Toward Early and Objective Hand Osteoarthritis Detection by Using EMG during Grasps
Abstract
1. Introduction
2. Materials and Methods
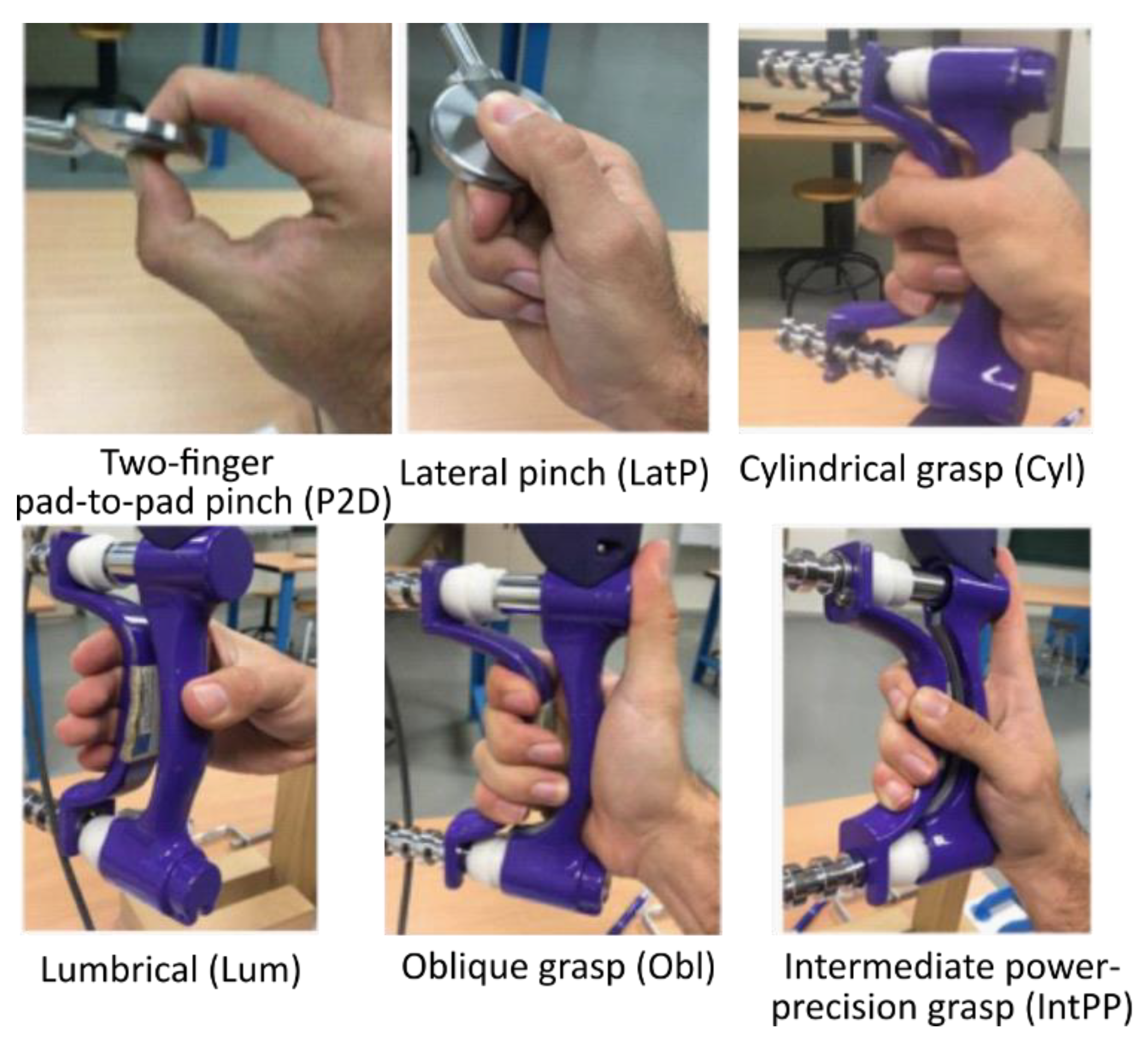
2.1. Experimental Study
2.2. Data Analysis
2.2.1. Computed Parameters
2.2.2. Global Description
2.2.3. Can EMG Characteristics Be Used for Early HOA Diagnosis?
3. Results
3.1. Are Forearm Muscles Significantly Affected or Differently Used by HOA in Terms of EMG Characteristics?
3.1.1. Gender Effect in the Control Group Subjects
3.1.2. HOA Effect
3.2. Can EMG Characteristics Be Used for the Early Detection of HOA?
4. Discussion
4.1. Are Forearm Muscles Significantly Affected or Differently Used by HOA in EMG Terms?
4.2. Can EMG Characteristics Be Used to Detect HOA Early?
- LDA2 and LDA5 were composed only of EWL values and required recording EMG signals from wrist flexors, ulnar deviators, thumb muscles, wrist extensors and radial deviators while performing all the grasps except the intermediate power–precision grasp. Not requiring MA characteristics would prevent MVC recordings and simplify the diagnosis method;
- LDA4, LDA9, LD10 and LDA14 required different combinations of EMG characteristics, but always from the same muscular forearm spots and grasps: digit flexors, thumb muscles, wrist extensors and radial deviators while performing the cylindrical, oblique-palmar grasp and intermediate power–precision grasp.
- NZC values did not well-discriminate HOA patients;
- Muscle activity (MA) did not require any other characteristic to discriminate HOA patients, but required MVC recordings;
- EWL could very accurately discriminate, but needed information of more grasps;
- EMAV could very accurately discriminate, but always had to be accompanied by other EMG characteristics (MA or EWL).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADLs | Activities of daily living |
| Cyl | Cylindrical grasp |
| DF | Digit flexion |
| EMAV | Enhanced mean absolute value |
| EMG | Electromyography |
| EWL | Enhanced wavelength |
| FE | Finger extension |
| HOA | Hand osteoarthritis |
| IntPP | Intermediate power–precision grasp |
| LatP | Lateral pinch |
| Lum | Lumbrical grasp |
| MGE | Maximum grasping effort |
| MVC | Maximum voluntary contraction |
| NZC | New zero crossing |
| Olb | Oblique palmar grasp |
| P2D | Two-finger pad-to-pad pinch |
| TM | Thumb extension and abduction/adduction |
| WE_UD | Wrist extension and ulnar deviation |
| WE_RD | Wrist extension and radial deviation |
| WF_RD | Wrist flexion and radial deviation |
| WF_UD | Wrist flexion and ulnar deviation |
References
