Evaluation of Spatial Gas Temperature and Water Vapor Inhomogeneities in TDLAS in Circular Multipass Absorption Cells Used for the Analysis of Dynamic Tube Flows
Abstract
:1. Introduction
2. Materials and Methods
2.1. dTDLAS
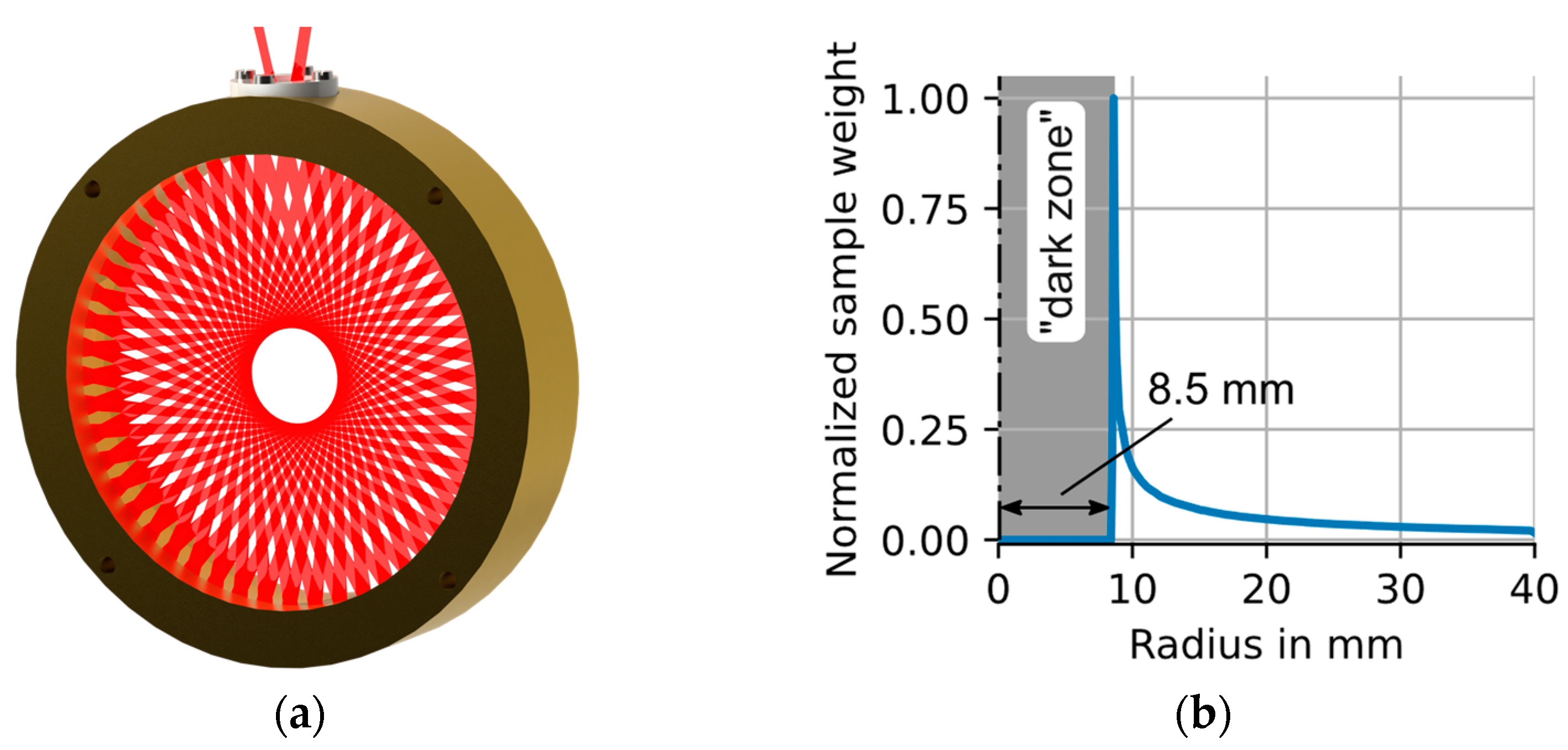
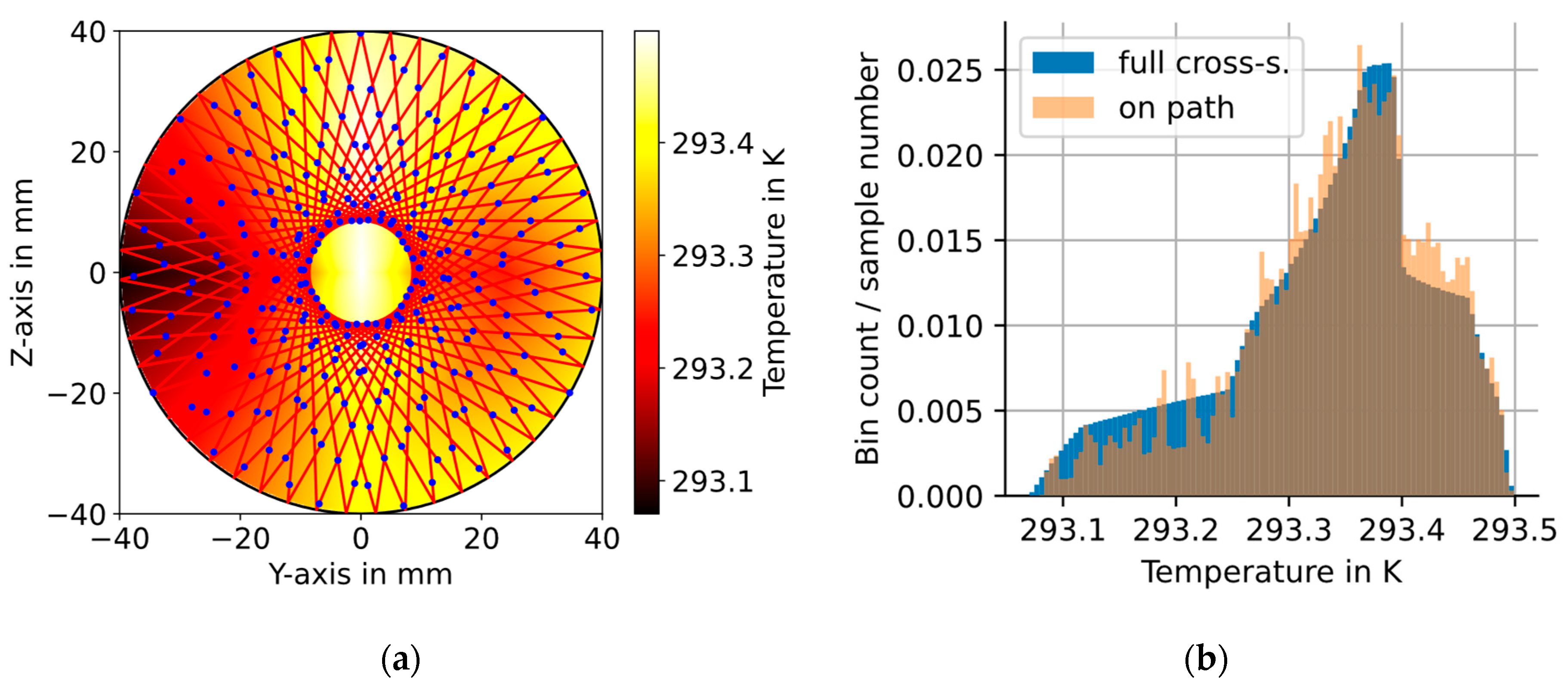
