Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals, and Instruments
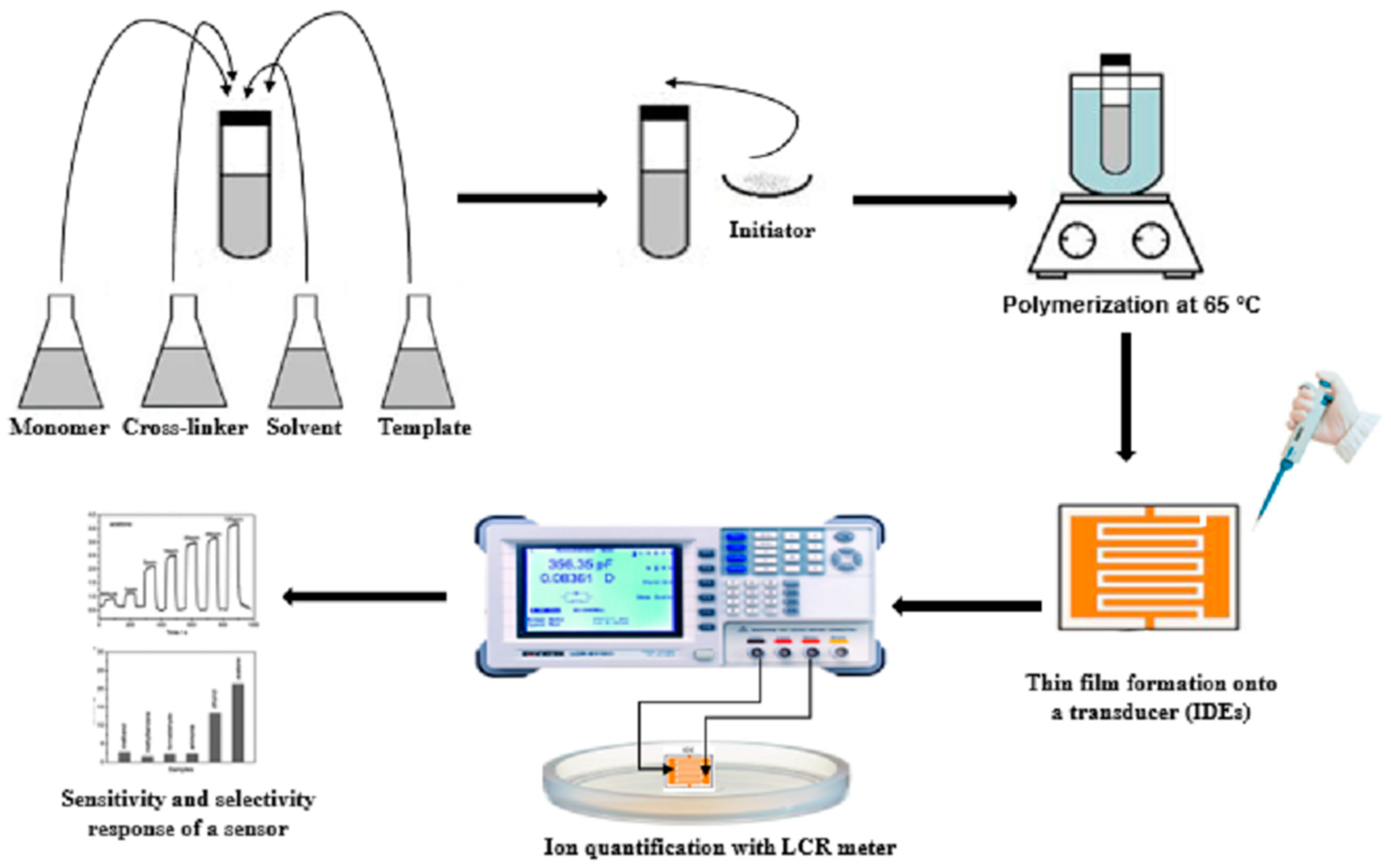
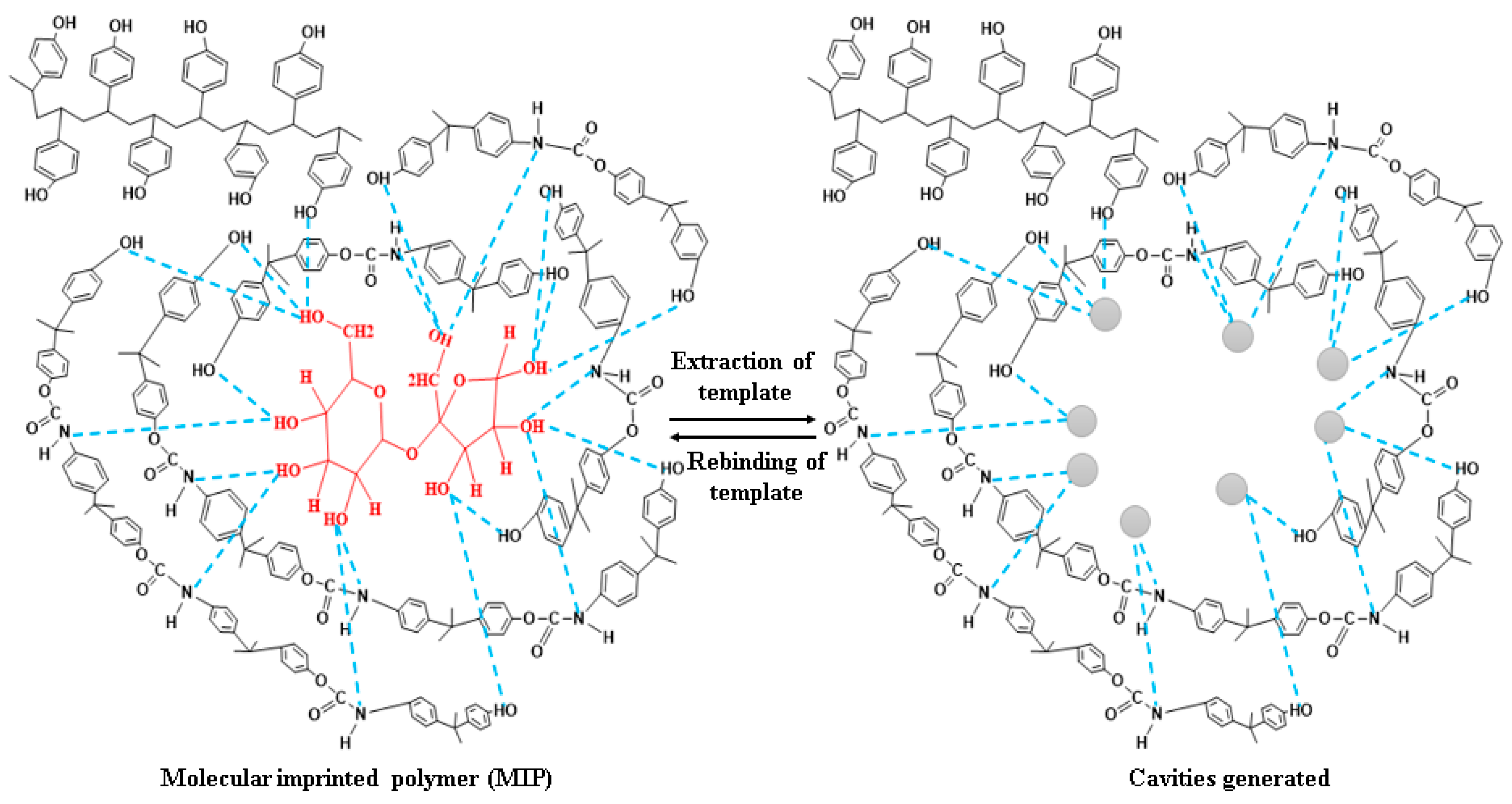
2.2. Synthesis of Molecular Imprinted Polymer (MIP) and GO-Composite
2.2.1. Synthesis of Molecular-Imprinted Polyurethane Receptors (PU-MIP)
2.2.2. Molecular-Imprinted Polymer-Functionalized Graphene Composite (GO-Sucrose) Synthesis
2.2.3. Non-Imprinted Polymer (NIP) Synthesis
2.2.4. Immobilization of Artificially Designed Receptors onto the Transducing Surface (IDEs) and Measurements
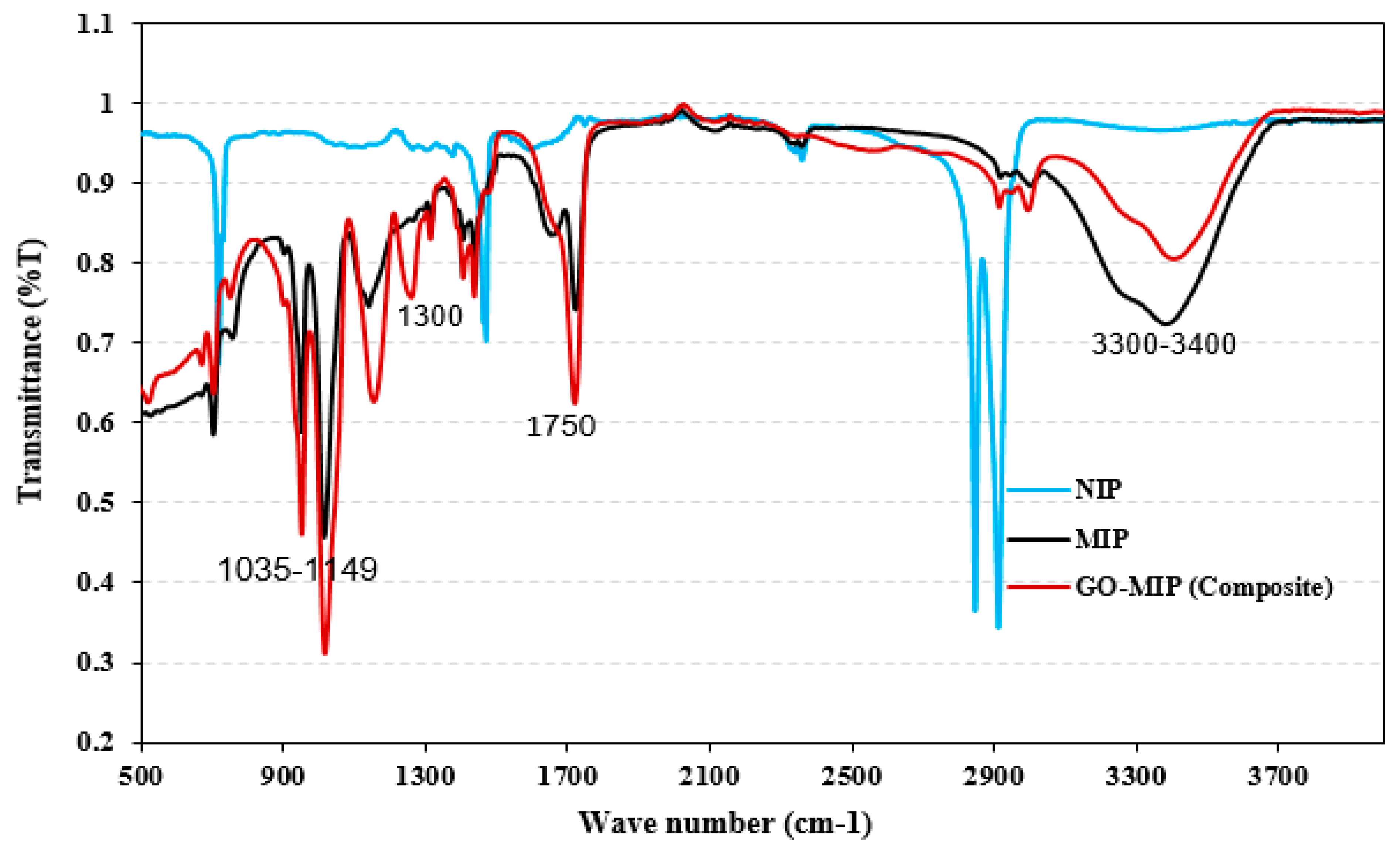
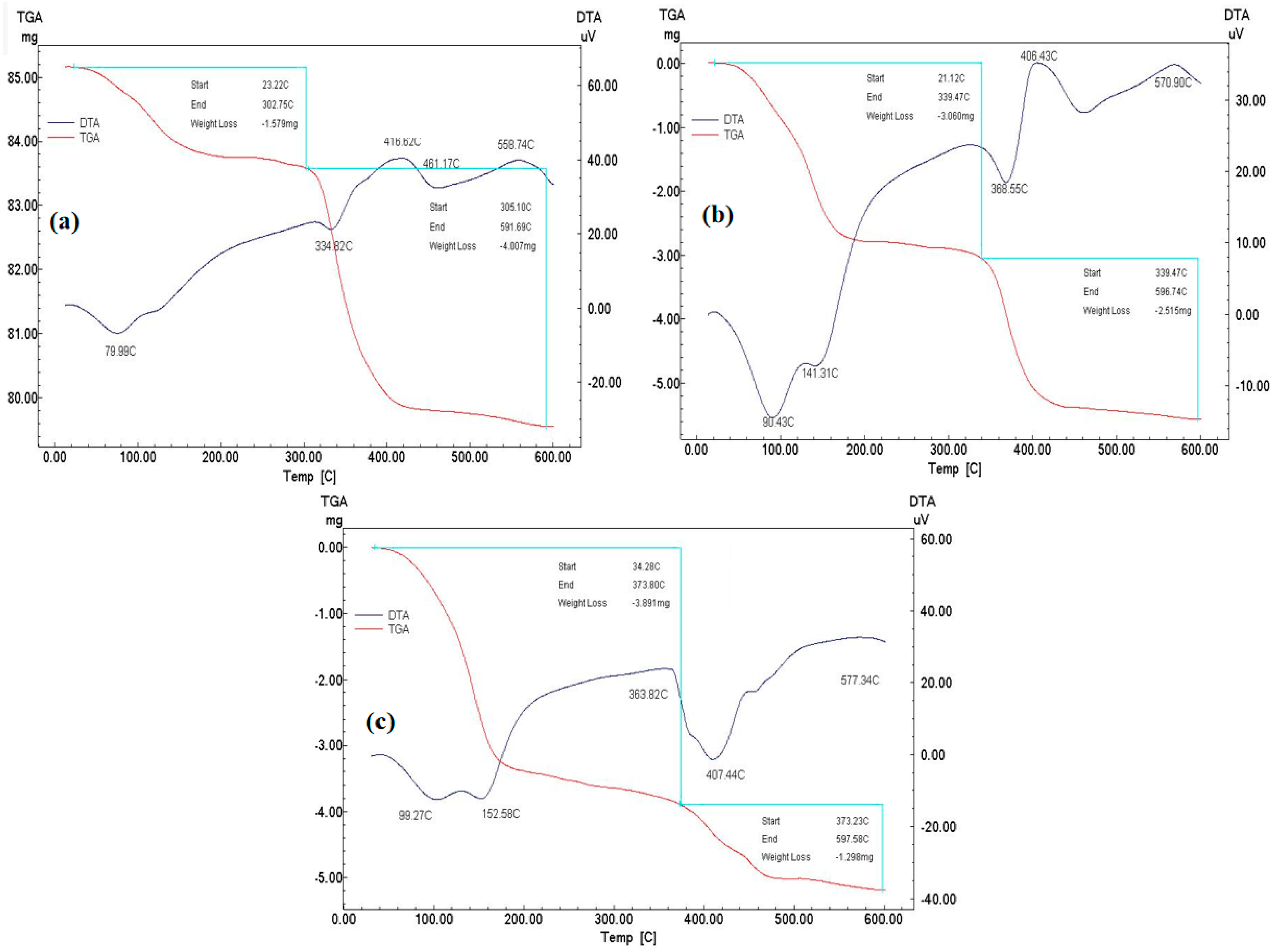
2.2.5. Characterization Methods
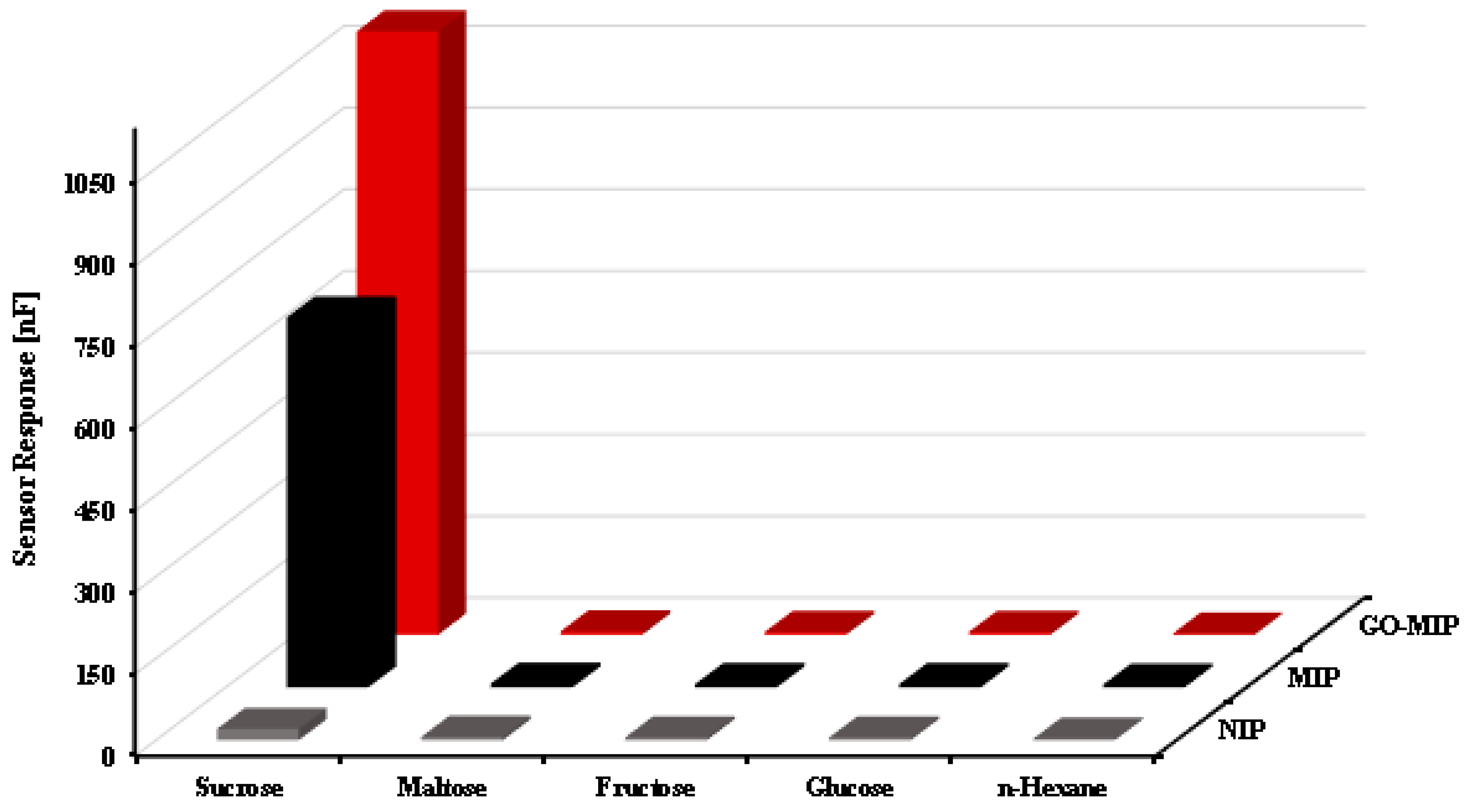
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido, C.; Clavijo, E.; Copaja, S.; Gómez-Jeria, J.; Campos-Vallette, M. Vibrational and electronic spectroscopic detection and quantification of carminic acid in candies. Food Chem. 2019, 283, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Weng, C.; Ruan, Y.; Li, K.; Cai, G.; Song, C.; Lin, Q. An optical chiral sensor based on weak measurement for the real-time monitoring of sucrose hydrolysis. Sensors 2021, 21, 1003. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Rupérez, P.; Villanueva-Suarez, M.J.; Mateos-Aparicio, I. High hydrostatic pressure assisted by food-grade enzymes as a sustainable approach for the development of an antioxidant ingredient. LWT 2022, 169, 113968. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Ma, J.; Wei, S.; Zhang, H. A novel electrochemical sensor based on molecularly imprinted polymer with binary functional monomers at Fe-doped porous carbon decorated Au electrode for the sensitive detection of lomefloxacin. Ionics 2020, 26, 4183–4192. [Google Scholar] [CrossRef]
- Janjua, S.; Hassan, I.; Ali, M.U.; Ibrahim, M.M.; Zafar, A.; Kim, S. Addressing Social Inequality and Improper Water Distribution in Cities: A Case Study of Karachi, Pakistan. Land 2021, 10, 1278. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. A molecularly imprinted electrochemical biosensor based on hierarchical Ti2Nb10O29 (TNO) for glucose detection. Microchim. Acta 2022, 189, 24. [Google Scholar] [CrossRef]
- Ko, H.Y.; Ho, L.H.; Neuhaus, H.E.; Guo, W.J. Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato. Plant Physiol. 2021, 187, 2230–2245. [Google Scholar] [CrossRef]
- Rahmayanti, H.; Ichsan, I.Z.; Oktaviani, V.; Syani, Y.; Hadi, W.; Marhento, G. Environmental attitude for smart city technology: Need assessment to develop smart trash in environmental education. Int. J. Adv. Sci. Technol. 2020, 29, 8374–8383. [Google Scholar]
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Ainsworth, S.; Menzies, S.K.; Casewell, N.R.; Harrison, R.A. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl. Trop. Dis. 2020, 14, e0008579. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Santini, A. Recent advances in metabolic engineering and synthetic biology for microbial production of isoprenoid-based biofuels: An overview. In Bioprocessing for Biofuel Production; Springer: Singapore, 2021; pp. 183–201. [Google Scholar]
