Abstract
In this work, acetone gas sensors were fabricated using pre-annealing metal oxide zinc oxide (pa-ZnO)-doped perovskite cesium lead bromide (CsPbBr3). The ZnO nanopowder, before it was doped into CsPbBr3 solution, was first put into a furnace to anneal at different temperatures, and formed the pa-ZnO. The properties of pa-ZnO were different from ZnO. The optimized doping conditions were 2 mg of pa-ZnO nanopowder and pre-annealing at 300 °C. Under these conditions, the highest sensitivity (gas signal current-to-air background current ratio) of the ZnO-doped CsPbBr3 perovskite acetone sensor was 1726. In addition, for the limit test, 100 ppm was the limit of detection of the ZnO-doped CsPbBr3 perovskite acetone sensor and the sensitivity was 101.
1. Introduction
There are more than 3500 chemical components in the breath exhaled by the human body, most of which are volatile organic compounds (VOCs). Among the breath VOCs, acetone is a by-product of lipid metabolism that is closely related to blood glucose levels. Acetone in exhaled breath can thus monitor the metabolic state of the human body. Various techniques have been employed to measure the acetone of very low concentrations, such as gas chromatography–mass spectrometry, proton transfer reaction mass spectrometry, vacuum-free ion mobility spectrometry, laser absorption spectroscopy, and colorimetric sensors [1,2]. However, these techniques require bulky equipment and complex measurement procedures, making real-time monitoring impossible for widespread use throughout the human body. Therefore, it is of great significance to develop a convenient and low-cost method to accurately detect acetone at extremely low concentrations.
The boiling point and autoignition temperature of acetone are 56 °C and 465 °C, respectively. Metal oxide semiconductors are commonly used as sensor materials for sensing chemical gases, but they need to have good sensitivity in high-temperature environments, while perovskite can have a good gas sensing effect in room-temperature environments [3,4,5,6,7,8]. Halide perovskite materials have been widely used in light-emitting-diodes (LEDs) and photovoltaic devices due to their fascinating properties, including a high absorption coefficient, high photoluminescence quantum yield, and low non-radiative recombination rate [9,10,11,12,13,14], and as such, a lot of articles have published on LED and photovoltaic devices. In addition, owing to their excellent hydration–dehydration, electronic transition, adsorption–desorption, phase transition, and ion intercalaltion-decalationthe, the perovskite materials have a high sensitivity to the environment, such as temperature, humidity, VOC, etc., so perovskite materials are also suitable as environmental probes [15,16,17,18,19,20]. However, few articles have investigated their optoelectronic applications. Furthermore, acetone gas sensors made of zinc oxide (ZnO) had a good performance [8,21]. Therefore, in this study, complex materials of ZnO nanopowders and perovskites were prepared, and the ZnO nanopowders were annealed in a high-temperature furnace to increase their oxygen vacancies and conductivity, and then fabricated into a resistance-type acetone sensor with a high sensitivity when operated in room temperature.
2. Materials and Methods
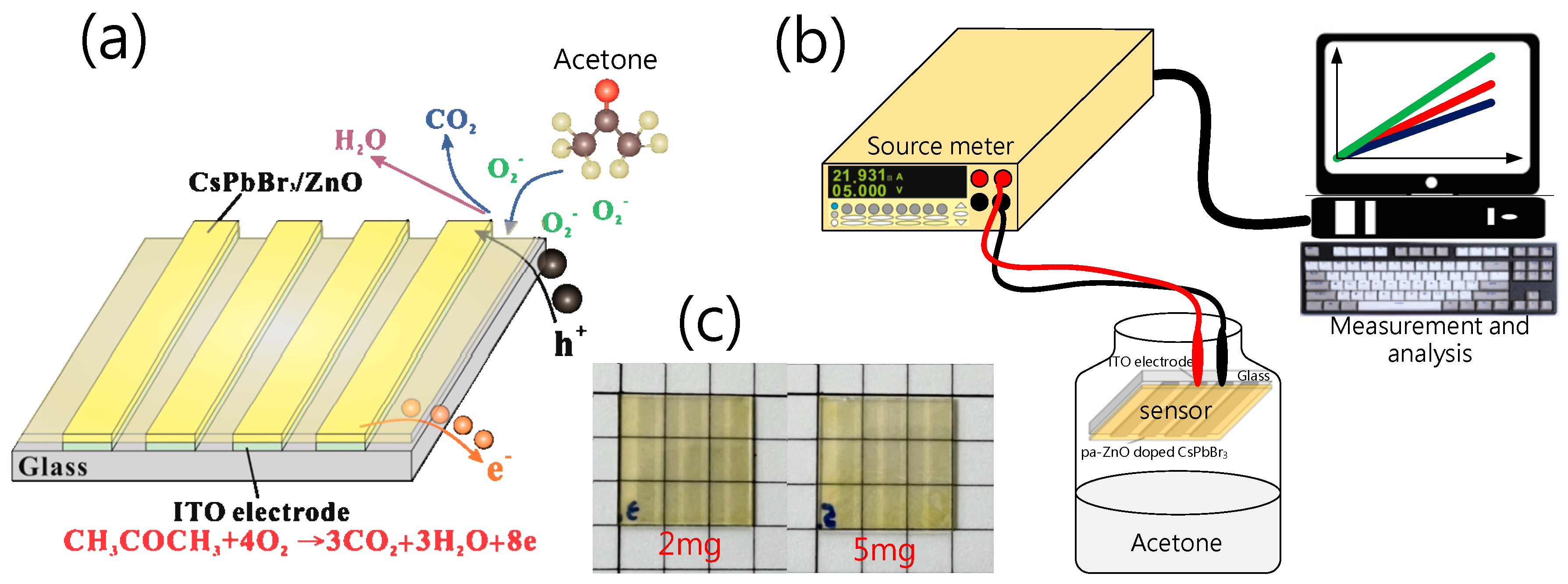
In the device preparation, the ZnO nanopowder with a size of 30 nm (Gredmann, 99.9%) was first put into a furnace to anneal at different temperatures, and formed the pa-ZnO. The second step was to prepare the pa-ZnO-doped CsPbBr3 solution. In total, 0.1835 g of PbBr2 powder (Alfa Aesar, 99.9999%), 0.1064 g of CsBr powder (Alfa Aesar, 99.9999%), and 1 mL of DMSO solvent (Alfa Aesar, 99.5%) were put into beaker and stirred with 500 ppm at a temperature of 70 °C for 30 min. Then, pa-ZnO nanopowders with various weights (1, 2, 5, 10 mg) were added into the beaker and stirred with 500 ppm at temperature of 70 °C for 1 day. Next, the third step was the formation of pa-ZnO-doped CsPbBr3 perovskite film for the acetone sensor. A total of 60 mL of pa-ZnO-doped CsPbBr3 solution was spin-coated on a glass substrate with an ITO (Ruilong, 7 ohm/cm2) pattern at 5000 ppm for 30 s. Additionally, the pa-ZnO-doped CsPbBr3 perovskite film was baked on a hot plate at 100 °C for 10 min to complete the electro-resistance-type pa-ZnO-doped CsPbBr3 perovskite acetone sensor, as shown in Figure 1a.
