Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Setup and Configuration
2.1.1. Sensor
2.1.2. Pre-Processing, Communication, and Interfacing
2.1.3. Embedded System
2.2. Experiment Setup and Study Design
2.2.1. Bed and Mattress
2.2.2. Sensors Deployment and Distributions
2.2.3. Subject Description
2.2.4. Data Acquisition
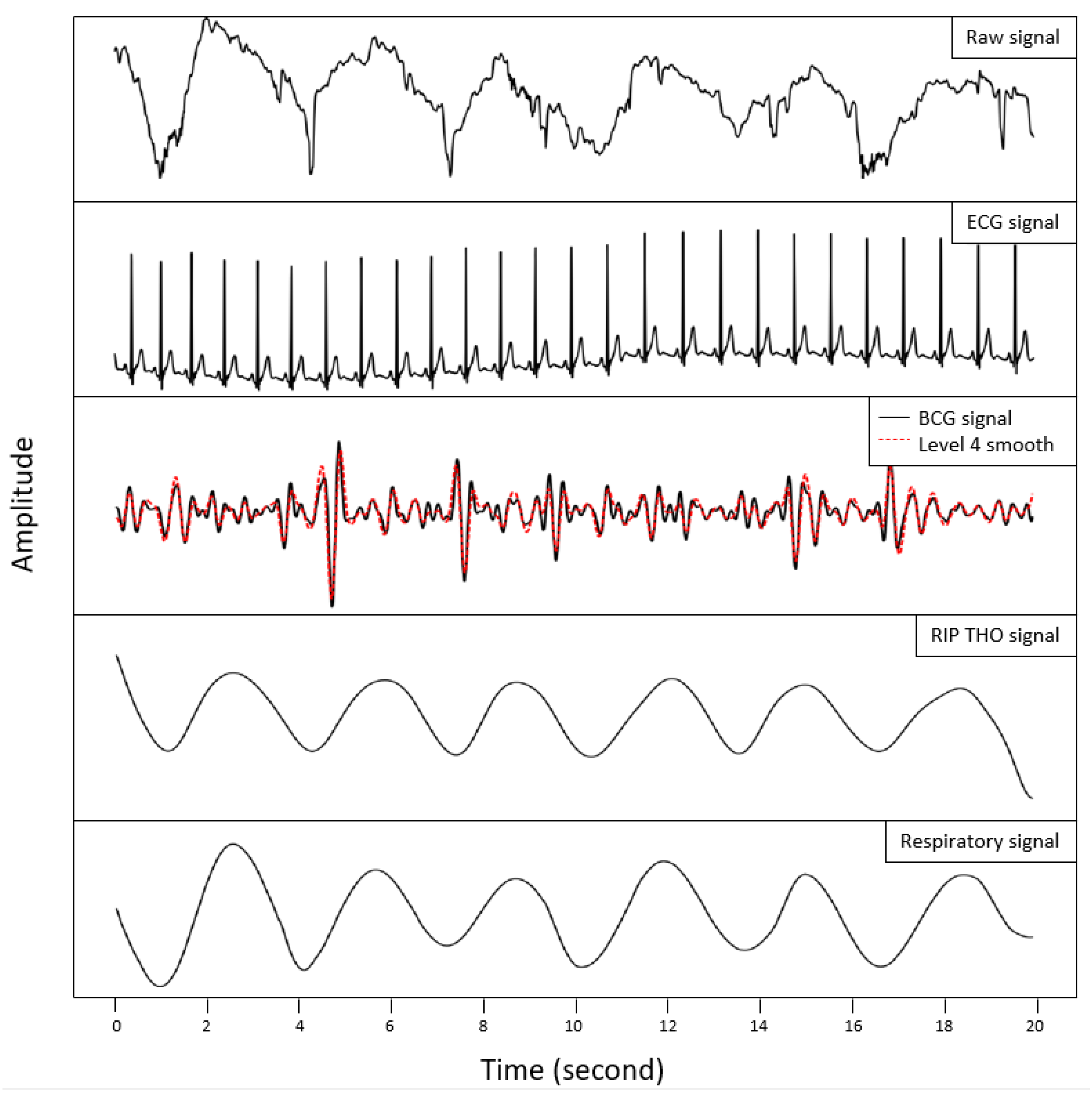
2.3. Data Processing and Analysis
3. Results
4. Discussion
4.1. Challenges and Drawbacks
4.2. Improvements, Applications, and Opportunities
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pozuelo, I.; Zhai, B.; Palotti, J.; Mall, R.; Aupetit, M.; Garcia-Gomez, J.M.; Taheri, S.; Guan, Y.; Fernandez-Luque, L. The future of sleep health: A data-driven revolution in sleep science and medicine. NPJ Digit. Med. 2020, 3, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drigas, A.; Mitsea, E. Metacognition, Stress-Relaxation Balance & Related Hormones. Int. J. Recent Contrib. Eng. Sci. IT 2021, 9, 4–16. [Google Scholar]
- Liu, H.; Chen, A. Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. 2019, 219, 231–237. [Google Scholar] [CrossRef]
- Cable, J.; Schernhammer, E.; Hanlon, E.C.; Vetter, C.; Cedernaes, J.; Makarem, N.; Dashti, H.S.; Shechter, A.; Depner, C.; Ingiosi, A.; et al. Sleep and circadian rhythms: Pillars of health—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2021, 1506, 18–34. [Google Scholar] [CrossRef]
- Faulkner, S.M.; Bee, P.E.; Meyer, N.; Dijk, D.J.; Drake, R.J. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 46, 108–123. [Google Scholar] [CrossRef]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive sleep apnea in adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, H.; Li, J.; Yang, J.; Luo, K.; Chang, L. The relationship between sleep disorders, anxiety, depression, and cognitive function with restless legs syndrome (RLS) in the elderly. Sleep Breath. 2022, 26, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Schenck, C.H.; Howell, M.J. NonREM disorders of arousal and related parasomnias: An updated review. Neurotherapeutics 2021, 18, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.; Daubian-Nosé, P.; De-Mello, M.T.; Esteves, A.M. Exercise as a favorable non-pharmacologic treatment to Sleep-Related Movement Disorders: A review. Sleep Sci. 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, W.T. Diagnosis of obstructive sleep apnea in adults. Proc. Am. Thorac. Soc. 2008, 5, 154–160. [Google Scholar] [CrossRef]
- Mindell, J.A.; Kuhn, B.; Lewin, D.S.; Meltzer, L.J.; Sadeh, A. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 2006, 29, 1263–1276. [Google Scholar]
- Harrison, Y.; Horne, J.A. The impact of sleep deprivation on decision making: A review. J. Exp. Psychol. Appl. 2000, 6, 236. [Google Scholar] [CrossRef]
- Watson, N.F.; Fernandez, C.R. Artificial intelligence and sleep: Advancing sleep medicine. Sleep Med. Rev. 2021, 59, 101512. [Google Scholar] [CrossRef] [PubMed]