2.2. Circular Multipass Absorption Cells—CMPAC
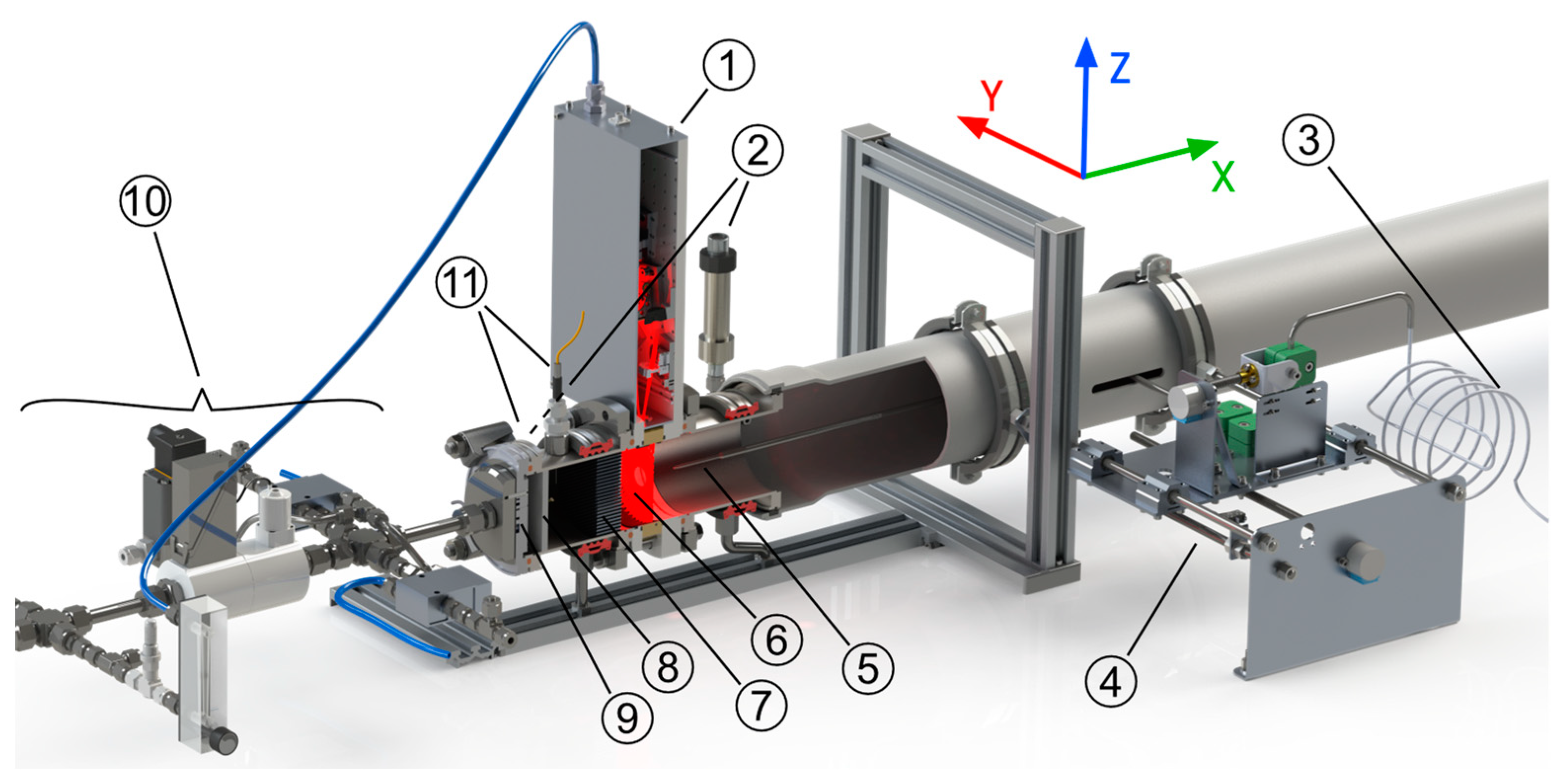
2.3. Experimental Setup
3. D-Temperature and H2O Concentration Distribution Measurements
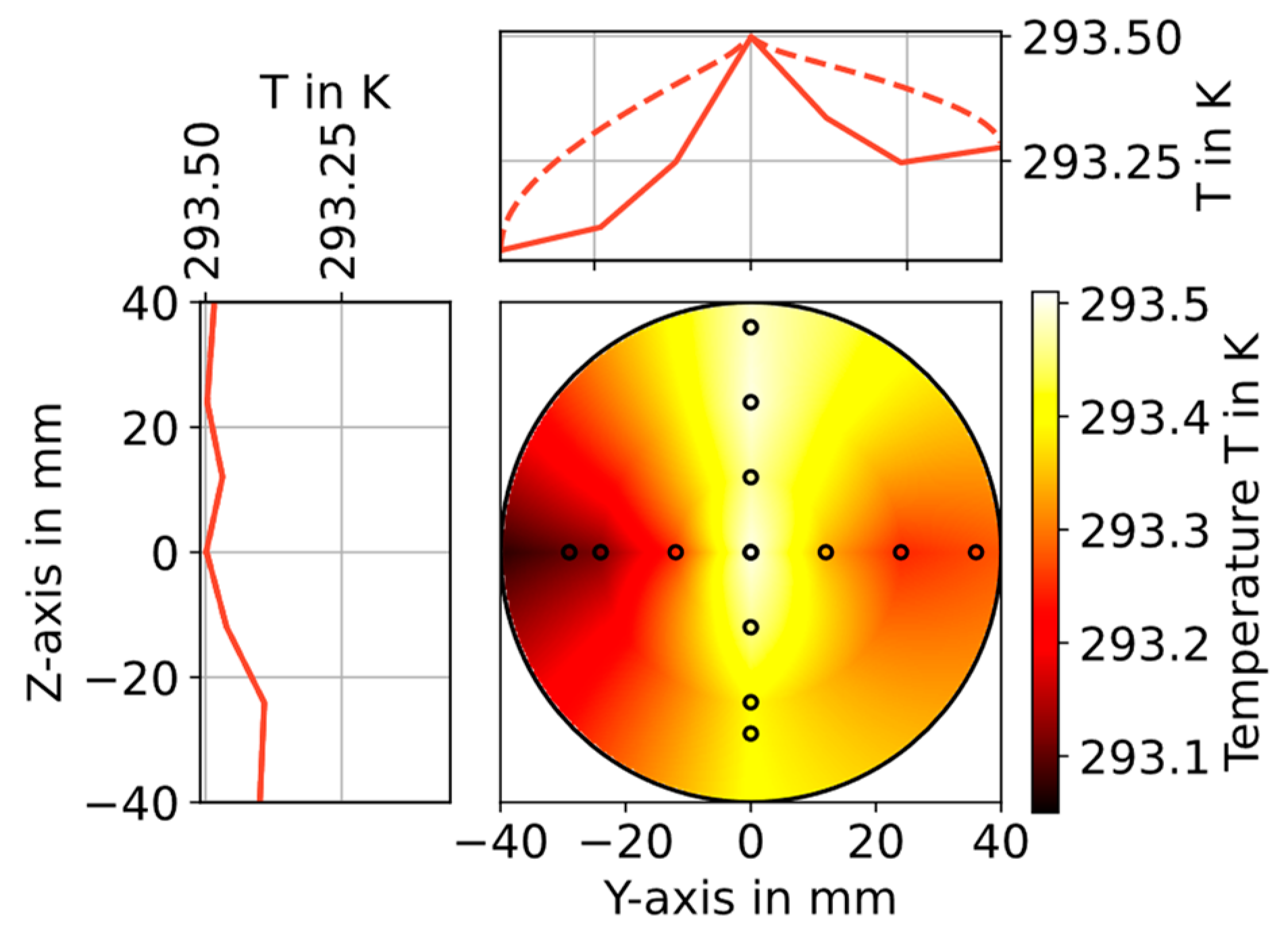
3.1. Temperature Measurements
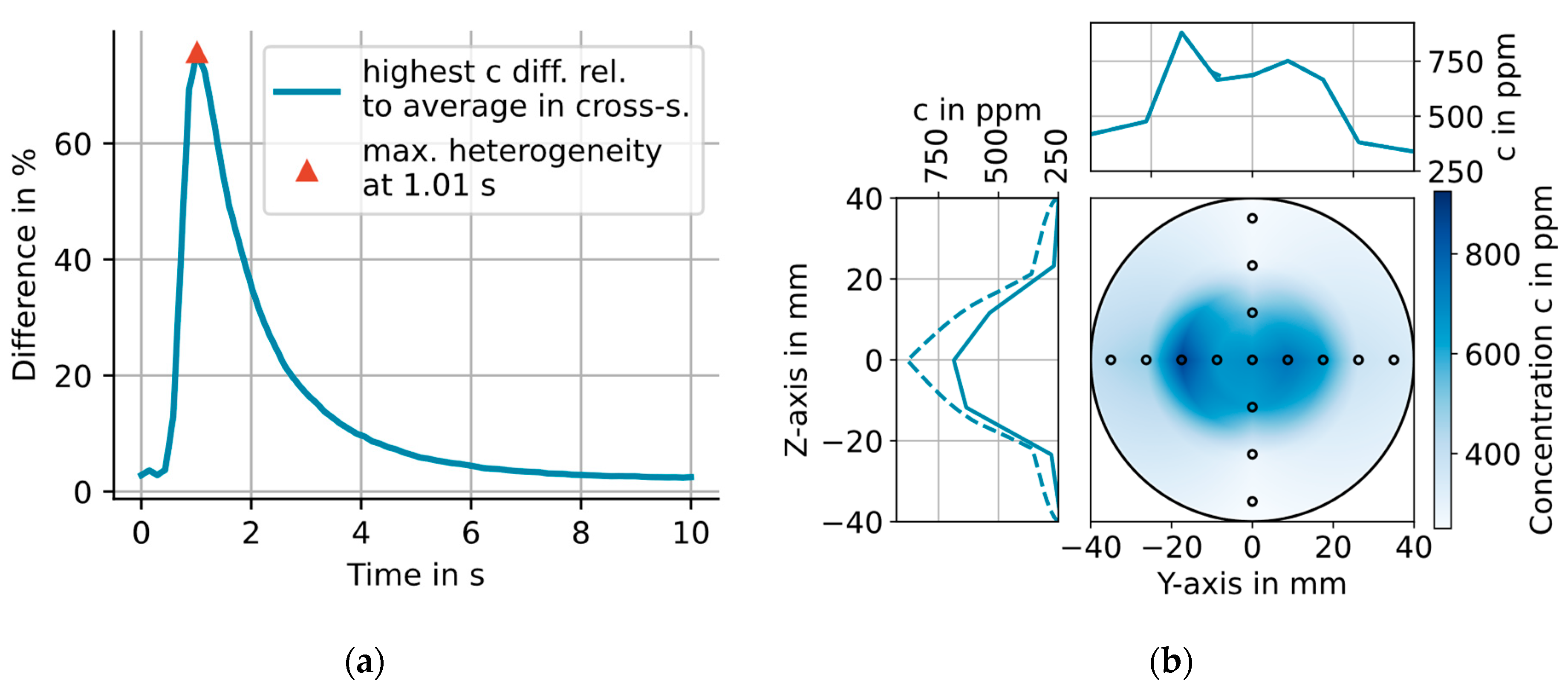
