Automatic Crop Canopy Temperature Measurement Using a Low-Cost Image-Based Thermal Sensor: Application in a Pomegranate Orchard under a Permanent Shade Net House
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Weather Station
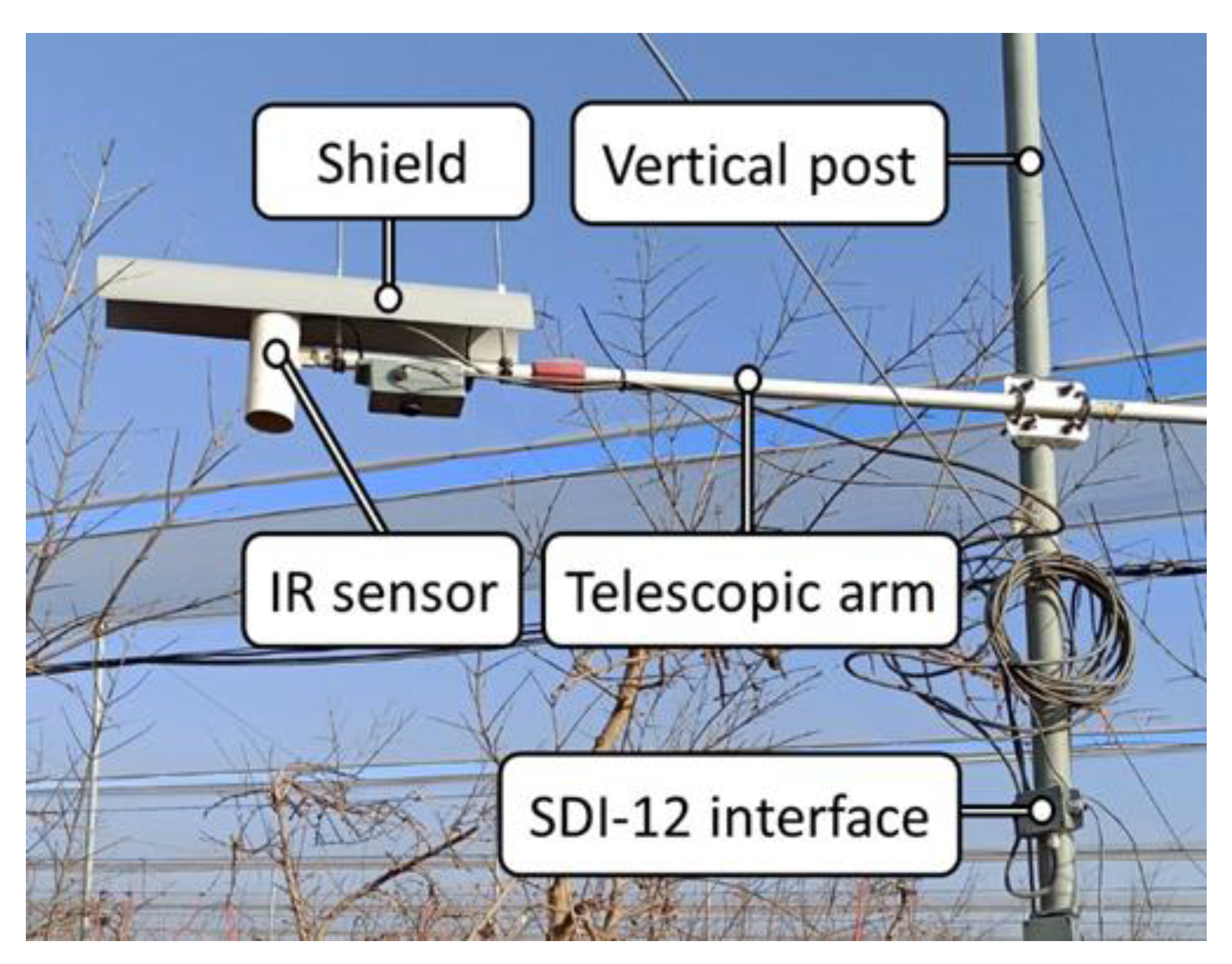
2.3. IR Sensor
2.4. Datalogger