- Arand, D.L.; Bonnet, M.H. The multiple sleep latency test. Handb. Clin. Neurol. 2019, 160, 393–403. [Google Scholar]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-based assessment of sleep parameters. Ann. Work. Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef] [Green Version]
- Caples, S.M.; Anderson, W.M.; Calero, K.; Howell, M.; Hashmi, S.D. Use of polysomnography and home sleep apnea tests for the longitudinal management of obstructive sleep apnea in adults: An American Academy of Sleep Medicine clinical guidance statement. J. Clin. Sleep Med. 2021, 17, 1287–1293. [Google Scholar] [CrossRef]
- Moridian, P.; Shoeibi, A.; Khodatars, M.; Jafari, M.; Pachori, R.B.; Khadem, A.; Alizadehsani, R.; Ling, S.H. Automatic diagnosis of sleep apnea from biomedical signals using artificial intelligence techniques: Methods, challenges, and future works. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2022, 12, e1478. [Google Scholar] [CrossRef]
- Hussain, Z.; Sheng, Q.Z.; Zhang, W.E.; Ortiz, J.; Pouriyeh, S. Non-invasive techniques for monitoring different aspects of sleep: A comprehensive review. ACM Trans. Comput. Healthc. (HEALTH) 2022, 3, 1–26. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, S.H. Smart technologies toward sleep monitoring at home. Biomed. Eng. Lett. 2019, 9, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Makarem, N.; Liao, M.; St-Onge, M.P.; Aggarwal, B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J. Am. Heart Assoc. 2020, 9, e014587. [Google Scholar] [CrossRef]
- Gaiduk, M.; Perea Rodríguez, J.J.; Seepold, R.; Martínez Madrid, N.; Penzel, T.; Glos, M.; Ortega, J.A. Estimation of sleep stages analyzing respiratory and movement signals. IEEE J. Biomed. Health Inform. 2022, 26, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Serrano Alarcón, Á.; Martínez Madrid, N.; Seepold, R. A minimum set of physiological parameters to diagnose obstructive sleep apnea syndrome using non-invasive portable monitors. A systematic review. Life 2021, 11, 1249. [Google Scholar] [CrossRef]
- Azimi, I.; Oti, O.; Labbaf, S.; Niela-Vilen, H.; Axelin, A.; Dutt, N.; Liljeberg, P.; Rahmani, A.M. Personalized maternal sleep quality assessment: An objective iot-based longitudinal study. IEEE Access 2019, 7, 93433–93447. [Google Scholar] [CrossRef]
- Fino, E.; Plazzi, G.; Filardi, M.; Marzocchi, M.; Pizza, F.; Vandi, S.; Mazzetti, M. (Not so) Smart sleep tracking through the phone: Findings from a polysomnography study testing the reliability of four sleep applications. J. Sleep Res. 2020, 29, e12935. [Google Scholar] [CrossRef] [PubMed]
- Agar, G.; Oliver, C.; Trickett, J.; Licence, L.; Richards, C. Sleep disorders in children with Angelman and Smith-Magenis syndromes: The assessment of potential causes of disrupted settling and night time waking. Res. Dev. Disabil. 2020, 97, 103555. [Google Scholar] [CrossRef] [PubMed]
- Jakkaew, P.; Onoye, T. Non-contact respiration monitoring and body movements detection for sleep using thermal imaging. Sensors 2020, 20, 6307. [Google Scholar] [CrossRef] [PubMed]