3.2. 2D H2O Concentration Measurements
4. Simulating the Effects of Temperature and H2O Concentration Inhomogeneities on the Line-of-Sight Averaged Concentration Measured with the CMPAC
4.1. Simulating the Effects of the Measured Spatial Gas-T and H2O Distributions
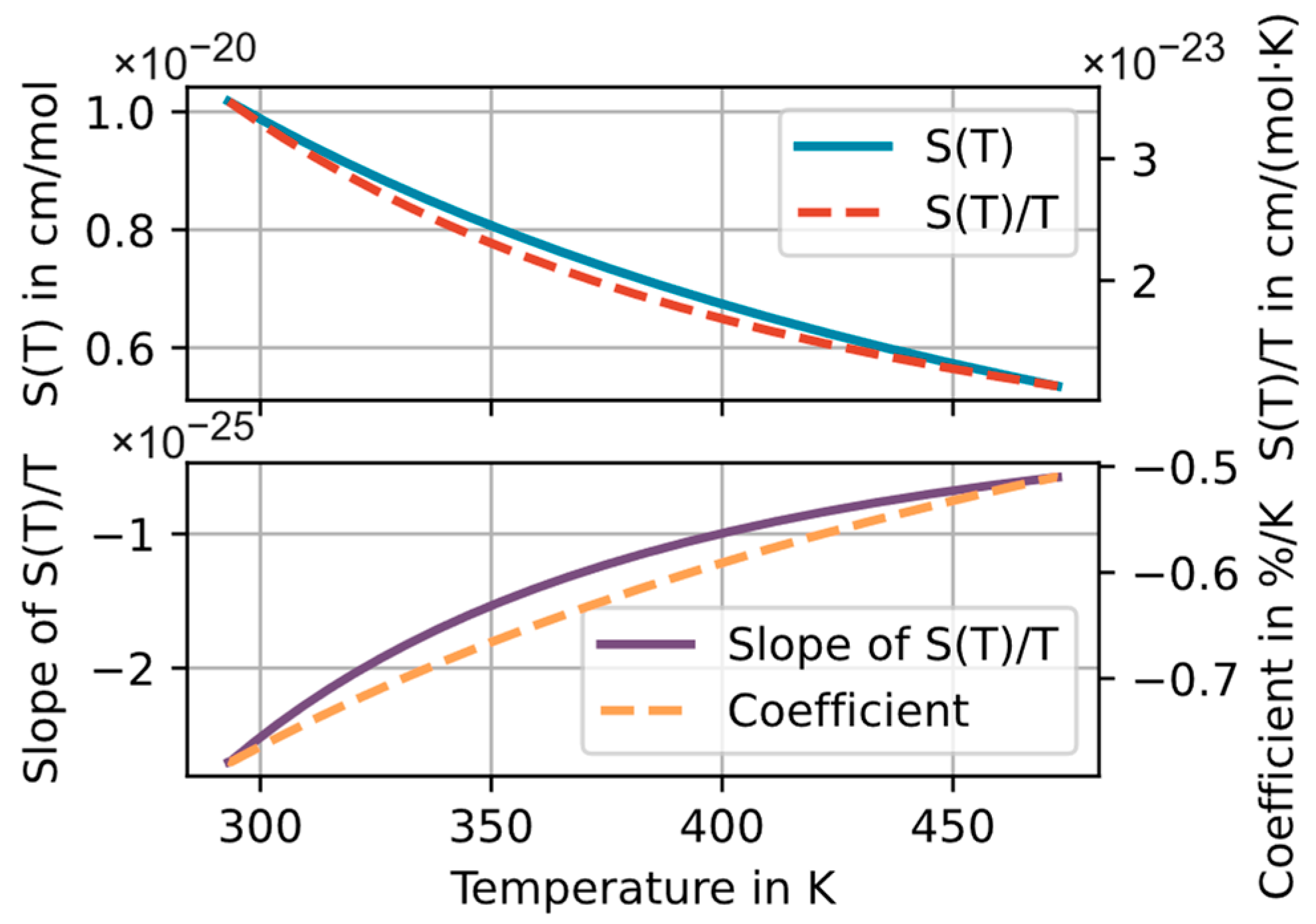
- Using the average temperature/concentration on the optical path. The deviations found in this scenario can be seen as the pure “spectroscopic effects”, e.g., from the nonlinear temperature dependence of the line intensity discussed in Section 2.1.
- Using an average temperature/concentration calculated for the entire cross section.
- Using a single temperature/concentration at the center of the ring cell. This scenario is especially relevant for practical applications of the circular cell where the temperature is often measured with a single temperature sensor at the center.