- Özbek, O.; Berkel, C.; Isildak, Ö.; Isildak, I. Potentiometric urea biosensors. Clin. Chim. Acta 2022, 524, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ponnaiah, S.K.; Prakash, P. Carbon dots doped tungstic anhydride on graphene oxide nanopanels: A new picomolar-range creatinine selective enzymeless electrochemical sensor. Mater. Sci. Eng. C 2020, 113, 111010. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, M.; Singh, G. Recent advancements in urea biosensors for biomedical applications. IET Nanobiotechnology 2021, 15, 358–379. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Aksorn, J.; Teepoo, S. Development of the simultaneous colorimetric enzymatic detection of sucrose, fructose and glucose using a microfluidic paper-based analytical device. Talanta 2020, 207, 120302. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Gershenzon, J.; van Dam, N.M.; Sala, A.; McDowell, N.G.; Chowdhury, S.; Gleixner, G.; Trumbore, S.; Hartmann, H. Storage of carbon reserves in spruce trees is prioritized over growth in the face of carbon limitation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023297118. [Google Scholar] [CrossRef]
- Soyseven, M.; Sezgin, B.U.R.C.U.; Arli, G. A novel, rapid and robust HPLC-ELSD method for simultaneous determination of fructose, glucose and sucrose in various food samples: Method development and validation. J. Food Compos. Anal. 2022, 107, 104400. [Google Scholar] [CrossRef]