- Crivello, A.; La Rosa, D.; Wilhelm, E.; Palumbo, F. A Sensing Platform to Monitor Sleep Efficiency. In Proceedings of the Ambient Assisted Living: Italian Forum 2020; Springer: Berlin/Heidelberg, Germany, 2022; pp. 335–345. [Google Scholar]
- Yang, C.; Ku, G.W.; Lee, J.G.; Kim, K. Improving the Accuracy of Biosignal Analysis Using BCG by Applying a Signal-to-Noise Ratio and Similarity-Based Channel Selection Algorithm. J. Electr. Eng. Technol. 2021, 16, 1043–1050. [Google Scholar] [CrossRef]
- Clausen, T.; Jura, T.; Jähne-Raden, N.; Wolf, M.C.; Wolf, L.; Kulau, U. A precise, parallel and scalable measurement system for ballistocardiographic research. Smart Health 2021, 19, 100169. [Google Scholar] [CrossRef]
- Watanabe, K.; Kurihara, Y.; Kobayashi, K.; Suzuki, K. Ballistocardiogram (BCG) measurement by a differential pressure sensor. IEEE Sens. J. 2020, 21, 8583–8592. [Google Scholar] [CrossRef]
- Haghi, M.; Seepold, R.; Martínez Madrid, N. Designing a sensor interface for cardiorespiratory measurement in sleep monitoring. In Proceedings of the Hardware and Software Supporting Physiological Measurement (HSPM-2022), Konstanz, Germany, 27–28 October 2022; pp. 9–12. [Google Scholar]
- Gaiduk, M.; Orcioni, S.; Seepold, R.; Martínez Madrid, N.; Pierleoni, P.; Gentili, A.; Burattini, L.; Sbrollini, A.; Marcantoni, I.; Morettini, M.; et al. Heart and breathing rate measurement using low intrusive monitoring systems. In Social Innovation in Long-Term Care through Digitalization: Proceedings of the German-Italian Workshop LTC-2021; Springer: Cham, Switzerland, 2022; pp. 37–49. [Google Scholar]
- Jung, H.; Kimball, J.P.; Receveur, T.; Agdeppa, E.D.; Inan, O.T. Accurate ballistocardiogram based heart rate estimation using an array of load cells in a hospital bed. IEEE J. Biomed. Health Inform. 2021, 25, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Kulau, U.; Rust, J.; Albrecht, U.V. BCG Measurement by differential Sensing in Real-Time. In Proceedings of the 2022 18th International Conference on Distributed Computing in Sensor Systems (DCOSS), Los Angeles, CA, USA, 30 May–1 June 2022; pp. 75–78. [Google Scholar]
- Kulau, U.; Rust, J.; Szafranski, D.; Drobczyk, M.; Albrecht, U.V. A Differential BCG Sensor System for Long Term Health Monitoring Experiment on the ISS. In Proceedings of the 2022 18th International Conference on Distributed Computing in Sensor Systems (DCOSS), Los Angeles, CA, USA, 30 May–1 June 2022; pp. 85–92. [Google Scholar]
- He, Y.; Wan, H.; Jiang, X.; Peng, C. Piezoelectric Micromachined Ultrasound Transducer Technology: Recent Advances and Applications. Biosensors 2022, 13, 55. [Google Scholar] [CrossRef]
- So, S.; Jain, D.; Kanayama, N. Piezoelectric sensor-based continuous monitoring of respiratory rate during sleep. J. Med. Biol. Eng. 2021, 41, 241–250. [Google Scholar] [CrossRef]