- Blanco, F.J.; Silva-Díaz, M.; Quevedo Vila, V.; Seoane-Mato, D.; Pérez Ruiz, F.; Juan-Mas, A.; Pego-Reigosa, J.M.; Narváez, J.; Quilis, N.; Cortés, R.; et al. Prevalence of symptomatic osteoarthritis in Spain: EPISER2016 study. Reumatol. Clin. 2020, 17, 461–470. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Kwok, W.Y. Hand osteoarthritis a heterogeneous disorder. Nat. Rev. Rheumatol. 2012, 8, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Fautrel, B.; Hilliquin, P.; Rozenberg, S.; Allaert, F.A.; Coste, P.; Leclerc, A.; Rossignol, M. Impact of osteoarthritis: Results of a nationwide survey of 10,000 patients consulting for OA. Jt. Bone Spine 2005, 72, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.M.; de Oliveira, D.G.; Aruin, A.S.; dos Santos, M.J. Relationship between hand function and grip force control in women with hand osteoarthritis. J. Rehabil. Res. Dev. 2012, 49, 855. [Google Scholar] [PubMed]
- Hermann, W.; Lambova, S.; Müller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.J.; Rainsford, K.D.; Kean, W.F. Osteoarthritis of the hand I: Aetiology and pathogenesis, risk factors, investigation and diagnosis. J. Pharm. Pharmacol. 2014, 66, 339–346. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dincer, F.; Dziedzic, K.; Hauselmann, H.J.; Kaklamanis, P.; et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: Report of a task force of ESCISIT. Ann. Rheum. Dis. 2008, 68, 8–17. [Google Scholar] [CrossRef]
- Zhang, Y. Prevalence of Symptomatic Hand Osteoarthritis and Its Impact on Functional Status among the Elderly: The Framingham Study. Am. J. Epidemiol. 2002, 156, 1021–1027. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Boersma, A.; Boonen, A.; van Beest, S.; Damman, W.; van der Heijde, D.; Rosendaal, F.R.; Kloppenburg, M. Performance of the Michigan Hand Outcomes Questionnaire in hand osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1627–1635. [Google Scholar] [CrossRef]
- Sierakowska, M.; Wysocka-Skurska, I.; Kułak, W. Identification of demographic factors and health problems that affect the acceptance of disease and health behaviors of patients with osteoarthritis. PeerJ 2017, 2017, e3276. [Google Scholar] [CrossRef]
- Bagis, S.; Gunsah, A.E.; Ae, S.; Yapici, Y.; Bolgen, O.; Ae, C.; Erdogan, C. The effect of hand osteoarthritis on grip and pinch strength and hand function in postmenopausal women. Clin. Rheumatol. 2003, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Hernández-Molina, G. Hand Osteoarthritis: An Epidemiological Perspective. Semin. Arthritis Rheum. 2010, 39, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lewek, M.D.; Rudolph, K.S.; Snyder-Mackler, L. Quadriceps Femoris Muscle Weakness and Activation Failure in Patients with Knee Osteoarthritis. J. Orthop. Res. 2011, 22, 110–115. [Google Scholar] [CrossRef]
