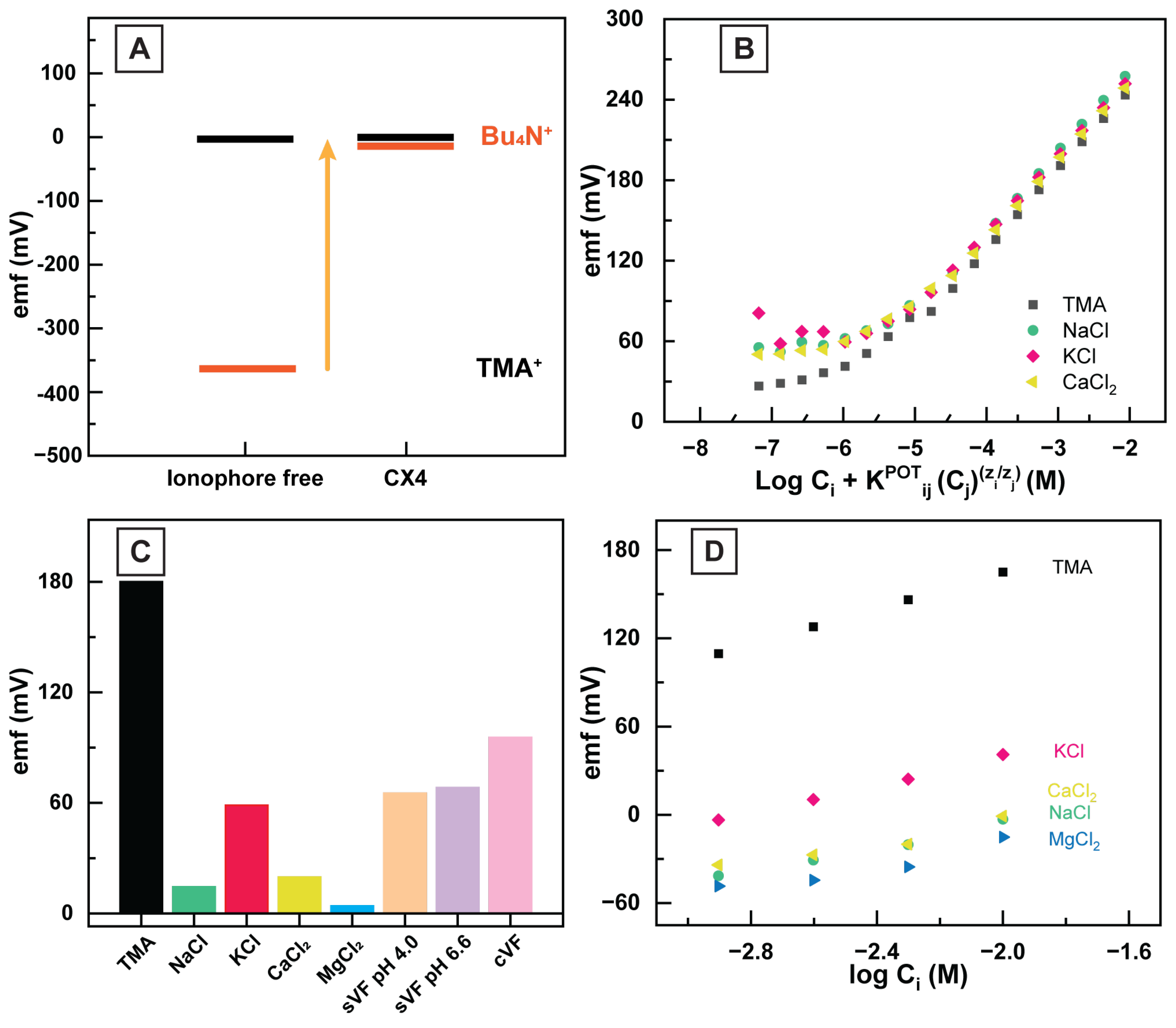
3.2. The Sensing Mechanism in the HERS and Sensor Response
3.3. TMA and Ionophore Interaction
3.4. Quantifying the Selectivity of HERS
3.5. Application of HERS in Vaginal Fluid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BV | bacterial vaginosis |
| TMA | Trimethylamine |
| TMA | Trimethylammonium |
| LOD | limit of detection |
| sVF | simulated vaginal fluid |
| cVF | commercial vaginal fluid |
| HERS | Home-Based Electrochemical Rapid Sensor |
| DI | deionized |
References
- Anderson, M.R.; Klink, K.; Cohrssen, A. Evaluation of vaginal complaints. JAMA 2004, 291. [Google Scholar] [CrossRef]
- Peebles, K.; Velloza, J.; Balkus, J.E.; McClelland, R.S.; Barnabas, R.V. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2019, 46, 304–311. [Google Scholar] [PubMed]
- Jones, A. Bacterial Vaginosis: A Review of Treatment, Recurrence, and Disparities. J. Nurse Pract. 2019, 15, 420–423. [Google Scholar] [CrossRef]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations With Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- STD Facts-Bacterial Vaginosis. 2 June 2022. Available online: https://www.cdc.gov/std/bv/stdfact-bacterial-vaginosis.htm (accessed on 21 October 2022).