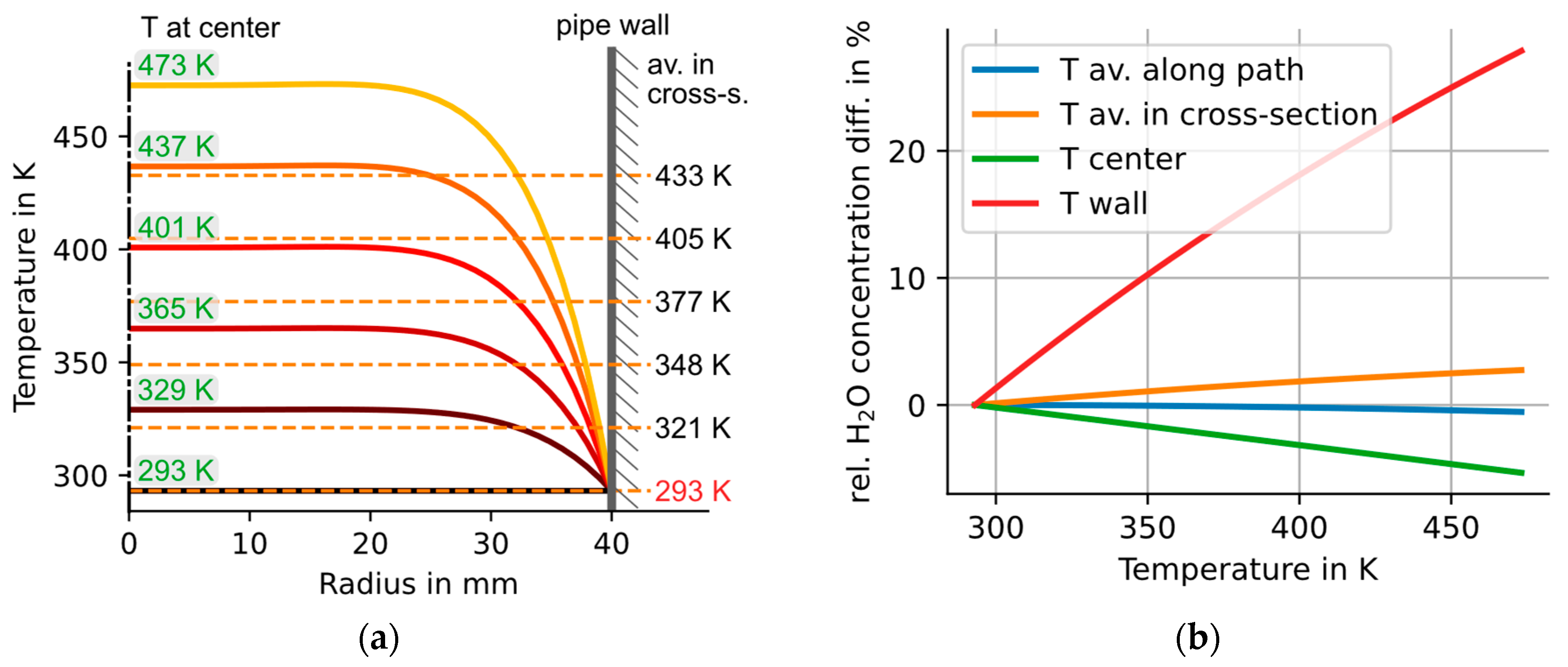
4.2. Simulating the Effects of Severe T Inhomogeneities at Center Temperatures of up to 473 K
5. Discussion of Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diskin, G.S.; Podolske, J.R.; Sachse, G.W.; Slate, T.A. Open-path airborne tunable diode laser hygrometer. In Diode Lasers and Applications in Atmospheric Sensing, Proceedings of the International Symposium on Optical Science and Technology, Seattle, WA, USA, 10–11 July 2002; International Society for Optics and Photonics: Ringham, WA, USA, 2002; Volume 4871, pp. 196–204. [Google Scholar]
- Lampert, A.; Hartmann, J.; Pätzold, F.; Lobitz, L.; Hecker, P.; Kohnert, K.; Larmanou, E.; Serafimovich, A.; Sachs, T. Comparison of Lyman-alpha and LI-COR infrared hygrometers for airborne measurement of turbulent fluctuations of water vapour. Atmos. Meas. Tech. 2018, 11, 2523–2536. [Google Scholar] [CrossRef]
- Witt, F.; Nwaboh, J.; Bohlius, H.; Lampert, A.; Ebert, V. Towards a Fast, Open-Path Laser Hygrometer for Airborne Eddy Covariance Measurements. Appl. Sci. 2021, 11, 5189. [Google Scholar] [CrossRef]
- May, R.D. Open-path, near-infrared tunable diode laser spectrometer for atmospheric measurements of H2O. J. Geophys. Res. 1998, 103, 19161–19172. [Google Scholar] [CrossRef]
- Manninen, A.; Tuzson, B.; Looser, H.; Bonetti, Y.; Emmenegger, L. Versatile multipass cell for laser spectroscopic trace gas analysis. Appl. Phys. B 2012, 109, 461–466. [Google Scholar] [CrossRef]
- Tuzson, B.; Mangold, M.; Looser, H.; Manninen, A.; Emmenegger, L. Compact multipass optical cell for laser spectroscopy. Opt. Lett. OL 2013, 38, 257–259. [Google Scholar] [CrossRef]
- Gurlit, W.; Zimmermann, R.; Giesemann, C.; Fernholz, T.; Ebert, V.; Wolfrum, J.; Platt, U.; Burrows, J.P. Lightweight diode laser spectrometer CHILD (Compact High-altitude In-situ Laser Diode) for balloonborne measurements of water vapor and methane. Appl. Opt. 2005, 44, 91–102. [Google Scholar] [CrossRef]
- Kühnreich, B.; Höh, M.; Wagner, S.; Ebert, V. Direct single-mode fibre-coupled miniature White cell for laser absorption spectroscopy. Rev. Sci. Instrum. 2016, 87, 23111. [Google Scholar] [CrossRef]
- Teichert, H.; Fernholz, T.; Ebert, V. Simultaneous in situ measurement of CO, H2O, and gas temperatures in a full-sized coal-fired power plant by near-infrared diode lasers. Appl. Opt. 2003, 42, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Scheidegger, P.; Kupferschmid, A.; Looser, H.; Peter, T.; Dirksen, R.; Emmenegger, L.; Tuzson, B. Compact and lightweight mid-infrared laser spectrometer for balloon-borne water vapor measurements in the UTLS. Atmos. Meas. Tech. 2021, 14, 1365–1378. [Google Scholar] [CrossRef]
- Witt, F.; Bubser, F.; Ebert, V.; Bergmann, D. C9.1 Temporal Hygrometer Characterization: Design and First Test of a New, Metrological Dynamic Testing Infrastructure. In System of Units and Metreological Infrastructure, Proceedings of the SMSI 2021, Digital, 3–6 May 2021; AMA Service GmbH: Wunstorf, Germany, 2021; pp. 308–309. [Google Scholar]
- Witt, F.; Schuchard, M.; Ebert, V. DynH2O: Aufbau zur metrologischen Charakterisierung der dynamischen Eigenschaften von Hygrometern. Tech. Mess. 2023, 90, 79–89. [Google Scholar] [CrossRef]