- Kristiansen, S.; Andersen, M.H.; Goebel, V.; Plagemann, T.; Traaen, G.M.; Øverland, B.; Akre, H.; Gullestad, L. Evaluating a Low-Cost Strain Gauge Breathing Sensor for Sleep Apnea Detection at Home. In Proceedings of the 2021 IEEE International Conference on Communications Workshops (ICC Workshops), Montreal, QC, Canada, 14–23 June 2021; pp. 1–6. [Google Scholar]
- Han, P.; Li, L.; Zhang, H.; Guan, L.; Marques, C.; Savović, S.; Ortega, B.; Min, R.; Li, X. Low-cost plastic optical fiber sensor embedded in mattress for sleep performance monitoring. Opt. Fiber Technol. 2021, 64, 102541. [Google Scholar] [CrossRef]
- Abro, Z.A.; Hong, C.; Zhang, Y.; Siddiqui, M.Q.; Abbasi, A.M.R.; Abro, Z.; Tariq, S.Q.B. Development of FBG pressure sensors using FDM technique for monitoring sleeping postures. Sens. Actuators A Phys. 2021, 331, 112921. [Google Scholar] [CrossRef]
- Li, Y.; Dong, B.; Chen, E.; Wang, X.; Zhao, Y.; Zhao, W.; Wang, Y. Breathing process monitoring with a biaxially oriented polypropylene film based fiber Fabry–Perot sensor. Opt. Commun. 2020, 475, 126292. [Google Scholar] [CrossRef]
- Wang, W.; Yiu, H.H.; Li, W.J.; Roy, V.A. The principle and architectures of optical stress sensors and the progress on the development of microbend optical sensors. Adv. Opt. Mater. 2021, 9, 2001693. [Google Scholar] [CrossRef]
- Ushakov, N.; Markvart, A.; Kulik, D.; Liokumovich, L. Comparison of pulse wave signal monitoring techniques with different fiber-optic interferometric sensing elements. Photonics 2021, 8, 142. [Google Scholar] [CrossRef]
- Buchmayer, F.; Monsberger, C.M.; Lienhart, W. Advantages of tunnel monitoring using distributed fibre optic sensing. J. Appl. Geod. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Koenig, S.M.; Mack, D.; Alwan, M. Sleep and sleep assessment technologies. In Eldercare Technology for Clinical Practitioners; Humana Press: Totowa, NJ, USA, 2008; pp. 77–120. [Google Scholar]
- Mack, D.C.; Patrie, J.T.; Suratt, P.M.; Felder, R.A.; Alwan, M. Development and preliminary validation of heart rate and breathing rate detection using a passive, ballistocardiography-based sleep monitoring system. IEEE Trans. Inf. Technol. Biomed. 2008, 13, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.C.; Alwan, M.; Turner, B.; Suratt, P.; Felder, R.A. A passive and portable system for monitoring heart rate and detecting sleep apnea and arousals: Preliminary validation. In Proceedings of the 1st Transdisciplinary Conference on Distributed Diagnosis and Home Healthcare, D2H2 2006, Arlington, VA, USA, 2–4 April 2006; pp. 51–54. [Google Scholar]
- Hwang, S.H.; Lee, H.J.; Yoon, H.N.; Lee, Y.J.G.; Lee, Y.J.; Jeong, D.U.; Park, K.S. Unconstrained sleep apnea monitoring using polyvinylidene fluoride film-based sensor. IEEE Trans. Biomed. Eng. 2014, 61, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Beattie, Z.T.; Hayes, T.L.; Guilleminault, C.; Hagen, C.C. Accurate scoring of the apnea–hypopnea index using a simple non-contact breathing sensor. J. Sleep Res. 2013, 22, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waltisberg, D.; Amft, O.; Brunner, D.P.; Tröster, G. Detecting disordered breathing and limb movement using in-bed force sensors. IEEE J. Biomed. Health Inform. 2016, 21, 930–938. [Google Scholar] [CrossRef]