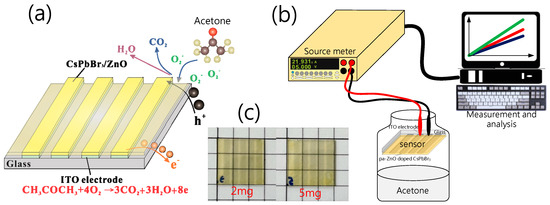
Figure 1.
(a) Electro-resistance-type pa-ZnO-doped CsPbBr3 perovskite acetone sensor and sensing mechanism. (b) Structure of experiment system. (c) The photos of 2- and 5-mg pa-ZnO-doped CsPbBr3 perovskite films on ITO glass.
In characteristic X-ray diffraction (XRD), a scanning electron microscope (SEM), photoluminescence (PL), UV/VIS spectrometers, and X-ray photoelectron spectroscopy (XPS) were used to measure and analyze the characteristics of pa-ZnO-doped CsPbBr3 perovskite films. Additionally, a Keithley 2400 source meter was employed to measure the I-V characteristics and to estimate the sensitivity of the electro-resistance-type pa-ZnO-doped CsPbBr3 perovskite acetone sensors. Figure 1b shows the structure of the experiment system for measurement.
3. Results and Discussion
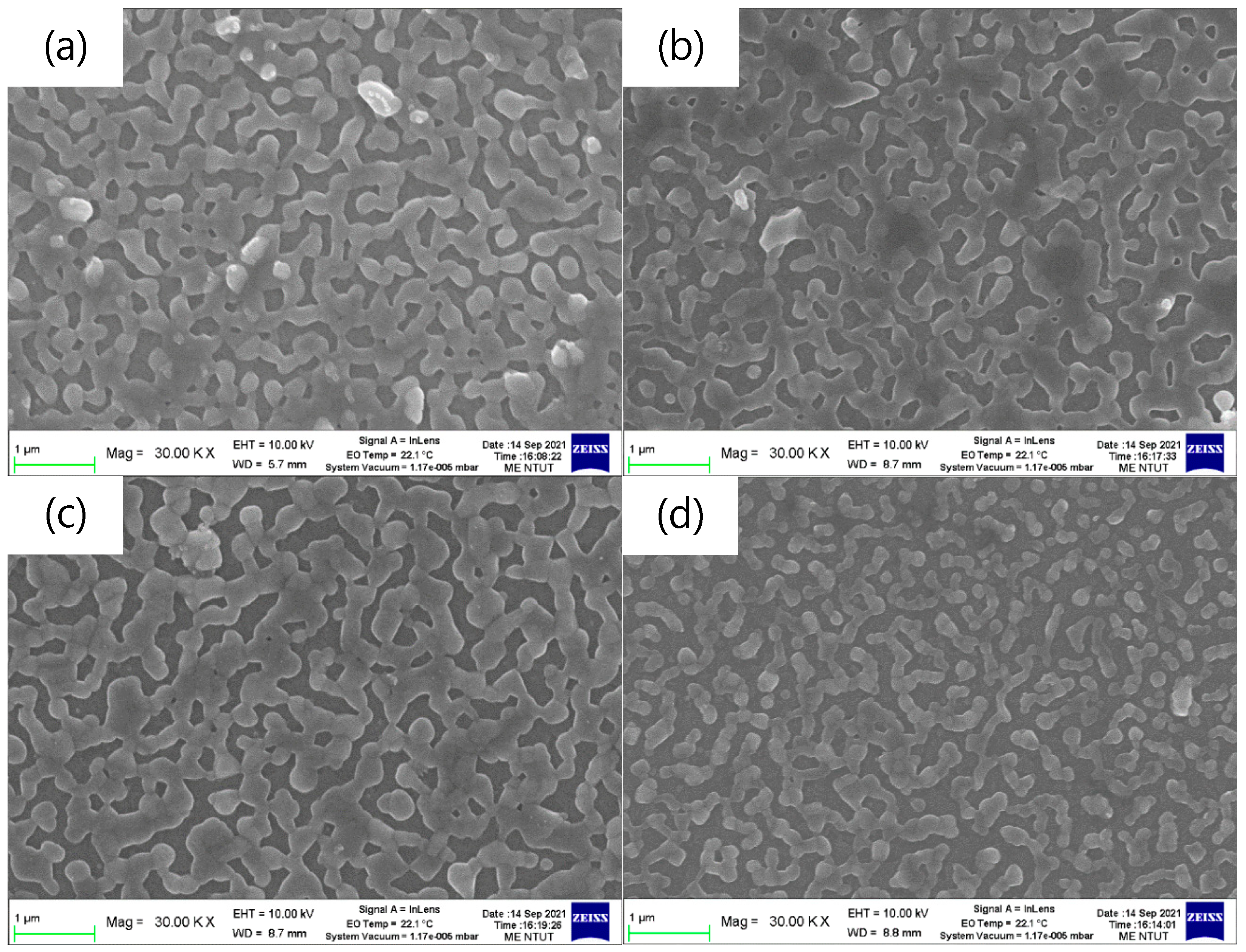
Figure 2 shows the top-view SEM images of the pa-ZnO-doped CsPbBr3 perovskite films on glass substrates. The coverages of all samples are around 83%, as estimated by Image J software. The aggregation domain sizes of the pa-ZnO-doped CsPbBr3 perovskite films, with 2 and 5 mg of pa-ZnO nanopowder doping, exhibited larger than that of the samples with 1 and 10 mg of pa-ZnO nanopowder doping, as show in Figure 2a–d. The pa-ZnO-doped CsPbBr3 perovskite films with 2 mg of the pa-ZnO nanopowder dopants had the largest aggregation domain size.