- Sanders, S.T.; Wang, J.; Jeffries, J.B.; Hanson, R.K. Diode-Laser Absorption Sensor for Line-of-Sight Gas Temperature Distributions. Appl. Opt. 2001, 40, 4404–4415. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jeffries, J.B.; Hanson, R.K. Measurement of Non-Uniform Temperature Distributions Using Line-of-Sight Absorption Spectroscopy. AIAA J. 2007, 45, 411–419. [Google Scholar] [CrossRef]
- Ouyang, X.; Varghese, P.L. Line-of-sight absorption measurements of high temperature gases with thermal and concentration boundary layers. Appl. Opt. 1989, 28, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Werhahn, O.; Ebert, V. Thermal Boundary Layer Effects on Line-of-Sight Tunable Diode Laser Absorption Spectroscopy (TDLAS) Gas Concentration Measurements. Appl. Spectrosc. 2018, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y. Tunable Diode Laser Absorption Spectroscopy Based Temperature Measurement with a Single Diode Laser Near 1.4 μm. Sensors 2022, 22, 6095. [Google Scholar] [CrossRef] [PubMed]
- Bürkle, S.; Biondo, L.; Ding, C.-P.; Honza, R.; Ebert, V.; Böhm, B.; Wagner, S. In-Cylinder Temperature Measurements in a Motored IC Engine using TDLAS. Flow Turbul. Combust 2018, 101, 139–159. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Hanson, R.K. Diode-laser measurements of linestrength and temperature-dependent lineshape parameters for H2O transitions near 1.4μm using Voigt, Rautian, Galatry, and speed-dependent Voigt profiles. J. Quant. Spectrosc. Radiat. Transf. 2015, 152, 127–139. [Google Scholar] [CrossRef]
- Durry, G.; Amarouche, N.; Joly, L.; Liu, X.; Parvitte, B.; Zéninari, V. Laser diode spectroscopy of H2O at 2.63 μm for atmospheric applications. Appl. Phys. B 2008, 90, 573–580. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Spearrin, R.M.; Schultz, I.A.; Jeffries, J.B.; Hanson, R.K. Wavelength-modulation spectroscopy near 1.4 µm for measurements of H2O and temperature in high-pressure and -temperature gases. Meas. Sci. Technol. 2014, 25, 55101. [Google Scholar] [CrossRef]
- Hanson, R.K.; Spearrin, R.M.; Goldenstein, C.S. Spectroscopy and Optical Diagnostics for Gases, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319232522. [Google Scholar]
- Ebert, V. In situ Absorption Spectrometers Using Near-IR Diode Lasers and Rugged Multi-Path-Optics for Environmental Field Measurements. In Proceedings of the Laser Applications to Chemical, Security and Environmental Analysis, Incline Village, NV, USA, 5–9 February 2006; p. WB1. [Google Scholar] [CrossRef]
- Ebert, V.; Wolfrum, J. Absorption. In Optical Measurements: Techniques and Applications, 2nd ed.; Mayinger, F., Feldmann, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 231–270. ISBN 978-3-642-63079-8. [Google Scholar]
- Buchholz, B.; Afchine, A.; Klein, A.; Schiller, C.; Krämer, M.; Ebert, V. HAI, a new airborne, absolute, twin dual-channel, multi-phase TDLAS-hygrometer: Background, design, setup, and first flight data. Atmos. Meas. Tech. 2017, 10, 35–57. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Delahaye, T.; Armante, R.; Scott, N.A.; Jacquinet-Husson, N.; Chédin, A.; Crépeau, L.; Crevoisier, C.; Douet, V.; Perrin, A.; Barbe, A.; et al. The 2020 edition of the GEISA spectroscopic database. J. Mol. Spectrosc. 2021, 380, 111510. [Google Scholar] [CrossRef]
- Hunsmann, S.; Wagner, S.; Saathoff, H.; Möhler, O.; Schurath, U.; Ebert, V. Messung der Temperaturabhängigkeit der Linienstärken und Druckverbreiterungskoeffizienten von H2O-Absorptionslinien im 1.4 µm-Band. VDI-Berichte 2006, 1959, 149–164. [Google Scholar]