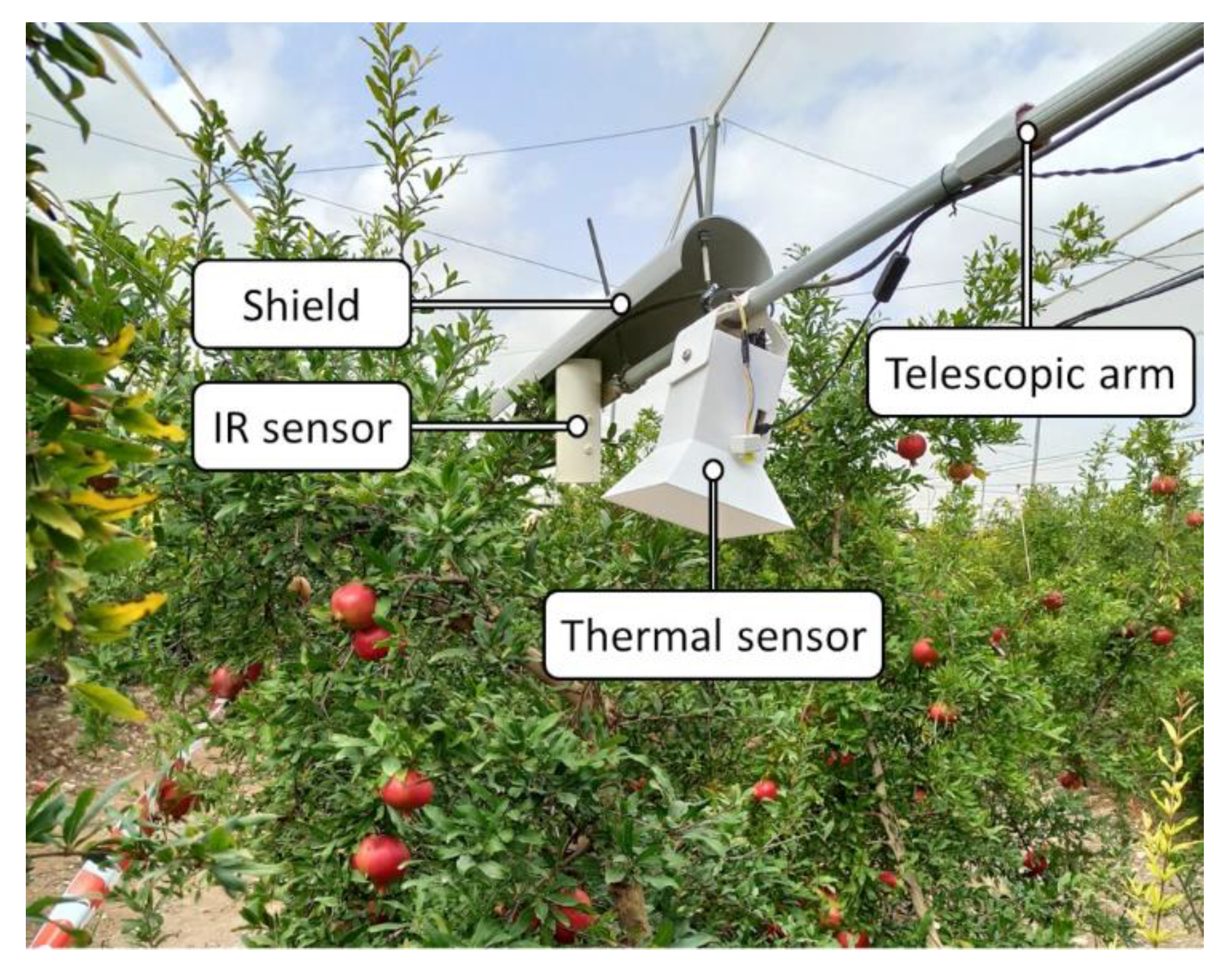
2.5. Thermal Sensor
3. Results and Discussion
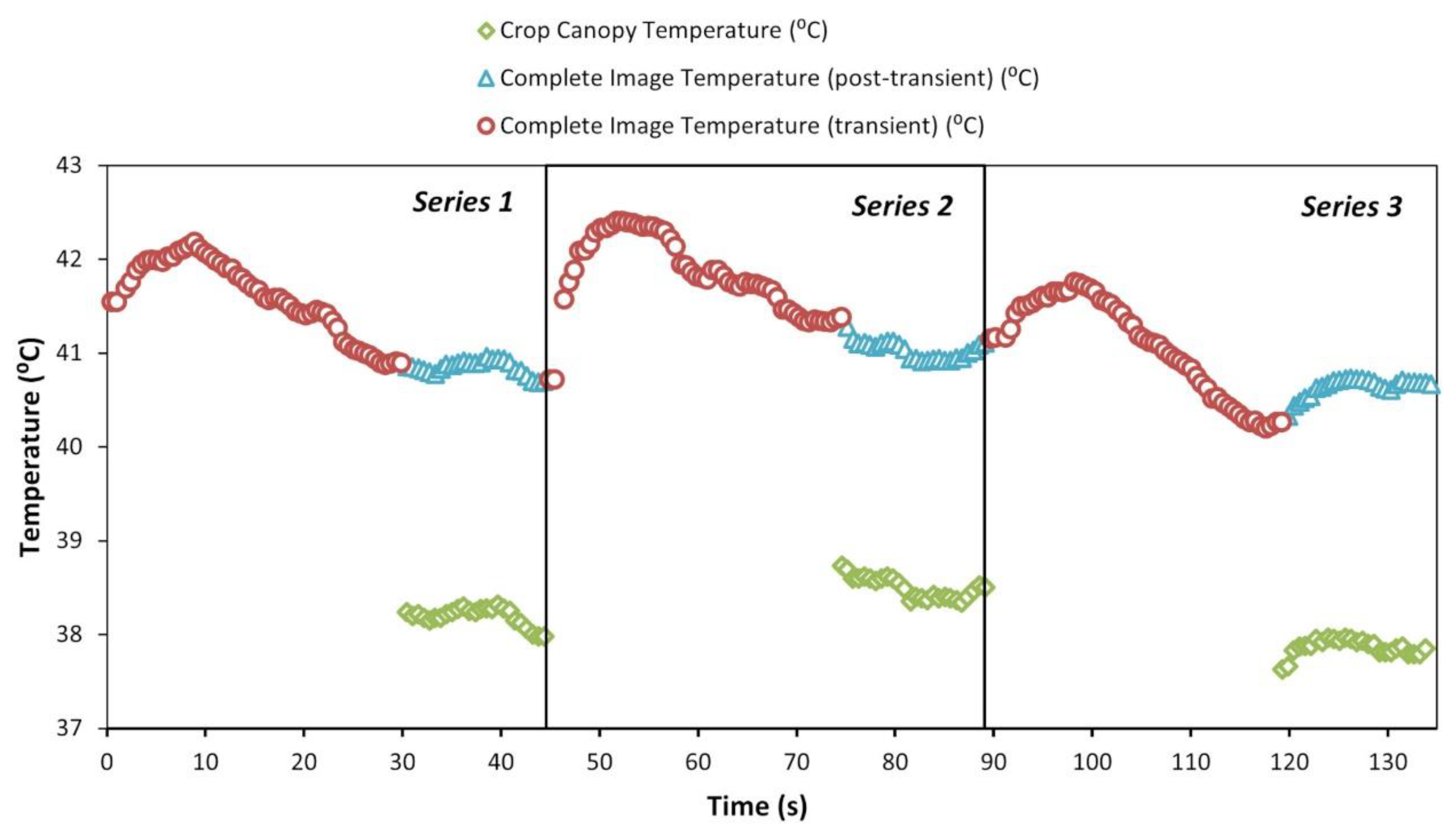
3.1. Time Series of Temperature Measurements
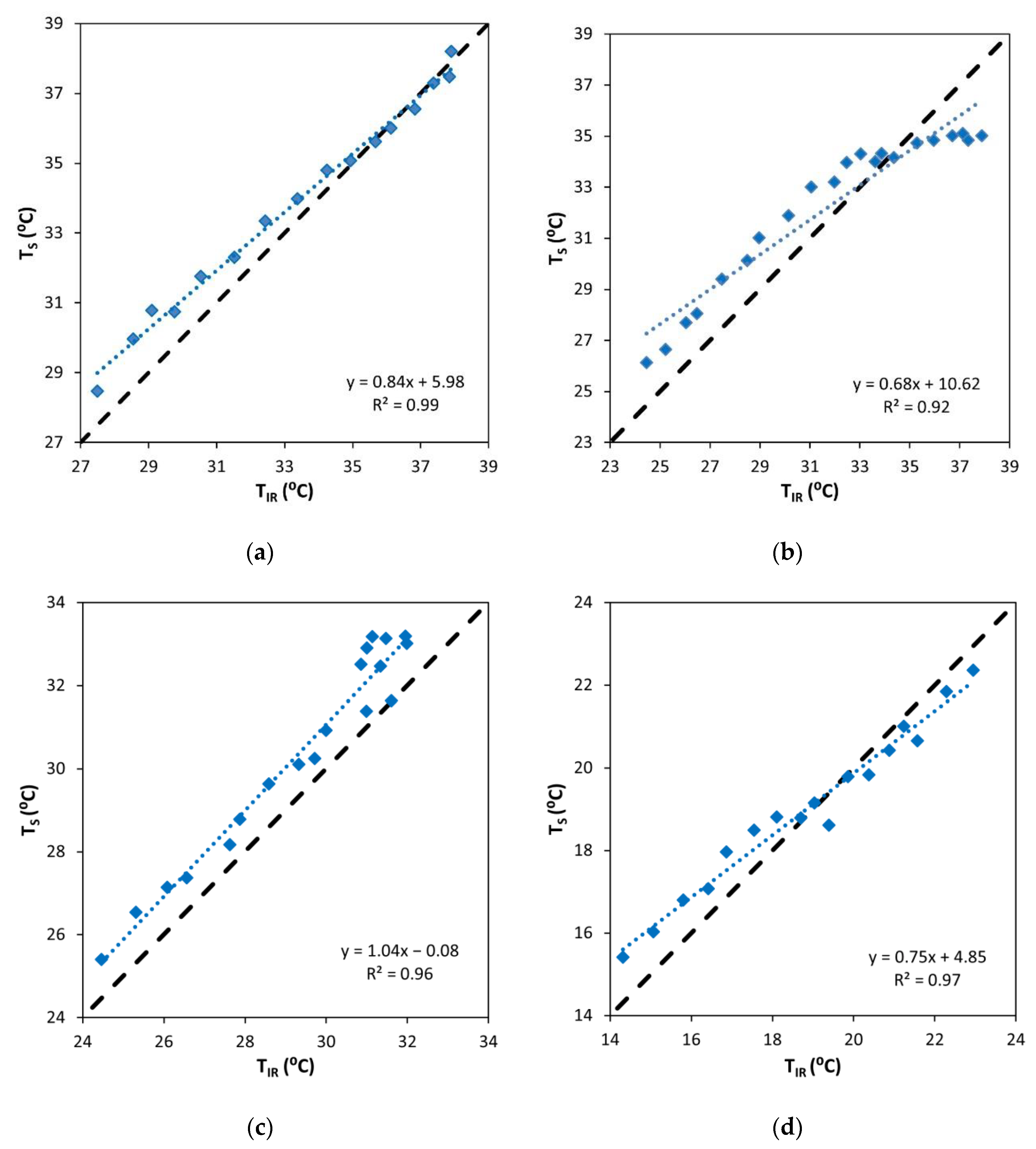
3.2. Comparative Analysis between Thermal and IR Sensors
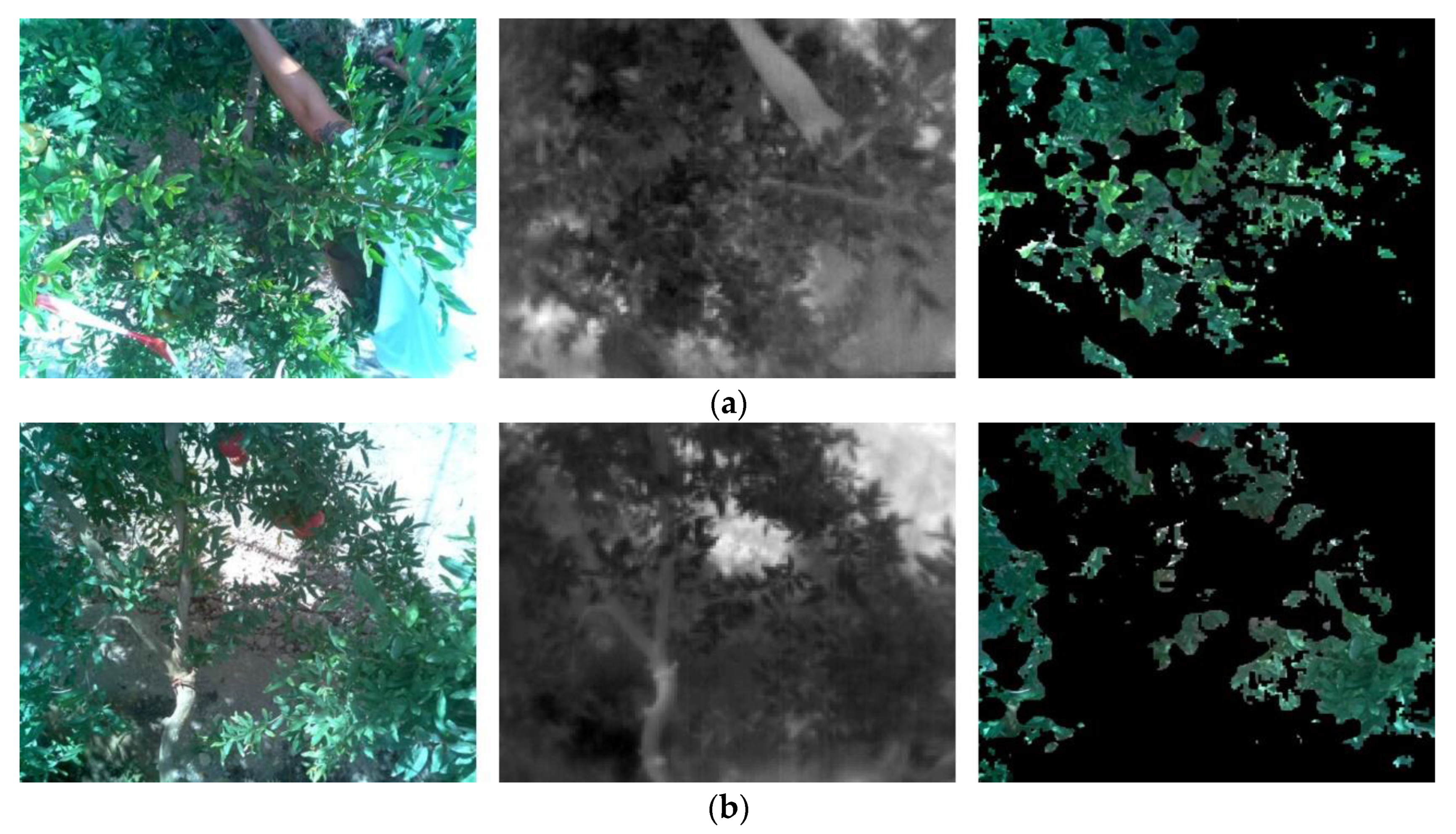
3.3. Analysis of Potential Factors Influencing the Thermal Sensor Performance
- Percentage of area covered by leaves in the image of the thermal sensor. In this study, the percentage ranged between 20 and 50%, depending on the orientation set on every day of experimentation, whereas in Giménez-Gallego et al. [61], the range was between 15 and 35%. However, in the case of the latter, additional tests were made by setting the sensor closer to the crop in order to increase the percentage of the canopy covered by the field of view, which did not result in a higher performance.