- Eron, J.; Hurt, C. Faculty Opinions recommendation of Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: A prospective cohort analysis among African couples. Fac. Opin.–Post-Publ. Peer Rev. Biomed. Lit. 2012, 9, e1001251. [Google Scholar] [CrossRef]
- Balkus, J.E.; Richardson, B.A.; Rabe, L.K.; Taha, T.E.; Mgodi, N.; Kasaro, M.P.; Ramjee, G.; Hoffman, I.F.; Karim, S.S.A. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV-1-negative women. Sex Trans Dis. 2014, 41, 123–128. [Google Scholar] [CrossRef]
- Nagot, N.; Ouedraogo, A.; Defer, M.C.; Vallo, R.; Mayaud, P.; Van de Perre, P. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin. Infect. Dis. 2003, 37, 319–325. [Google Scholar]
- Wiesenfeld, H.C.; Hillier, S.L.; Krohn, M.A.; Landers, D.V.; Sweet, R.L. Bacterial Vaginosis Is a Strong Predictor of Neisseria gonorrhoeae and Chlamydia trachomatis Infection. Clin. Infect. Dis. 2003, 36, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Hillier, S.L.; Kip, K.E.; Soper, D.E.; Stamm, C.A.; McGregor, J.A.; Bass, D.C.; Sweet, R.L.; Rice, P.; Richter, H.E. Bacterial Vaginosis and Risk of Pelvic Inflammatory Disease. Obstet. Gynecol. 2004, 104, 761–769. [Google Scholar] [CrossRef]
- Bacterial Vaginosis. 7 July 2022. Available online: https://www.cdc.gov/std/treatment-guidelines/bv.htm (accessed on 22 October 2022).
- Oleen-Burkey, M.A.; Hillier, S.L. Pregnancy Complications Associated With Bacterial Vaginosis and Their Estimated Costs. Infect. Dis. Obstet. Gynecol. 1995, 3, 149–157. [Google Scholar] [CrossRef]
- Reiter, S.; Spadt, S.K. Bacterial vaginosis: A primer for clinicians. Postgrad. Med. 2019, 131, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Cruden, D.L.; Galask, R.P. Trimethylamine Oxide to Trimethylamine by Mobiluncus Strains Isolated from Patients with Bacterial Vaginosis. Microb. Ecol. Health Dis. 1988, 1. [Google Scholar] [CrossRef]
- Sachdeva, S. Clue cell. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 392–393. [Google Scholar] [PubMed]
- Dewick, P.M. Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2013; p. 137. [Google Scholar]
- Ruben, E. Bacterial Vaginosis: 5 At-Home Tests and What to Know. Healthline. Available online: https://www.healthline.com/health/womens-health/bacterial-vaginosis-5-at-home-tests-and-what-to-know (accessed on 21 June 2022).
- Lin, Y.P.; Chen, W.-C.; Cheng, C.-M.; Shen, C.-J. Vaginal pH Value for Clinical Diagnosis and Treatment of Common Vaginitis. Diagnostics 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Eschenbach, D.A.; Thwin, S.S.; Patton, D.L.; Hooton, T.M.; Stapleton, A.E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W.E. Influence of the Normal Menstrual Cycle on Vaginal Tissue, Discharge, and Microflora. Clin. Infect. Dis. 2000, 30, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.P.; Grésenguet, G.; Bélec, L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 1997, 3, 19–23. [Google Scholar] [CrossRef]
- Myziuk, L.; Romanowski, B.; Johnson, S.C. BVBlue Test for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 2003, 41, 1925–1928. [Google Scholar] [CrossRef]
- Douaki, A.; Garoli, D.; Inam, A.S.; Angeli, M.A.C.; Cantarella, G.; Rocchia, W.; Wang, J.; Petti, L.; Lugli, P. Smart Approach for the Design of Highly Selective Aptamer-Based Biosensors. Biosensors 2022, 12, 574. [Google Scholar] [CrossRef]
- Pal, A.; Nadiger, V.G.; Goswami, D.; Martinez, R.V. Conformal, waterproof electronic decals for wireless monitoring of sweat and vaginal pH at the point-of-care. Biosens. Bioelectron. 2020, 160, 112206. [Google Scholar]
- Liu, Z.; Bian, L.; Yeoman, C.J.; Clifton, G.D.; Ellington, J.E.; Ellington-Lawrence, R.D.; Borgogna, J.L.C.; Star, A. Bacterial Vaginosis Monitoring with Carbon Nanotube Field-Effect Transistors. Anal. Chem. 2022, 94, 3849–3857. [Google Scholar]