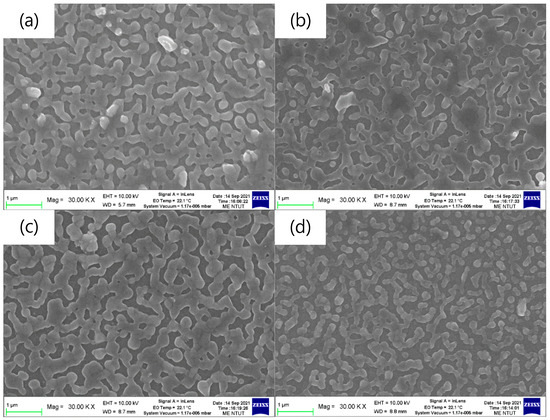
Figure 2.
Top-view SEM images of the pa-ZnO-doped CsPbBr3 perovskite films on glass substrates with various amounts of pa-ZnO nanopowder: (a) 1 mg, (b) 2 mg, (c) 5 mg, and (d) 10 mg.
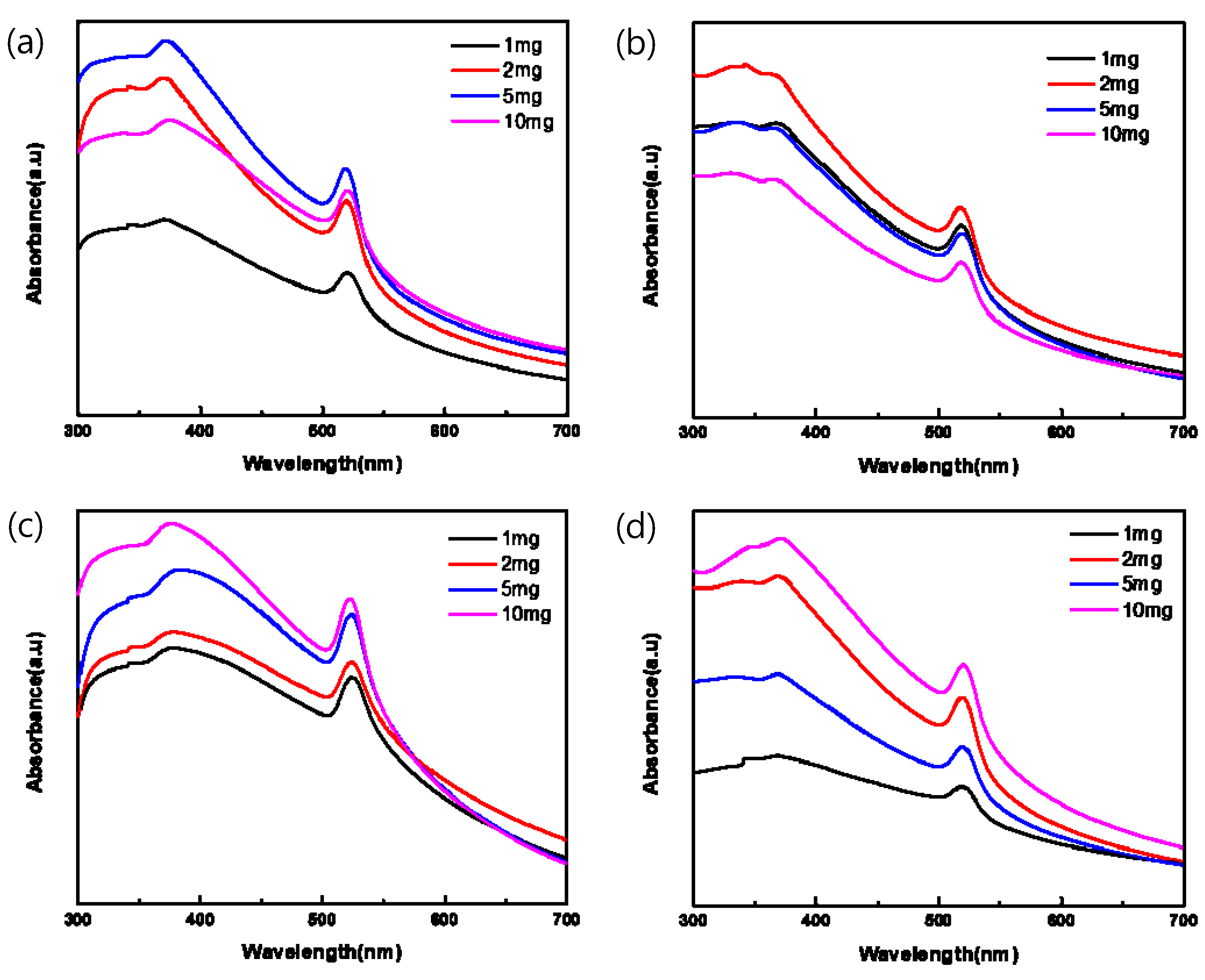
Figure 3 plots the absorption spectra of the pa-ZnO-doped CsPbBr3 perovskite films, with various amounts of pa-ZnO nanopowder dopants with treatments of different annealing temperatures of 200–500 °C in UV-vis range. In total, two absorption peaks at 372 and 519 nm were observed. They are corresponding to the absorption of pa-ZnO and CsPbBr3. The peak position of the absorption did not shift with the doping amount of the pa-ZnO dopant and annealing temperature. This means that the nature of the materials of the pa-ZnO-doped CsPbBr3 perovskite films did not change.
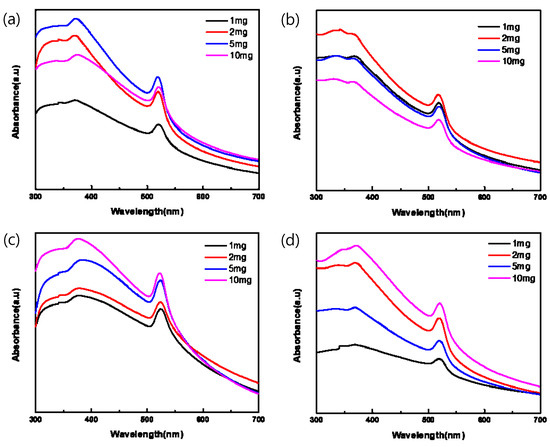
Figure 3.