- Lou, Y.R.; Pichersky, E.; Last, R.L. Deep roots and many branches: Origins of plant-specialized metabolic enzymes in general metabolism. Curr. Opin. Plant Biol. 2022, 66, 102192. [Google Scholar] [CrossRef]
- Ronzetti, M.H.; Baljinnyam, B.; Simeonov, A. Protein Refolding Guided by High-Throughput Differential Scanning Fluorimetry: A case study of an HtrA-Family Bacterial Protease. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, B.; Zhang, W.; Xie, Y.; Chen, Y.; Wang, L.; Wang, X. A colorimetric and fluorescence dual-signal determination for iron (II) and H2O2 in food based on sulfur quantum dots. Food Chem. 2022, 366, 130613. [Google Scholar] [CrossRef]
- Baig, N.; Waheed, A.; Sajid, M.; Khan, I.; Kawde, A.N.; Sohail, M. Porous graphene-based electrodes: Advances in electrochemical sensing of environmental contaminants. Trends Environ. Anal. Chem. 2021, 30, e00120. [Google Scholar] [CrossRef]
- Zaldarriaga Heredia, J.; Wagner, M.; Jofré, F.C.; Savio, M.; Azcarate, S.M.; Camiña, J.M. An overview on multi-elemental profile integrated with chemometrics for food quality assessment: Toward new challenges. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Szerlauth, A.; Szalma, L.; Muráth, S.; Sáringer, S.; Varga, G.; Li, L.; Szilágyi, I. Nanoclay-based sensor composites for the facile detection of molecular antioxidants. Analyst 2022, 147, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Perović, J.; Kojić, J.; Krulj, J.; Pezo, L.; Tumbas Šaponjac, V.; Ilić, N.; Bodroža-Solarov, M. Inulin determination by an improved HPLC-ELSD method. Food Anal. Methods 2022, 15, 1001–1010. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.; Ma, S.; Bo, C.; Ou, J.; Gong, B. Recent application of molecular imprinting technique in food safety. J. Chromatogr. A 2021, 1657, 462579. [Google Scholar] [CrossRef]
- Murtada, K.; Moreno, V. Nanomaterials-based electrochemical sensors for the detection of aroma compounds-towards analytical approach. J. Electroanal. Chem. 2020, 861, 113988. [Google Scholar] [CrossRef]
- Özkan, A.; Atar, N.; Yola, M.L. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: Development of molecularly imprinted SPR nanosensor for anticancer drug, etoposide. Biosens. Bioelectron. 2019, 130, 293–298. [Google Scholar] [CrossRef]
- Liu, S.; Kannegulla, A.; Kong, X.; Sun, R.; Liu, Y.; Wang, R.; Yu, Q.; Wang, A.X. Simultaneous colorimetric and surface-enhanced Raman scattering detection of melamine from milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118130. [Google Scholar] [CrossRef]
- Joshi, A.; Kim, K.H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M. Ultrasonication and electrochemically-assisted synthesis of reduced graphene oxide nanosheets for electrochemical sensor applications. FlatChem 2020, 23, 100183. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Li, T.; Ji, Y.; Li, R. Molecularly imprinted polymer-enhanced biomimetic paper-based analytical devices: A review. Anal. Chim. Acta 2021, 1148, 238196. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Li, S.; Qin, Y.; Mo, Q.; Chen, L.; Li, X. Highly sensitive molecular imprinted voltammetric sensor for resveratrol assay in wine via polyaniline/gold nanoparticles signal enhancement and polyacrylamide recognition. J. Electroanal. Chem. 2021, 895, 115455. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.; Correa, D.S. A review on the role and performance of cellulose nanomaterials in sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Mao, Y.; Zhang, S.; Li, H.; Qian, M.; Liu, D.; Qi, B. Electrochemical switch sensor toward ephedrine hydrochloride determination based on molecularly imprinted polymer/nafion-MWCNTs modified electrode. Microchem. J. 2021, 164, 105981. [Google Scholar] [CrossRef]
- Sala, A.; Brisset, H.; Margaillan, A.; Mullot, J.U.; Branger, C. Electrochemical sensors modified with ion-imprinted polymers for metal ion detection. TrAC Trends Anal. Chem. 2022, 148, 116536. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Bao, X.; Yang, M.; Liu, J.; Sun, K.; Li, Z.; Deng, G. Novel fluorescence sensor for the selective recognition of tetracycline based on molecularly imprinted polymer-capped N-doped carbon dots. RSC Adv. 2022, 12, 24778–24785. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. [Google Scholar] [CrossRef]