- Aspden, R.M. Osteoarthritis: A problem of growth not decay? Rheumatology 2008, 47, 1452–1460. [Google Scholar] [CrossRef]
- Brorsson, S.; Nilsdotter, A.; Thorstensson, C.; Bremander, A. Differences in muscle activity during hand-dexterity tasks between women with arthritis and a healthy reference group. BMC Musculoskelet. Disord. 2014, 15, 154. [Google Scholar] [CrossRef]
- Tossini, N.B.; Lessi, G.C.; Simões Zacharias, A.L.; Corrêa e Silva, G.R.; Seraphim Abrantes, L.S.; Mendes da Silva Serrão, P.R. Impairment of electrical activation of wrist flexor and extensor muscles during gripping and functional activities in the early stage of hand osteoarthritis: A cross-sectional study. J. Hand Ther. 2021, 34, 109–115. [Google Scholar] [CrossRef]
- Harada, T.; Miyagami, T.; Kunitomo, K.; Shimizu, T. Clinical decision support systems for diagnosis in primary care: A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 8435. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Guaragnella, C. A Decision Support System for Melanoma Diagnosis from Dermoscopic Images. Appl. Sci. 2022, 12, 7007. [Google Scholar] [CrossRef]
- Yu, H.-L.; Chase, R.A.; Robert, A.; Strauch, B. Atlas of Hand Anatomy and Clinical Implications; Mosby: St. Louis, MO, USA, 2004; ISBN 0815179278. [Google Scholar]
- Jarque-Bou, N.J.; Vergara, M.; Sancho-Bru, J.L.; Alba, R.-S.; Gracia-Ibáñez, V. Identification of forearm skin zones with similar muscle activation patterns during activities of daily living. J. NeuroEng. Rehabil. Under Rev. 2018, 15, 91. [Google Scholar] [CrossRef]
- Too, J.; Abdullah, A.R.; Saad, N.M.; Tee, W. EMG Feature Selection and Classification Using a Pbest-Guide Binary Particle Swarm Optimization. Computation 2019, 7, 12. [Google Scholar] [CrossRef]
- Tepe, C.; Demir, M.C. Real-Time Classification of EMG Myo Armband Data Using Support Vector Machine. IRBM 2022, 43, 300–308. [Google Scholar] [CrossRef]
- Phinyomark, A.; Quaine, F.; Charbonnier, S.; Serviere, C.; Tarpin-Bernard, F.; Laurillau, Y. EMG feature evaluation for improving myoelectric pattern recognition robustness. Expert Syst. Appl. 2013, 40, 4832–4840. [Google Scholar] [CrossRef]
- Too, J.; Abdullah, A.R.; Saad, N.M. Classification of Hand movements based on discrete wavelet transform and enhanced feature extraction. Int. J. Adv. Comput. Sci. Appl. 2019, 10, 83–89. [Google Scholar] [CrossRef]
- Arunraj, M.; Srinivasan, A.; Arjunan, S.P. A Real-Time Capable Linear Time Classifier Scheme for Anticipated Hand Movements Recognition from Amputee Subjects Using Surface EMG Signals. IRBM 2021, 42, 277–293. [Google Scholar] [CrossRef]