Absorption spectra of pa-ZnO-doped CsPbBr3 perovskite films with various amounts of pa-ZnO nanopowder dopants with treatment of annealing temperatures at (a) 200 °C, (b) 300 °C, (c) 400 °C, and (d) 500 °C.
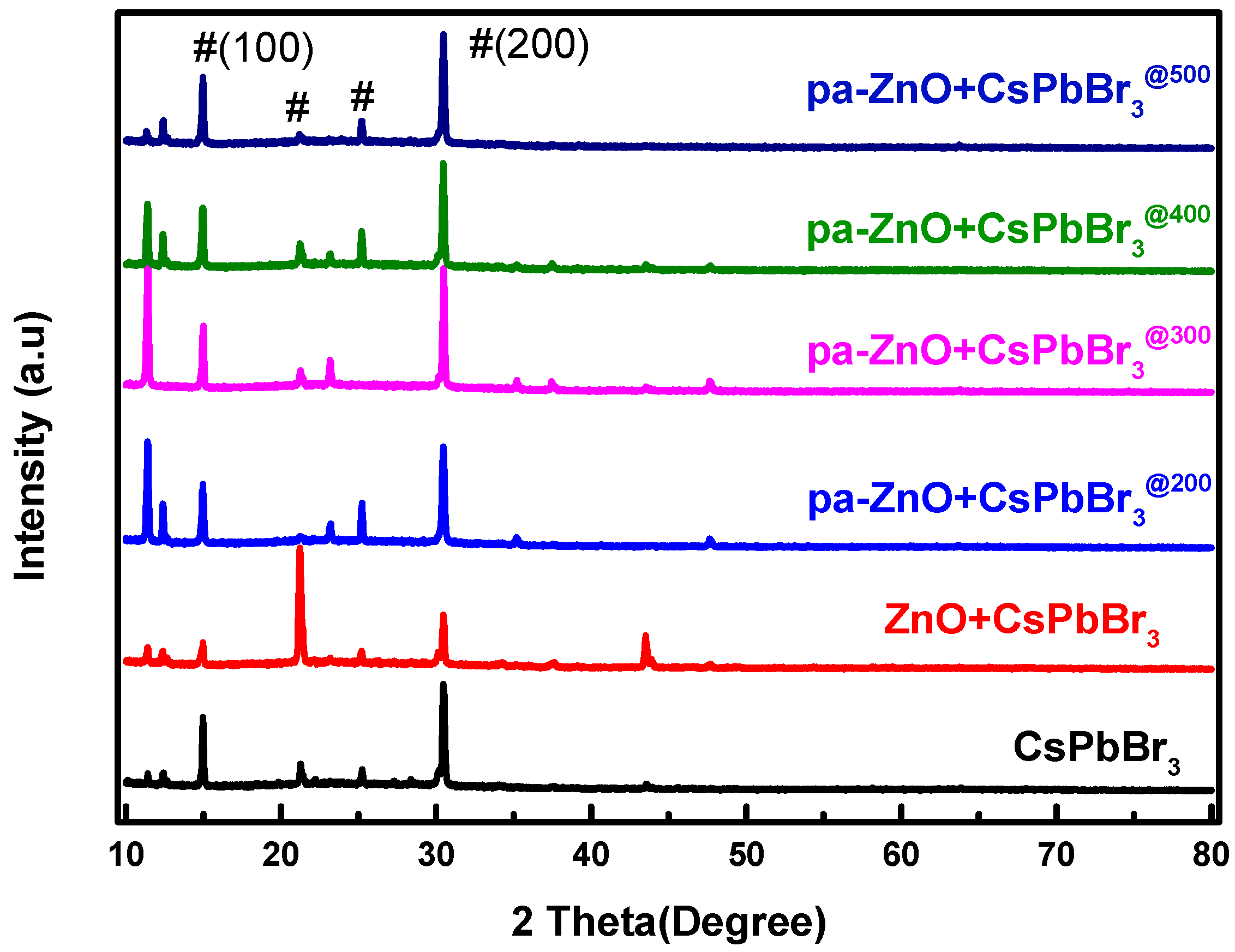
An XRD pattern was employed to understand the quality and composition variation of the pa-ZnO-doped CsPbBr3 perovskite films. As shown in Figure 4, there are four XRD characteristic peaks of perovskite CsPbBr3 that are observed. Their positions are at approximately 14.97°, 21.24°, 25.23°, and 30.47°, and are corresponding to the (100), (110), (111), and (200) phases of the cubic lattice structure, respectively [22,23,24]. A total of two weak diffraction peaks observed at the 2θ values of 35.24°and 37.45° have been indexed as (002) and (101) crystal planes of ZnO, respectively, for the sample of the pa-ZnO-doped CsPbBr3 perovskite film at an annealing temperature of 300 °C. This means that 300 °C is the optimized annealing condition.
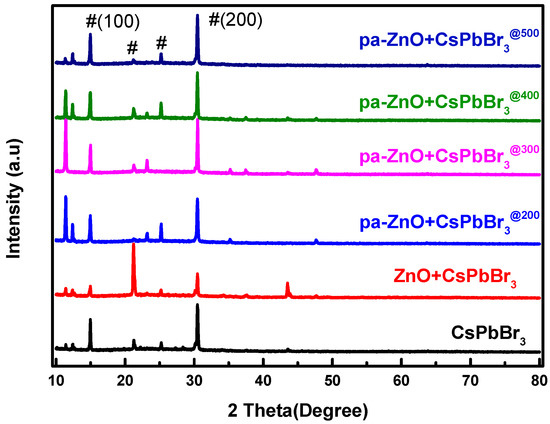
Figure 4.
XRD pattern of pa-ZnO-doped CsPbBr3 perovskite films with various pre-annealing temperatures.