- Protection against wind. The presence of a cover net above the orchard allowed the sensor to be less exposed to wind in this trial. The neighboring trees and the mounting, as the sensor was enveloped by the perimeter branches of the pomegranate tree, also contributed to this. In contrast, under the experimental setup in the work by Giménez-Gallego et al. [61], the sensor was fully exposed to wind, even so, it was shown that the wind did not affect the temperature measurement considerably, due to the high number of repetitions performed. This allowed us to average and make the TC determination independent of the standard deviation caused by the wind influence.
- Orientation of the sensor. In this paper, the sensor was placed vertically, mounted on the arm pointing downwards, whereas in the work by Giménez-Gallego et al. [61], the sensor was mounted on a horizontal bracket, pointing to the canopy from the side. The horizontal orientation caused the camera’s field of view to encompass the sky and other external elements of the experimental set up, such as roof tiles. These might be more problematic sources of radiation than the ground, which was the background in the case of vertical orientation. Moreover, in the vertical installation, the thermal sensor is less exposed to solar radiation, so the heating of the electronics inside the housing, including the thermal imaging camera, is lower.
- Location of the sensor. In this study, the sensor was placed within the tree canopy, allowing it to be immersed in an atmosphere equal to that of the leaves. This could have made it less sensitive to changes in environmental factors, such as air temperature, relative humidity, and wind, which might have affected the measurement.
3.4. Optimization of the Thermal Sensor Measurement Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannan, N.; Anandhi, A. Water Management for Sustainable Food Production. Water 2020, 12, 778. [Google Scholar] [CrossRef]
- Azorín, P.R.; García, J.G. The Productive, Economic, and Social Efficiency of Vineyards Using Combined Drought-Tolerant Rootstocks and Efficient Low Water Volume Deficit Irrigation Techniques Under Mediterranean Semiarid Conditions. Sustainability 2020, 12, 1930. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Fernández García, I.F.; Lecina, S.; Ruiz-Sánchez, M.C.; Vera, J.; Conejero, W.; Conesa, M.R.; Domínguez, A.; Pardo, J.J.; Léllis, B.C.; Montesinos, P. Trends and Challenges in Irrigation Scheduling in the Semi-Arid Area of Spain. Water 2020, 12, 785. [Google Scholar] [CrossRef]
- Noguera, M.; Millán, B.; Pérez-Paredes, J.J.; Ponce, J.M.; Aquino, A.; Andújar, J.M. A new low-cost device based on thermal infrared sensors for olive tree canopy temperature measurement and water status monitoring. Remote Sens. 2020, 12, 723. [Google Scholar] [CrossRef]
- Pardo, J.J.; Martínez-Romero, A.; Léllis, B.C.; Tarjuelo, J.M.; Domínguez, A. Effect of the optimized regulated deficit irrigation methodology on water use in barley under semiarid conditions. Agric. Water Manag. 2020, 228, 105925. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit irrigation in fruit trees and vines in Spain. Spanish J. Agric. Res. 2010, 8, S5–S20. [Google Scholar] [CrossRef]
- Torres-Sanchez, R.; Navarro-Hellin, H.; Guillamon-Frutos, A.; San-Segundo, R.; Ruiz-Abellón, M.C.; Domingo-Miguel, R. A decision support system for irrigation management: Analysis and implementation of different learning techniques. Water 2020, 12, 548. [Google Scholar] [CrossRef]
- de Lima, R.S.N.; de Assis Figueiredo, F.A.M.M.; Martins, A.O.; de Deus, B.C.D.S.; Ferraz, T.M.; de Assis Gomes, M.D.M.; de Sousa, E.F.; Glenn, D.M.; Campostrini, E. Partial rootzone drying (PRD) and regulated deficit irrigation (RDI) effects on stomatal conductance, growth, photosynthetic capacity, and water-use efficiency of papaya. Sci. Hortic. 2015, 183, 13–22. [Google Scholar] [CrossRef]
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Naor, A. Midday stem water potential as a plant water stress indicator for irrigation scheduling in fruit trees. Acta Hortic. 2000, 537, 447–454. [Google Scholar] [CrossRef]
- Naor, A.; Klein, I.; Doron, I. Stem Water Potential and Apple Size. J. Am. Soc. Hortic. Sci. 1995, 120, 577–582. [Google Scholar] [CrossRef]