- Sadek, I.; Seet, E.; Biswas, J.; Abdulrazak, B.; Mokhtari, M. Nonintrusive vital signs monitoring for sleep apnea patients: A preliminary study. IEEE Access 2017, 6, 2506–2514. [Google Scholar] [CrossRef]
- Gaiduk, M.; Wehrle, D.; Seepold, R.; Ortega, J.A. Non-obtrusive system for overnight respiration and heartbeat tracking. Procedia Comput. Sci. 2020, 176, 2746–2755. [Google Scholar] [CrossRef]
- Albukhari, A.; Lima, F.; Mescheder, U. Bed-embedded heart and respiration rates detection by longitudinal ballistocardiography and pattern recognition. Sensors 2019, 19, 1451. [Google Scholar] [CrossRef] [Green Version]
- Lokavee, S.; Tantrakul, V.; Pengjiam, J.; Kerdcharoen, T. A sleep monitoring system using force sensor and an accelerometer sensor for screening sleep apnea. In Proceedings of the 2021 13th International Conference on Knowledge and Smart Technology (KST), Bangsaen, Thailand, 21–24 January 2021; pp. 208–213. [Google Scholar]
- Wang, Z.; Sui, Z.; Zhang, A.; Wang, R.; Zhang, Z.; Lin, F.; Chen, J.; Gao, S. A piezoresistive array based force sensing technique for sleeping posture and respiratory rate detection for SAS patients. IEEE Sens. J. 2021. [Google Scholar] [CrossRef]
- Gaiduk, M.; Vunderl, B.; Seepold, R.; Ortega, J.A.; Penzel, T. Sensor-mesh-based system with application on sleep study. In Proceedings of the Bioinformatics and Biomedical Engineering: 6th International Work-Conference, IWBBIO 2018, Granada, Spain, 25–27 April 2018; pp. 371–382. [Google Scholar]
- Wen, X.; Huang, Y.; Wu, X.; Zhang, B. A feasible feature extraction method for atrial fibrillation detection from BCG. IEEE J. Biomed. Health Inform. 2019, 24, 1093–1103. [Google Scholar] [CrossRef]
- Gaiduk, M.; Seepold, R.; Martínez Madrid, N.; Orcioni, S.; Conti, M. Recognizing breathing rate and movement while sleeping in home environment. In Proceedings of the Applications in Electronics Pervading Industry, Environment and Society: APPLEPIES 2019; Springer: Cham, Switzerland, 2020; pp. 333–339. [Google Scholar]
- Hsu, M.H.; Fang, S.C.; Wang, F.T.; Chan, H.L.; Huang, H.E.; Yang, S.C. Sleep apnea assessment using declination duration-based global metrics from unobtrusive fiber optic sensors. Physiol. Meas. 2019, 40, 075005. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.; Long, X.; Radha, M.; Haakma, R.; Aarts, R.M.; Rolink, J. Sleep stage classification with ECG and respiratory effort. Physiol. Meas. 2015, 36, 2027. [Google Scholar] [CrossRef] [Green Version]
- Da Estrela, C.; McGrath, J.; Booij, L.; Gouin, J.P. Heart rate variability, sleep quality, and depression in the context of chronic stress. Ann. Behav. Med. 2021, 55, 155–164. [Google Scholar] [CrossRef]
- Scott, H.; Lack, L.; Lovato, N. A systematic review of the accuracy of sleep wearable devices for estimating sleep onset. Sleep Med. Rev. 2020, 49, 101227. [Google Scholar] [CrossRef]