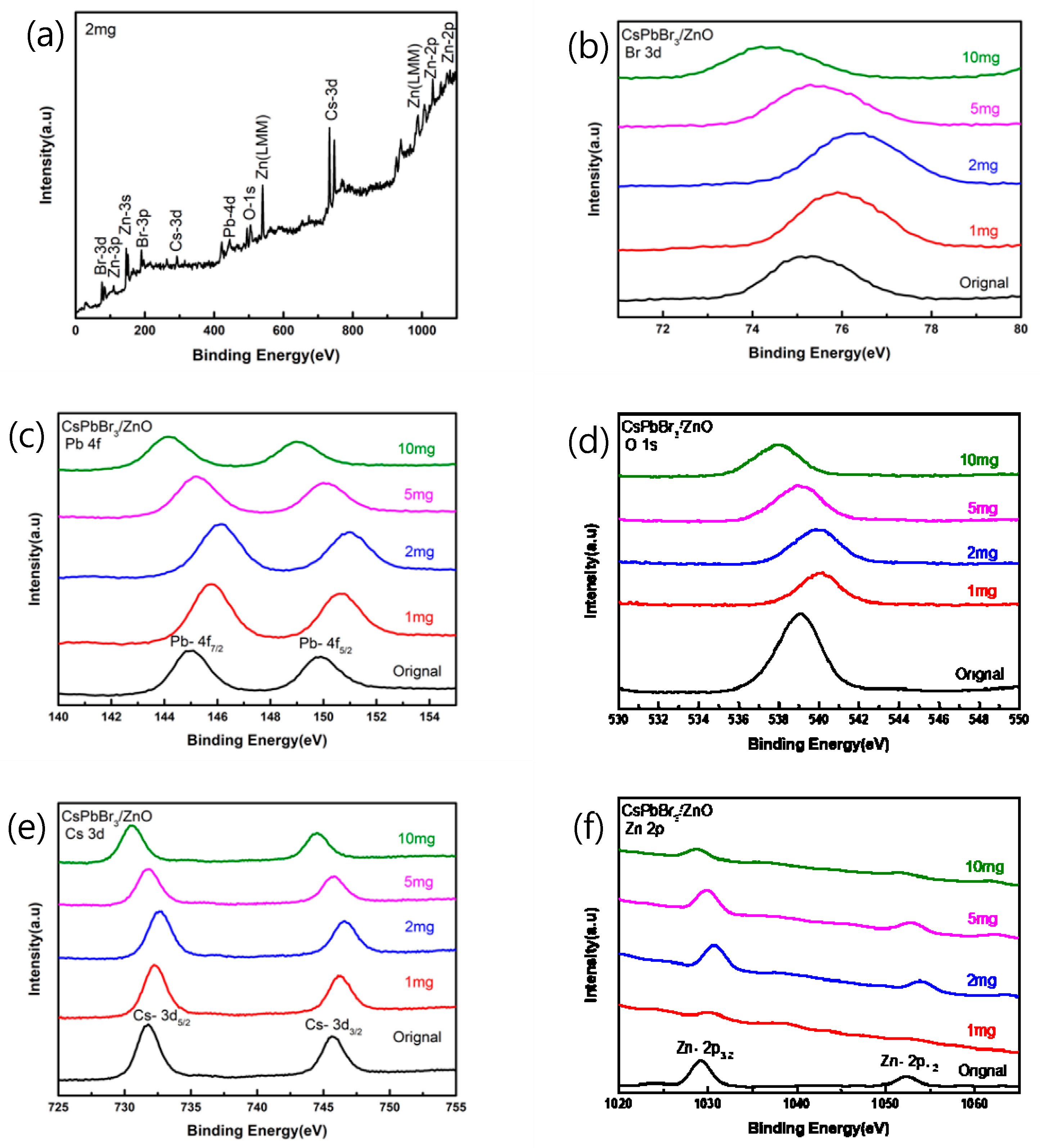
Figure 5a shows the XPS spectrum of CsPbBr3 added with 2 mg of pa-ZnO annealed at 300 °C. As shown in Figure 5b, the binding energy of Br-3d was increased from 75.21 eV of the original sample (undoped CsPbBr3 film) to 76.35 eV. As shown in Figure 5c, the binding energy of Pb-4f5/2 increased from 149.87 eV of the original sample to 150.97 eV, and the binding energy of Pb-4f7/2 increased from 144.97 eV of the original sample to 146.11 eV. As shown in Figure 5d, the binding energy of O-1s increased from 539.04 eV of the original sample to 540.11 eV. As shown in Figure 5e, the binding energy of Cs-3d5/2 increased from 731.68 eV of the original sample to 732.56 eV, and the binding energy of Cs-3d3/2 increased again from 745.65 eV of the original sample to 746.61 eV. As shown in Figure 5f, the binding energy of Zn-2p3/2 increased from 1029.22 eV of the original sample to 1030.78 eV. The binding energy of Zn-2p1/2 was increased from 1052.36 eV to 1054.07 eV to compare with the original sample. Therefore, it can be found that when 2 mg of pa-ZnO is added, for any different atoms, the binding energy is increased in comparison to the original sample, which is the highest in all samples. The phenomenon of this binding energy displacement may be that when the atoms are oxidized, the bond energy will be enhanced, and the oxidation will increase the binding energy of the inner layer electrons. The more electrons that are lost during oxidation, the greater this increase, and the peak position of the O-1s is related to the oxygen vacancies of the crystal lattice. It can be seen that, compared with the original sample of XPS spectra, the intensity after annealing has a downward trend, and it is known that the annealing heating causes oxygen vacancies [25,26].
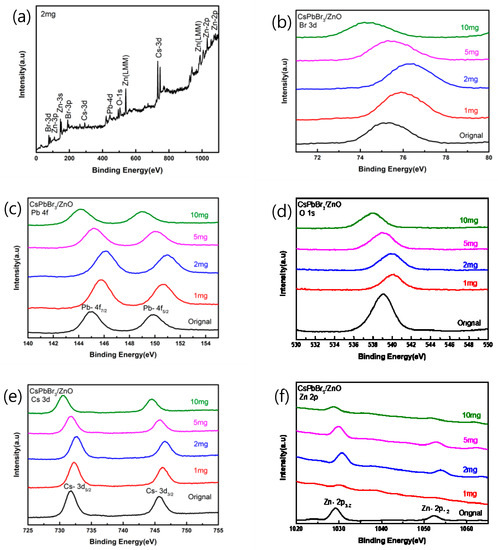
Figure 5.
XPS spectra of pa-ZnO-doped CsPbBr3 films (a) added 2 mg of pa-ZnO nanopowder, and (b–f) in different binding energy range.