- González-Teruel, J.D.; Ruiz-Abellon, M.C.; Blanco, V.; Blaya-Ros, P.J.; Domingo, R.; Torres-Sánchez, R. Prediction of Water Stress Episodes in Fruit Trees Based on Soil and Weather Time Series Data. Agronomy 2022, 12, 1422. [Google Scholar] [CrossRef]
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and plant water indicators for deficit irrigation management of field-grown sweet cherry trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
- Blonquist, J.M.; Norman, J.M.; Bugbee, B. Automated measurement of canopy stomatal conductance based on infrared temperature. Agric. For. Meteorol. 2009, 149, 1931–1945. [Google Scholar] [CrossRef]
- González-Teruel, J.D.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Toledo-Moreo, A.B.; Jiménez-Buendía, M.; Soto-Valles, F. Design and Calibration of a Low-Cost SDI-12 Soil Moisture Sensor. Sensors 2019, 19, 491. [Google Scholar] [CrossRef]
- Brown, H.O.-T. Researches on some of the physiological processes of green leaves, with special reference to the interchange of energy between the leaf and its surroundings. Proc. R. Soc. London. Ser. B Contain. Pap. Biol. Character 1905, 76, 29–111. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy Temperature as a Crop Water Stress Indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Camino, C.; Zarco-Tejada, P.J.; Gonzalez-Dugo, V. Effects of heterogeneity within tree crowns on airborne-quantified SIF and the CWSI as indicators of water stress in the context of precision agriculture. Remote Sens. 2018, 10, 604. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Ortega-Arévalo, C.J.; Iglesias-Contreras, M.; Moreno, J.M.; Souza, L.; Tavira, S.C.; Durán-Zuazo, V.H. Assessing the crop-water status in almond (Prunus dulcis mill.) trees via thermal imaging camera connected to smartphone. Sensors 2018, 18, 1050. [Google Scholar] [CrossRef]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Patra, H.; Bajpai, V.; Das, B.; Kumar, S.; Dhandapani, R.; Vishwakarma, C.; Pal, M.; et al. Application of thermal imaging and hyperspectral remote sensing for crop water deficit stress monitoring. Geocarto Int. 2021, 36, 481–498. [Google Scholar] [CrossRef]
- Kullberg, E.G.; DeJonge, K.C.; Chávez, J.L. Evaluation of thermal remote sensing indices to estimate crop evapotranspiration coefficients. Agric. Water Manag. 2017, 179, 64–73. [Google Scholar] [CrossRef]
- Poblete, T.; Ortega-Farías, S.; Ryu, D. Automatic coregistration algorithm to remove canopy shaded pixels in UAV-borne thermal images to improve the estimation of crop water stress index of a drip-irrigated cabernet sauvignon vineyard. Sensors 2018, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- Blaya-Ros, P.J.; Blanco-Montoya, V.; Torres-Sánchez, R.; González-Teruel, J.D.; Soto-Valles, F.; Toledo-Moreo, A.B.; Jiménez-Buendía, M.; Domingo-Miguel, R. Sistema para la asistencia en la orientación de termo-radiómetros para procesos de medida de temperatura foliar. In Proceedings of the XXXVII National Irrigation Congress, Badajoz, Spain, 4–6 June 2019. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: A review of sensor technology, measurement procedures, and data correction workflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Berni, J.A.J. Determinación del Estado Hídrico de la Vegetación mediante Teledetección Basada en Vehículos Aéreos No Tripulados; Universidad de Córdoba: Córdoba, Spain, 2009. [Google Scholar]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant-environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef]
- Fuentes, S.; De Bei, R.; Pech, J.; Tyerman, S. Computational water stress indices obtained from thermal image analysis of grapevine canopies. Irrig. Sci. 2012, 30, 523–536. [Google Scholar] [CrossRef]
- Blaya-Ros, P.J.; Blanco, V.; Domingo, R.; Soto-Valles, F.; Torres-Sánchez, R. Feasibility of Low-Cost Thermal Imaging for Monitoring Water Stress in Young and Mature Sweet Cherry Trees. Appl. Sci. 2020, 10, 5461. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, X.; Wheaton, A.; Cooley, N.; Moran, B. Automatic optical and IR image fusion for plant water stress analysis. In Proceedings of the 12th International Conference on Information Fusion, Seattle, WA, USA, 6–9 July 2009; pp. 1053–1059. [Google Scholar]
- Gutiérrez, S.; Diago, M.P.; Fernández-Novales, J.; Tardaguila, J. Vineyard water status assessment using on-the-go thermal imaging and machine learning. PLoS ONE 2018, 13, e0192037. [Google Scholar] [CrossRef] [PubMed]