- Yasinzai, M.; Mustafa, G.; Asghar, N.; Ullah, I.; Zahid, M.; Lieberzeit, P.; Han, D.; Latif, U. Ion-Imprinted Polymer-Based Receptors for Sensitive and Selective Detection of Mercury Ions in Aqueous Environment. J. Sens. 2018, 2018, 8972549. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.G.; Ge, R. Electrospun nanofiber-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef] [PubMed]
- Bonatti, A.F.; De Maria, C.; Vozzi, G. Molecular imprinting strategies for tissue engineering applications: A review. Polymers 2021, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Asghar, N.; Mustafa, G.; Yasinzai, M.; Al-Soud, Y.A.; Lieberzeit, P.A.; Latif, U. Real-time and online monitoring of glucose contents by using molecular imprinted polymer-based IDEs sensor. Appl. Biochem. Biotechnol. 2019, 189, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Sroysee, W.; Chunta, S.; Amatatongchai, M.; Lieberzeit, P.A. Molecularly imprinted polymers to detect profenofos and carbofuran selectively with QCM sensors. Phys. Med. 2019, 7, 100016. [Google Scholar] [CrossRef]
- Yin, S.N.; Yao, T.; Wu, T.H.; Zhang, Y.; Wang, P. Novel metal nanoparticle-enhanced fluorescence for determination of trace amounts of fluoroquinolone in aqueous solutions. Talanta 2017, 174, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bagal-Kestwal, D.R.; Chiang, B.H. Platinum nanoparticle-carbon nanotubes dispersed in gum Arabic-corn flour composite-enzymes for an electrochemical sucrose sensing in commercial juice. Ionics 2019, 25, 5551–5564. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Zhang, W.W.; Zhang, H.W.; Yuan, R.; He, H. Elaborately manufacturing an electrochemical aptasensor based on gold nanoparticle/COF composites for amplified detection performance. J. Mater. Chem. C 2020, 8, 16984–16991. [Google Scholar] [CrossRef]





| Serial No. | Sensor Type | Linear Range | Lower Limit of Detection (LoD) | Reference |
|---|---|---|---|---|
| 1 | Florescent | 0.025–1.0 mg/L | 90,000 ppb | [45] |
| 2 | Colimetric | 1 × 10−4–1 × 10−9 mol/L | 1 × 10−5 ppb | [46] |
| 3 | Florescent | 0.12–12 mg/L | 1000 ppb | [47] |
| 4 | Electrochemical (MIP) | 31 ppb–528 ppb | 31 ppb | Present work |
| 5 | Electrochemical (Go-MIP) | 16–600 ppb | 16 ppb | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, N.; Mustafa, G.; Jabeen, N.; Dawood, A.; Rida; Jabeen, Z.; Malik, Q.H.; Khan, M.A.; Khan, M.U. Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose. Sensors 2023, 23, 2008. https://doi.org/10.3390/s23042008
Asghar N, Mustafa G, Jabeen N, Dawood A, Rida, Jabeen Z, Malik QH, Khan MA, Khan MU. Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose. Sensors. 2023; 23(4):2008. https://doi.org/10.3390/s23042008
Chicago/Turabian StyleAsghar, Nazia, Ghulam Mustafa, Nawishta Jabeen, Asadullah Dawood, Rida, Zeenat Jabeen, Qaiser Hameed Malik, Muhammad Asad Khan, and Muhammad Usman Khan. 2023. "Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose" Sensors 23, no. 4: 2008. https://doi.org/10.3390/s23042008