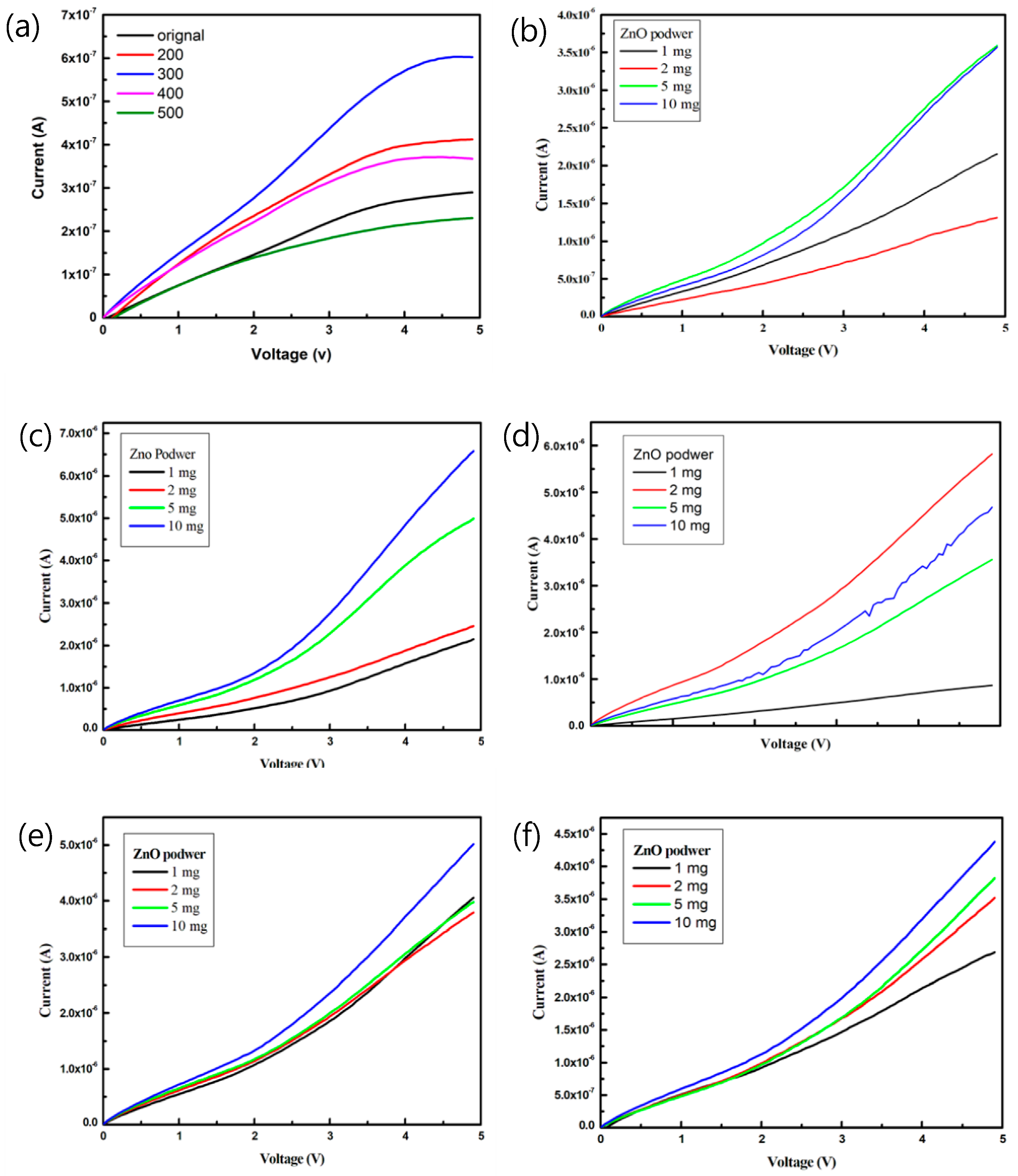
Figure 6 plots the current-voltage (I–V) characteristics of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with the treatment of annealing at various temperatures and pa-ZnO doping amounts. The sensitivity, S, can be used to estimate the performance of the gas sensor [5,27]:
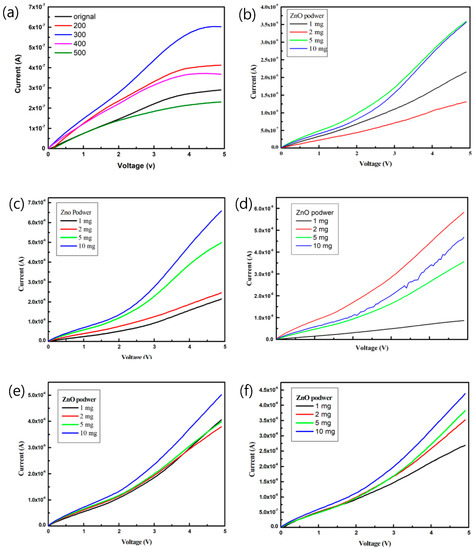
Figure 6.
I–V characteristics of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with treatment of annealing at various temperatures and pa-ZnO doping amounts: (a) pa-ZnO sensor, (b) doping pa-ZnO without anneal, (c) doping pa-ZnO annealed at 200 °C, (d) doping pa-ZnO annealed at 300 °C, (e) doping pa-ZnO annealed at 400 °C, and (f) doping pa-ZnO annealed at 500 °C.
The sensitivity of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with the treatment of annealing at various temperatures and pa-ZnO doping amounts at 4.9 V, are summarized in Table 1. The sensitivity of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with the treatment of annealing at 300 °C and a doping amount of 2 mg shows the highest sensitivity, owing to the best conductivity, caused by a lot of oxygen vacancies. When the annealing temperature increases to 400 and 500 °C, the sensitivity decreases because of the description of oxygen vacancies, owing to the oxidation effect.

Table 1.
Sensitivity of pa-ZnO-doped CsPbBr3 perovskite acetone sensors with treatment of annealing at various temperatures and pa-ZnO doping amounts at 4.9 V.