- Pan, Q.; Brulin, D.; Campo, E. Current status and future challenges of sleep monitoring systems: Systematic review. JMIR Biomed. Eng. 2020, 5, e20921. [Google Scholar] [CrossRef]
- Sadek, I.; Heng, T.T.S.; Seet, E.; Abdulrazak, B. A new approach for detecting sleep apnea using a contactless bed sensor: Comparison study. J. Med. Internet Res. 2020, 22, e18297. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Pereira, F.; Carvalho, N.; Lopes, S. Designing a Smart Pillow for Sleep Quality Remote Monitoring. Res. Sq. 2022. Preprint. [Google Scholar] [CrossRef]
- Yu, M.C.; Wu, H.; Liou, J.L.; Lee, M.S.; Hung, Y.P. Multiparameter sleep monitoring using a depth camera. In Proceedings of the Biomedical Engineering Systems and Technologies: 5th International Joint Conference, BIOSTEC 2012, Vilamoura, Portugal, 1–4 February 2012; pp. 311–325. [Google Scholar]
- Van Gastel, M.; Stuijk, S.; Overeem, S.; van Dijk, J.P.; van Gilst, M.M.; de Haan, G. Camera-based vital signs monitoring during sleep–A proof of concept study. IEEE J. Biomed. Health Inform. 2020, 25, 1409–1418. [Google Scholar] [CrossRef]
- Falie, D.; Ichim, M. Sleep monitoring and sleep apnea event detection using a 3D camera. In Proceedings of the 2010 8th International Conference on Communications, Bucharest, Romania, 10–12 June 2010; pp. 177–180. [Google Scholar]





| Male | Female | All Subjects | ||||
|---|---|---|---|---|---|---|
| Position | Se1 | Se2 | Se1 | Se2 | Se1 | Se2 |
| P1 | 2.19 | 2.86 | 1.85 | 3.17 | 2.09 | 2.95 |
| P2 | 2.04 | 2.86 | 2.34 | 2.52 | 2.13 | 2.76 |
| P3 | 2.50 | 3.11 | 2.74 | 3.11 | 2.57 | 3.11 |
| P4 | 2.53 | 2.62 | 2.41 | 2.27 | 2.49 | 2.52 |
| Average | 2.32 | 2.86 | 2.33 | 2.77 | 2.32 | 2.83 |
| Male | Female | All Subjects | ||||
|---|---|---|---|---|---|---|
| Position | ||||||
| 3.58 | 3.58 | 3.20 | 3.46 | 3.47 | 3.55 | |
| 4.10 | 4.04 | 2.12 | 4.01 | 3.51 | 4.03 | |
| 2.91 | 3.57 | 2.71 | 4.45 | 2.85 | 3.83 | |
| 3.31 | 3.02 | 2.68 | 3.85 | 3.12 | 3.27 | |
| Average | 3.47 | 3.55 | 2.68 | 3.94 | 3.24 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghi, M.; Asadov, A.; Boiko, A.; Ortega, J.A.; Martínez Madrid, N.; Seepold, R. Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study. Sensors 2023, 23, 3973. https://doi.org/10.3390/s23083973
Haghi M, Asadov A, Boiko A, Ortega JA, Martínez Madrid N, Seepold R. Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study. Sensors. 2023; 23(8):3973. https://doi.org/10.3390/s23083973
Chicago/Turabian StyleHaghi, Mostafa, Akhmadbek Asadov, Andrei Boiko, Juan Antonio Ortega, Natividad Martínez Madrid, and Ralf Seepold. 2023. "Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study" Sensors 23, no. 8: 3973. https://doi.org/10.3390/s23083973
APA StyleHaghi, M., Asadov, A., Boiko, A., Ortega, J. A., Martínez Madrid, N., & Seepold, R. (2023). Validating Force Sensitive Resistor Strip Sensors for Cardiorespiratory Measurement during Sleep: A Preliminary Study. Sensors, 23(8), 3973. https://doi.org/10.3390/s23083973