- Osroosh, Y.; Khot, L.R.; Peters, R.T. Economical thermal-RGB imaging system for monitoring agricultural crops. Comput. Electron. Agric. 2018, 147, 34–43. [Google Scholar] [CrossRef]
- Awais, M.; Li, W.; Cheema, M.J.M.; Zaman, Q.U.; Shaheen, A.; Aslam, B.; Zhu, W.; Ajmal, M.; Faheem, M.; Hussain, S.; et al. UAV-based remote sensing in plant stress imagine using high-resolution thermal sensor for digital agriculture practices: A meta-review. Int. J. Environ. Sci. Technol. 2023, 20, 1135–1152. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Castillo, C.; Soto-Vallés, F.; Torres-Sánchez, R.; Domingo, R. Potential of UAS-Based Remote Sensing for Estimating Tree Water Status and Yield in Sweet Cherry Trees. Remote Sens. 2020, 12, 2359. [Google Scholar] [CrossRef]
- Chang, A.; Jung, J.; Maeda, M.M.; Landivar, J.A.; Carvalho, H.D.R.; Yeom, J. Measurement of Cotton Canopy Temperature Using Radiometric Thermal Sensor Mounted on the Unmanned Aerial Vehicle (UAV). J. Sens. 2020, 2020, 8899325. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Luo, Y.; Cao, J.; Tao, F. Combining optical, fluorescence, thermal satellite, and environmental data to predict county-level maize yield in China using machine learning approaches. Remote Sens. 2020, 12, 21. [Google Scholar] [CrossRef]
- Zhou, Z.; Majeed, Y.; Diverres Naranjo, G.; Gambacorta, E.M.T. Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications. Comput. Electron. Agric. 2021, 182, 106019. [Google Scholar] [CrossRef]
- Cerutti, G.; Kurtz, C.; Vacavant, A.; Tougne, L.; Weber, J.; Grand-Brochier, M. Tree Leaves Extraction in Natural Images: Comparative Study of Preprocessing Tools and Segmentation Methods. IEEE Trans. Image Process. 2015, 24, 1549–1560. [Google Scholar] [CrossRef]
- Giménez-Gallego, J.; González-Teruel, J.D.; Jiménez-Buendía, M.; Toledo-Moreo, A.B.; Soto-Valles, F.; Torres-Sánchez, R. Segmentation of Multiple Tree Leaves Pictures with Natural Backgrounds using Deep Learning for Image-Based Agriculture Applications. Appl. Sci. 2019, 10, 202. [Google Scholar] [CrossRef]
- Singh, V.; Misra, A.K. Detection of plant leaf diseases using image segmentation and soft computing techniques. Inf. Process. Agric. 2017, 4, 41–49. [Google Scholar] [CrossRef]
- Ward, D.; Moghadam, P.; Hudson, N. Deep leaf segmentation using synthetic data. arXiv 2019. [Google Scholar] [CrossRef]
- Koirala, A.; Walsh, K.B.; Wang, Z.; McCarthy, C. Deep learning—Method overview and review of use for fruit detection and yield estimation. Comput. Electron. Agric. 2019, 162, 219–234. [Google Scholar] [CrossRef]
- Azlah, M.A.F.; Chua, L.S.; Rahmad, F.R.; Abdullah, F.I.; Alwi, S.R.W. Review on techniques for plant leaf classification and recognition. Computers 2019, 8, 77. [Google Scholar] [CrossRef]
- Apolo-Apolo, O.E.; Martínez-Guanter, J.; Egea, G.; Raja, P.; Pérez-Ruiz, M. Deep learning techniques for estimation of the yield and size of citrus fruits using a UAV. Eur. J. Agron. 2020, 115, 126030. [Google Scholar] [CrossRef]
- Jia, W.; Tian, Y.; Luo, R.; Zhang, Z.; Lian, J.; Zheng, Y. Detection and segmentation of overlapped fruits based on optimized mask R-CNN application in apple harvesting robot. Comput. Electron. Agric. 2020, 172, 105380. [Google Scholar] [CrossRef]
- Li, H.; Lee, W.S.; Wang, K. Immature green citrus fruit detection and counting based on fast normalized cross correlation (FNCC) using natural outdoor colour images. Precis. Agric. 2016, 17, 678–697. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Y.; Zou, X.; Cheng, J.; Xiong, J. Fruit detection in natural environment using partial shape matching and probabilistic Hough transform. Precis. Agric. 2019, 21, 160–177. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Y.; Zou, X.; Xiong, J.; Fang, Y. Color-, depth-, and shape-based 3D fruit detection. Precis. Agric. 2019, 21, 1–17. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T. Detecting fruit surface wetness using a custom-built low-resolution thermal-RGB imager. Comput. Electron. Agric. 2019, 157, 509–517. [Google Scholar] [CrossRef]
- Fashi, M.; Naderloo, L.; Javadikia, H. Pomegranate grading based on pH using image processing and artificial intelligence. J. Food Meas. Charact. 2020, 14, 3112–3121. [Google Scholar] [CrossRef]
- Prospera Technologies. Autonomous Crop Management. Available online: https://prospera.ag/ (accessed on 3 November 2022).