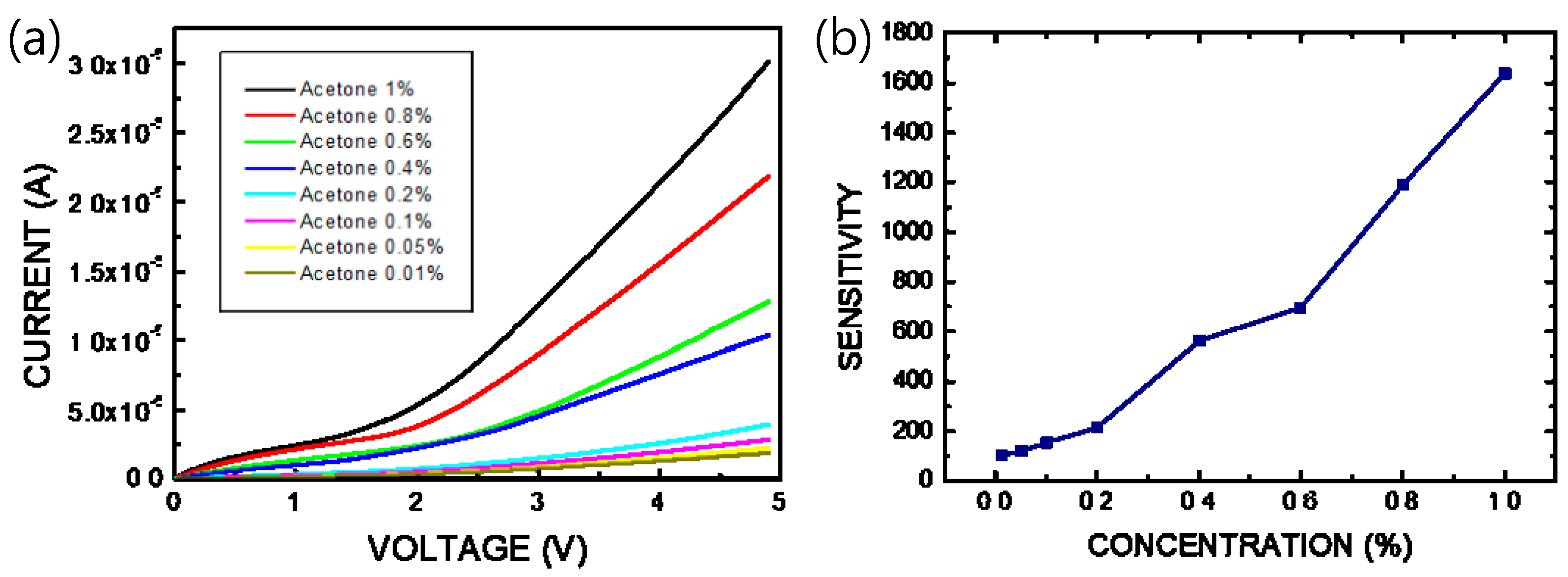
When the thin film of the pa-ZnO-doped CsPbBr3 perovskite is exposed to gas molecules to be measured, adsorbed oxygen on the pa-ZnO-doped CsPbBr3 perovskite film reacts with the acetone gas, thereby releasing electrons, as shown in Figure 1a. The electrons will fill the vacancies of the perovskite film and form electron and hole currents to improve the conductivity of the pa-ZnO-doped CsPbBr3 perovskite film. Therefore, we measured the current-voltage (I–V) characteristics to evaluate the performance of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors. Figure 7 shows the I–V characteristics of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with the treatment of annealing at 300 °C and a pa-ZnO doping amount of 2 mg, under the ambient of various concentrations of acetone. Under the treatment of the champion condition: an annealing temperature of 300 °C and a pa-ZnO doping amount of 2 mg, the current of the pa-ZnO-doped CsPbBr3 perovskite acetone sensor decreases as the concentration of the acetone decreases. When the concentration of the acetone decreases to 0.01% (100 ppm), the I-V curve of the pa-ZnO-doped CsPbBr3 perovskite acetone sensor is a near-background I-V curve. Therefore, the 100 ppm is the limit of detection for the pa-ZnO-doped CsPbBr3 perovskite acetone sensor, and the sensitivity is 101. To compare the results of other works [4], the performance of the pa-ZnO-doped CsPbBr3 perovskite acetone sensor in this work have a competitive edge.
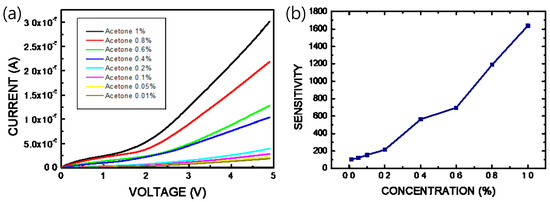
Figure 7.
(a) I–V characteristics and (b) sensitivity of the pa-ZnO-doped CsPbBr3 perovskite acetone sensors with treatment of annealing at 300 °C and pa-ZnO doping amount of 2 mg under ambient of various concentration of acetone.
4. Conclusions
In this work, pa-ZnO-doped CsPbBr3 perovskite acetone sensors have been investigated. We have studied the morphology, absorption spectra, and XRD pattern of pa-ZnO-doped CsPbBr3 perovskite films for pre-annealing temperatures and doping amounts, respectively. The optimized pre-annealing temperature and doping amount are 300 °C and 2 mg of ZnO, respectively. According to the XPS spectra, the binding energy shifts with the amount of pa-ZnO nanopowder added. The effect this binding energy displacement may be that the atoms will be oxidized, the bond energy will be enhanced, and the oxidation will increase the binding energy of the inner layer electrons. Under the treatment of the champion condition, the highest sensitivity of the pa-ZnO-doped CsPbBr3 perovskite acetone sensor is 1726. In addition, 100 ppm is the limit of detection of the pa-ZnO-doped CsPbBr3 perovskite acetone sensor, and the sensitivity is 101. This work is not suitable for medical applications (1 ppm-level) [28,29,30,31]. However, the devices with an introduction of low-dimensional structure (quantum dots, nanorods, and nanosheets) may meet the requirement of biotechnology in the future.