- Pawley, D.C.; Dikici, E.; Deo, S.K.; Raccamarich, P.; Fischl, M.A.; Alcaide, M.; Daunert, S. Rapid Point-of-Care Test Kit for Bacterial Vaginosis: Detection of Vaginolysin and Clue Cells Using Paper Strips and a Smartphone. Anal. Chem. 2022, 94, 11619–11626. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.P.; El-Rahman, M.K.A.; Mahmoud, A.M.; Abdelsalam, R.M.; Bühlmann, P. In situ sensing of the neurotransmitter acetylcholine in a dynamic range of 1 nM to 1 mM. ACS Sens. 2018, 3, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Tietz, K.; Klein, S. Simulated Genital Tract Fluids and Their Applicability in Drug Release/Dissolution Testing of Vaginal Dosage Forms. Dissolution Technol. 2018, 25, 40–51. [Google Scholar] [CrossRef]
- Mousavi, M.P.; Ainla, A.; Tan, E.K.; Abd El-Rahman, M.K.; Yoshida, Y.; Yuan, L.; Sigurslid, H.H.; Arkan, N.; Yip, M.C.; Abrahamsson, C.K.; et al. Ion sensing with thread-based potentiometric electrodes. Lab Chip 2018, 18, 2279–2290. [Google Scholar]
- Bühlmann, P.; Chen, L.D. Ion-selective electrodes with ionophore-doped sensing membranes. Supramol. Chem. Mol. Nanomater. 2012, 5, 2539. [Google Scholar]
- Wolrath, H.; Ståhlbom, B.; Hallén, A.; Forsum, U. Trimethylamine and trimethylamine oxide levels in normal women and women with bacterial vaginosis reflect a local metabolism in vaginal secretion as compared to urine. Apmis 2005, 113, 513–516. [Google Scholar] [CrossRef]
- Johnson, R.D.; Bachas, L.G. Ionophore-based ion-selective potentiometric and optical sensors. Anal. Bioanal. Chem. 2003, 376, 328–341. [Google Scholar] [CrossRef]
- El-Rahman, M.K.A.; Mazzone, G.; Mahmoud, A.M.; Sicilia, E.; Shoeib, T. Novel choline selective electrochemical membrane sensor with application in milk powders and infant formulas. Talanta 2021, 221, 121409. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Calixarenes: An Introduction; The Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-258-6. [Google Scholar]
- Ceresa, A.; Pretsch, E. Determination of formal complex formation constants of various Pb2+ ionophores in the sensor membrane phase. Anal. Chim. Acta 1999, 395, 41–52. [Google Scholar] [CrossRef]
- Geshnizgani, A.M.; Onderdonk, A.B. Defined medium simulating genital tract secretions for growth of vaginal microflora. J. Clin. Microbiol. 1992, 30, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Miao, M.; Chen, J.; Chen, K.; Wu, B.; Dai, Q.; Wang, J.; Sun, F.; Shi, H.; Yuan, W. The Association Between Calcium, Magnesium, and Ratio of Calcium/Magnesium in Seminal Plasma and Sperm Quality. Biol. Trace Elem. Res. 2016, 174, 1–7. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Köhler, A.; Rohr, G.; Runnebaum, B. The pH as an important determinant of sperm-mucus interaction. Fertil. Steril. 1993, 59, 617–628. [Google Scholar] [CrossRef]



| Ions | ||
|---|---|---|
| Sodium | −2.69 | −1.25 |
| Potassium | −2.03 | −1.12 |
| Calcium | −3.99 | −1.09 |
| Magnesium | −4.28 |
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| Whiff test | Low-cost, rapid | Requires reagent addition, subjective, non-quantitative, low sensitivity/specificity |
| Gram stain | Gold standard, excellent sensitivity/specificity | Time intensive, requires skilled personnel, laboratory facility, and reagent addition |
| Sialidase detection (BVBlue) | Rapid, moderate sensitivity/selectivity | Requires reagent addition, costly, non-quantitative, cannot be used by women who have recently engaged in sexual intercourse |
| HERS | Low-cost, rapid, quantitative, good sensitivity/selectivity | Not yet clinically validated, requires off-body analysis (not fully wearable) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banks, M.; Amirghasemi, F.; Mitchell, E.; Mousavi, M.P.S. Home-Based Electrochemical Rapid Sensor (HERS): A Diagnostic Tool for Bacterial Vaginosis. Sensors 2023, 23, 1891. https://doi.org/10.3390/s23041891
Banks M, Amirghasemi F, Mitchell E, Mousavi MPS. Home-Based Electrochemical Rapid Sensor (HERS): A Diagnostic Tool for Bacterial Vaginosis. Sensors. 2023; 23(4):1891. https://doi.org/10.3390/s23041891
Chicago/Turabian StyleBanks, Melissa, Farbod Amirghasemi, Evelyn Mitchell, and Maral P. S. Mousavi. 2023. "Home-Based Electrochemical Rapid Sensor (HERS): A Diagnostic Tool for Bacterial Vaginosis" Sensors 23, no. 4: 1891. https://doi.org/10.3390/s23041891