- Vicente-Guijalba, F.; Martinez-Marin, T.; Lopez-Sanchez, J.M. Crop Phenology Estimation Using a Multitemporal Model and a Kalman Filtering Strategy. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1081–1085. [Google Scholar] [CrossRef]
- Fisher, D.K.; Kebede, H. A low-cost microcontroller-based system to monitor crop temperature and water status. Comput. Electron. Agric. 2010, 74, 168–173. [Google Scholar] [CrossRef]
- Martínez, J.; Egea, G.; Agüera, J.; Pérez-Ruiz, M. A cost-effective canopy temperature measurement system for precision agriculture: A case study on sugar beet. Precis. Agric. 2017, 18, 95–110. [Google Scholar] [CrossRef]
- Petrie, P.R.; Wang, Y.; Liu, S.; Lam, S.; Whitty, M.A.; Skewes, M.A. The accuracy and utility of a low cost thermal camera and smartphone-based system to assess grapevine water status. Biosyst. Eng. 2019, 179, 126–139. [Google Scholar] [CrossRef]
- Giménez-Gallego, J.; González-Teruel, J.D.; Soto-Valles, F.; Jiménez-Buendía, M.; Navarro-Hellín, H.; Torres-Sánchez, R. Intelligent thermal image-based sensor for affordable measurement of crop canopy temperature. Comput. Electron. Agric. 2021, 188, 106319. [Google Scholar] [CrossRef]
- InfluxData. InfluxDB Times Series Data Platform. Available online: https://www.influxdata.com/ (accessed on 24 November 2022).
- Grafana Labs. Grafana: The Open Observability Platform. Available online: https://grafana.com/ (accessed on 24 November 2022).
- SDI-12 Support Group. Available online: https://sdi-12.org/ (accessed on 25 November 2022).
- Giménez-Gallego, J.; Jimenez-Buendia, M.; Toledo-Moreo, A.B.; Soto-Valles, F.; González-Teruel, J.D.; Blaya-Ros, P.J.; Domingo-Miguel, R.; Torres-Sánchez, R. Optimización del despliegue a gran escala de sensores en ensayos con tratamientos múltiples de riego. In Proceedings of the III Symposium Ibérico de Ingeniería Hortícola, Cartagena, Spain, 6–8 April 2022; ISBN 978-84-95556-37-0. [Google Scholar]
- IR120 Product Manual. Available online: https://s.campbellsci.com/documents/es/manuals/ir100_ir120%20-%20708.pdf (accessed on 1 March 2023).
- Python Official Website. Available online: https://www.python.org/ (accessed on 11 December 2019).
- Hamrelius, T. Accurate temperature measurement in thermography. In Proceedings of the Eurotherm Seminar, Châtenay-Malabry, France, 7–9 July 1992. [Google Scholar]


| ΔT (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measurement Approach | Series | Repetitions | Total Measurements | Series Time (s) | Total Measurement Time (s) | R2 | Mean | Median | Standard Deviation | Maximum |
| Original | 3 | 25 | 75 | 45 | 135 | 0.978 | 0.995 | 0.96 | 0.623 | 2.87 |
| Alternative 1 | 3 | 3 | 9 | 45 | 135 | 0.977 | 0.998 | 0.96 | 0.624 | 2.93 |
| Alternative 2 | 3 | 3 (initial) | 9 | 32 | 96 | 0.977 | 1.061 | 1.05 | 0.653 | 2.81 |
| Alternative 3 | 1 | 3 (initial) | 3 | 32 | 32 | 0.976 | 1.103 | 1.06 | 0.686 | 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Gallego, J.; González-Teruel, J.D.; Blaya-Ros, P.J.; Toledo-Moreo, A.B.; Domingo-Miguel, R.; Torres-Sánchez, R. Automatic Crop Canopy Temperature Measurement Using a Low-Cost Image-Based Thermal Sensor: Application in a Pomegranate Orchard under a Permanent Shade Net House. Sensors 2023, 23, 2915. https://doi.org/10.3390/s23062915
Giménez-Gallego J, González-Teruel JD, Blaya-Ros PJ, Toledo-Moreo AB, Domingo-Miguel R, Torres-Sánchez R. Automatic Crop Canopy Temperature Measurement Using a Low-Cost Image-Based Thermal Sensor: Application in a Pomegranate Orchard under a Permanent Shade Net House. Sensors. 2023; 23(6):2915. https://doi.org/10.3390/s23062915
Chicago/Turabian StyleGiménez-Gallego, Jaime, Juan D. González-Teruel, Pedro J. Blaya-Ros, Ana B. Toledo-Moreo, Rafael Domingo-Miguel, and Roque Torres-Sánchez. 2023. "Automatic Crop Canopy Temperature Measurement Using a Low-Cost Image-Based Thermal Sensor: Application in a Pomegranate Orchard under a Permanent Shade Net House" Sensors 23, no. 6: 2915. https://doi.org/10.3390/s23062915
APA StyleGiménez-Gallego, J., González-Teruel, J. D., Blaya-Ros, P. J., Toledo-Moreo, A. B., Domingo-Miguel, R., & Torres-Sánchez, R. (2023). Automatic Crop Canopy Temperature Measurement Using a Low-Cost Image-Based Thermal Sensor: Application in a Pomegranate Orchard under a Permanent Shade Net House. Sensors, 23(6), 2915. https://doi.org/10.3390/s23062915