Author Contributions
Conceptualization, L.-C.C. and K.-Y.L.; methodology, L.-C.C. and A.-N.S.; formal analysis, L.-C C.; investigation, L.-C.C. and K.-Y.L.; data curation, A.-N.S.; writing—original draft preparation, L.-C.C.; writing—review and editing, L.-C.C. and K.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Technology (Taiwan) under Contract No. 111-2221-E-027-040-MY3. This work was also supported by the NTUT-Industry Joint Research Program under contract No. 211A171.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amor, R.E.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, C.C.; Wu, C.L.; Lin, C.W. CsPbBr3 Perovskite Powder, a Robust and Mass-Producible Single-Source Precursor: Synthesis, Characterization, and Optoelectronic Applications. ACS Omega 2019, 4, 8081–8086. [Google Scholar] [CrossRef] [PubMed]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Liu, J.; Liu, F.J.; Liu, H.N.; Yue, J.Y.; Jin, J.Y.; Impundu, J.; Liu, H.; Yang, Z.; Peng, Z.S.; Wei, H.N.; et al. Mixed-dimensional CsPbBr3@ZnO heterostructures for high-performance p-n diodes and photodetectors. Nano Today 2021, 36, 101055. [Google Scholar] [CrossRef]
- Neogi, S.; Ghosh, R. Ion-dipole interaction for selective detection of acetone by perovskite BiFeO3 chemi-resistive sensor. Anal. Chim. Acta 2022, 1206, 339788. [Google Scholar] [CrossRef]
- Solanki, V.; Banerjee, A.; Nanda, K.K. Conductometric room temperature ammonia sensor based on porous tin oxide. Sens. Actuators B-Chem. 2022, 366, 131942. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566. [Google Scholar] [CrossRef]
- Schanze, K.S.; Kamat, P.V.; Yang, P.; Bisquert, J. Progress in perovskite photocatalysis. ACS Energy Lett. 2020, 5, 2602–2604. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Liu, Z.; Krückemeier, L.; Krogmeier, B.; Klingebiel, B.; Márquez, J.; Levcenko, S.; Öz, S.; Mathur, S.; Rau, U.; Unold, T.; et al. Open-circuit voltages exceeding 1.26 V in planar methylammonium lead iodide perovskite solar cells. ACS Energy Lett. 2019, 4, 110–117. [Google Scholar] [CrossRef]
- Gao, B.; Meng, J.; Lu, J.; Zhao, R. CH3NH3PbI3 perovskite solar cells with efficiency over 22% fabricated by green antisolvent method. Mater. Lett. 2020, 274, 127995. [Google Scholar] [CrossRef]
- Park, J.; Jang, H.M.; Kim, S.; Jo, S.H.; Lee, T.W. Electroluminescence of Perovskite Nanocrystals with Ligand Engineering. Trends Chem. 2020, 2, 837–849. [Google Scholar] [CrossRef]
- He, Q.; Mei, E.; Liang, X.; Xiang, W. Ultrastable PVB films-protected CsPbBr3/Cs4PbBr6 perovskites with high color purity for nearing Rec. 2020 standard. Chem. Eng. J. 2021, 419, 129529. [Google Scholar] [CrossRef]
- Ahmad, S.; Husain, A.; Khan, M.M.A.; Khan, I.; Khan, A.; Asiri, A.M. Perovskite-based material for sensor applications. Hybrid Perovskite Compos. Mater. 2021, 135–145. [Google Scholar]
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Hu, L.; Shao, G.; Jiang, T.; Li, D.; Lv, X.; Wang, H.; Liu, X.; Song, H.; Tang, J.; Liu, H. Investigation of the Interaction between Perovskite Films with Moisture via in Situ Electrical Resistance Measurement. ACS Appl. Mater. Interface 2015, 7, 25113–25120. [Google Scholar] [CrossRef]
- Zhao, M.; Shi, Y.; Dai, J.; Lian, J. Ellipsometric study of the complex optical constants of a CsPbBr3 perovskite thin film. J. Mater. Chem. C 2018, 6, 10450–10455. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; He, W.; Peng, H.; Dai, Q. CsPbBr3 perovskite nanowires and their optical properties. Opt. Mater. 2020, 109, 110399. [Google Scholar] [CrossRef]
- Chen, L.C.; Kao, C.H. Improved extraction efficiency of CsPbBr3 perovskite light-emitting diodes due to anodic aluminum oxide nanopore structure. Sci. Rep. 2022, 12, 14750. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.; Güntner, A.T.; Park, Y.; Rim, H.J.; Lee, H.S.; Lee, W. Sensing of acetone by Al-doped ZnO. Sens. Actuators B Chem. 2019, 283, 107–115. [Google Scholar] [CrossRef]
- Yen, M.C.; Lee, C.J.; Liu, K.H.; Peng, Y.; Leng, J.; Chang, T.H.; Chang, C.C.; Tamada, K.; Lee, Y.J. All-inorganic perovskite quantum dot light-emitting memories. Nat. Commun. 2021, 12, 4460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.C.; Tang, A.C.; Lin, S.Y.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Robust and Stable Narrow-Band Green Emitter: An Option for Advanced Wide-Color-Gamut Backlight Display. Chem. Mater. 2016, 28, 8493–8497. [Google Scholar] [CrossRef]
- Shen, H.; Nan, R.; Jian, Z.; Li, X. Defect step controlled growth of perovskite MAPbBr3 single crystal. J. Mater. Sci. 2019, 54, 11596–11603. [Google Scholar] [CrossRef]
- Yuan, B.; Li, N.; Liu, J.; Xu, F.; Li, C.; Juan, F.; Yu, H.; Li, C.; Cao, B. Improving the performances of CsPbBr3 solar cells fabricated in ambient condition. J. Mater. Sci. 2020, 31, 21154–21167. [Google Scholar] [CrossRef]
- Son, D.; Moon, B.J.; Lee, A.; Rho, H.; Lee, H.J.; Kim, T.W.; Ha, J.S.; Lee, S.H. Polarity effects of ZnO on charge recombination at CsPbBr3 nanoparticles/ZnO interfaces. App. Sur. Sci. 2019, 483, 165–169. [Google Scholar] [CrossRef]
- Tien, C.H.; Lee, K.L.; Tao, C.C.; Lin, Z.Q.; Lin, Z.H.; Chen, L.C. Two-Dimensional (PEA)2PbBr4 Perovskites Sensors for Highly Sensitive Ethanol Vapor Detection. Sensors 2022, 22, 8155. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. IEEE Sens. J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, D.; Singh, A.; Gupta, M.; Thapa, K.B.; Yadav, B.C. Detection of acetone via exhaling human breath for regular monitoring of diabetes by low-cost sensing device based on perovskite BaSnO3 nanorods. Sens. Actuators B Chem. 2022, 361, 131708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).