- Sravani, C.; Bajaj, V.; Taran, S.; Sengur, A. Flexible Analytic Wavelet Transform Based Features for Physical Action Identification Using sEMG Signals. IRBM 2020, 41, 18–22. [Google Scholar] [CrossRef]
- Cutkosky, M.R. On grasp choice, grasp models, and the design of hands for manufacturing tasks. IEEE Trans. Robot. Autom. 1989, 5, 269–279. [Google Scholar] [CrossRef]
- Cipriani, C.; Zaccone, F.; Micera, S.; Carrozza, M.C. On the shared control of an EMG-controlled prosthetic hand: Analysis of user-prosthesis interaction. IEEE Trans. Robot. 2008, 24, 170–184. [Google Scholar] [CrossRef]
- Feix, T.; Romero, J.; Schmiedmayer, H.-B.; Dollar, A.M.; Kragic, D. The GRASP Taxonomy of Human Grasp Types. IEEE Trans. Hum.-Mach. Syst. 2016, 46, 66–77. [Google Scholar] [CrossRef]
- Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibáñez, V.; Pérez-González, A. An introductory study of common grasps used by adults during performance of activities of daily living. J. Hand Ther. 2014, 27, 1–28. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Toledo-Perez, D.C.; Rodriguez-Resendiz, J.; Gomez-Loenzo, R.A. A study of computing zero crossing methods and an improved proposal for EMG signals. IEEE Access 2020, 8, 8783–8790. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG A Practical Introduction to Kinesiological Electromyography; Noraxon: Scottsdale, AZ, USA, 2005. [Google Scholar]
- Haugen, I.K.; Aaserud, J.; Kvien, T.K. Get a Grip on Factors Related to Grip Strength in Persons With Hand Osteoarthritis: Results From an Observational Cohort Study. Arthritis Care Res. 2021, 73, 794–800. [Google Scholar] [CrossRef]
- Calder, K.M.; Galea, V.; Wessel, J.; MacDermid, J.C.; MacIntyre, N.J. Muscle activation during hand dexterity tasks in women with hand osteoarthritis and control subjects. J. Hand Ther. 2011, 24, 207–215. [Google Scholar] [CrossRef]







| NZC | EWL | EMAV | MA | |
|---|---|---|---|---|
| LDA1 | X | |||
| LDA2 | X | |||
| LDA3 | X | |||
| LDA4 | X | |||
| LDA5 | X | X | ||
| LDA6 | X | X | ||
| LDA7 | X | X | ||
| LDA8 | X | X | ||
| LDA9 | X | X | ||
| LDA10 | X | X | ||
| LDA11 | X | X | X | |
| LDA12 | X | X | X | |
| LDA13 | X | X | X | |
| LDA14 | X | X | X | |
| LDA15 | X | X | X | X |
| Spot | |||||||
|---|---|---|---|---|---|---|---|
| Factor | WF_UD | WF_RD | DF | TM | FE | WE_UD | WE_RD |
| Gender | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | NZC | ||
| Grasp type | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA |
| Interaction | EWL EMAV MA | ||||||
| Spot | |||||||
|---|---|---|---|---|---|---|---|
| Factor | WF_UD | WF_RD | DF | TM | FE | WE_UD | WE_RD |
| Sample | NZC EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA |
| Grasp type | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | NZC EMAV MA | EWL EMAV MA | NZC EWL EMAV MA |
| Interaction | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | MA | EMAV MA | |
| Spot | |||||||
|---|---|---|---|---|---|---|---|
| Grasp Type | WF_UD | WF_RD | DF | TM | FE | WE_UD | WE_RD |
| P2D | EWL | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | |
| LatP | EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | |
| CyL | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA |
| Lum | EWL | EWL EMAV MA | NZC | NZC EWL | |||
| Obl | EWL EMAV MA | EWL EMAV | EWL EMAV MA | EWL EMAV MA | EWL EMAV MA | NZC EWL EMAV MA | NZC EWL EMAV MA |
| IntPP | EWL EMAV MA | EWL EMAV MA | NZC | NZC | NZC EWL EMAV MA |
| Success Ratio | Model | |
|---|---|---|
| LDA1 | 73.3% | 0.013·NZCWE_RD,Lum -10.383 |
| LDA2 & LDA5 | 100% | 0.002·EWLWF_UD,Obl + 0.003·EWLTM,LatP + 0.004·EWLTM,Cyl − 0.004·EWLTM,Lum − 0.002·EWLWE_RD,P2D-4.198 |
| LDA3 & LDA8 | 93.3% | 8.065·EMAVTM,Cyl − 4.399 |
| LDA4 | 100% | 3.163·MADF,Obl + 8.121·MATM,Cyl − 4.986·MAWE_RD,IntPP − 3.232 |
| LDA6 & LDA11 | 93.3% | 7.902·EMAVTM,Cyl + 0.005·NZCWE_RD,IntPP − 8.483 |
| LDA7 | 93.3% | 6.514·MATM,Cyl + 0.006·NZCWE_RD,IntPP − 7.512 |
| LDA9 | 100% | 0.002·EWLDF,Obl + 8.542·MATM,Cyl − 5.566·MAWE_RD,IntPP − 4.140 |
| LDA10 | 100% | 3.277·MADF,Obl + 8.215 MATM,Cyl − 6.313·EMAVWE_RD,IntPP − 2.127 |
| LDA12, LDA13 & LDA15 | 93.3% | 6.514·MATM,Cyl + 0.006·NZCWE_RD,IntPP − 7.512 |
| LDA14 | 100% | 0.002·EWLDF,Obl + 8.681·MATM,Cyl − 7.112·EMAVWE_RD,IntPP − 2.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarque-Bou, N.J.; Gracia-Ibáñez, V.; Roda-Sales, A.; Bayarri-Porcar, V.; Sancho-Bru, J.L.; Vergara, M. Toward Early and Objective Hand Osteoarthritis Detection by Using EMG during Grasps. Sensors 2023, 23, 2413. https://doi.org/10.3390/s23052413
Jarque-Bou NJ, Gracia-Ibáñez V, Roda-Sales A, Bayarri-Porcar V, Sancho-Bru JL, Vergara M. Toward Early and Objective Hand Osteoarthritis Detection by Using EMG during Grasps. Sensors. 2023; 23(5):2413. https://doi.org/10.3390/s23052413
Chicago/Turabian StyleJarque-Bou, Néstor J., Verónica Gracia-Ibáñez, Alba Roda-Sales, Vicente Bayarri-Porcar, Joaquín L. Sancho-Bru, and Margarita Vergara. 2023. "Toward Early and Objective Hand Osteoarthritis Detection by Using EMG during Grasps" Sensors 23, no. 5: 2413. https://doi.org/10.3390/s23052413
APA StyleJarque-Bou, N. J., Gracia-Ibáñez, V., Roda-Sales, A., Bayarri-Porcar, V., Sancho-Bru, J. L., & Vergara, M. (2023). Toward Early and Objective Hand Osteoarthritis Detection by Using EMG during Grasps. Sensors, 23(5), 2413. https://doi.org/10.3390/s23052413