- Pogány, A.; Klein, A.; Ebert, V. Measurement of water vapor line strengths in the 1.4–2.7 µm range by tunable diode laser absorption spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 2015, 165, 108–122. [Google Scholar] [CrossRef]
- Nwaboh, J.A.; Werhahn, O.; Ebert, V. H2O Collisional Broadening Coefficients at 1.37 µm and Their Temperature Dependence: A Metrology Approach. Appl. Sci. 2021, 11, 5341. [Google Scholar] [CrossRef]
- Kuntz, M. A new implementation of the Humlicek algorithm for the calculation of the Voigt profile function. J. Quant. Spectrosc. Radiat. Transf. 1997, 57, 819–824. [Google Scholar] [CrossRef]
- Mayinger, F.; Feldmann, O. (Eds.) Optical Measurements: Techniques and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 978-3-642-63079-8. [Google Scholar]
- Gamache, R.R.; Vispoel, B.; Rey, M.; Nikitin, A.; Tyuterev, V.; Egorov, O.; Gordon, I.E.; Boudon, V. Total internal partition sums for the HITRAN2020 database. J. Quant. Spectrosc. Radiat. Transf. 2021, 271, 107713. [Google Scholar] [CrossRef]
- Buchholz, B.; Kallweit, S.; Ebert, V. SEALDH-II-An Autonomous, Holistically Controlled, First Principles TDLAS Hygrometer for Field and Airborne Applications: Design-Setup-Accuracy/Stability Stress Test. Sensors 2016, 17, 68. [Google Scholar] [CrossRef]
- Hunsmann, S.; Wunderle, K.; Wagner, S.; Rascher, U.; Schurr, U.; Ebert, V. Absolute, high resolution water transpiration rate measurements on single plant leaves via tunable diode laser absorption spectroscopy (TDLAS) at 1.37 μm. Appl. Phys. B 2008, 92, 393–401. [Google Scholar] [CrossRef]
- Coxeter, H.S.M. Introduction to Geometry, 2nd ed.; Wiley: New York, NY, USA, 1989; ISBN 9780471504580. [Google Scholar]
- Graf, M.; Emmenegger, L.; Tuzson, B. Compact, circular, and optically stable multipass cell for mobile laser absorption spectroscopy. Opt. Lett. OL 2018, 43, 2434–2437. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.; Böse, N.; Ebert, V. Absolute validation of a diode laser hygrometer via intercomparison with the German national primary water vapor standard. Appl. Phys. B 2014, 116, 883–899. [Google Scholar] [CrossRef]
- Buchholz, B.; Kühnreich, B.; Smit, H.G.J.; Ebert, V. Validation of an extractive, airborne, compact TDL spectrometer for atmospheric humidity sensing by blind intercomparison. Appl. Phys. B 2013, 110, 249–262. [Google Scholar] [CrossRef]
- Buerkle, S.; Ebert, V.; Rauen, D.; Dreizler, A.; Moeller, G.; Hees, J.; Zabrodiec, D.; Massmeyer, A.; Kneer, R.; Wagner, S. Laser-based Measurement of Temperature and Species Concentrations during the Combustion of torrefied Biomass in a 100 KWth-Dust Firing Test Facility. In 28. Deutscher Flammentag: Verbrennung und Feuerung, 1st ed.; VDI Verlag: Düsseldorf, Germany, 2017; pp. 69–84. ISBN 9783181023020. [Google Scholar]

| Scenario 1 Av. Along Path | Scenario 2 Av. in Cross Section | Scenario 3 Center | |
|---|---|---|---|
| Temperature | 0.00000161% | 0.000544% | −0.00838% |
| Concentration | 0.0146% | −16.1% | 29.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witt, F.; Bohlius, H.; Ebert, V. Evaluation of Spatial Gas Temperature and Water Vapor Inhomogeneities in TDLAS in Circular Multipass Absorption Cells Used for the Analysis of Dynamic Tube Flows. Sensors 2023, 23, 4345. https://doi.org/10.3390/s23094345
Witt F, Bohlius H, Ebert V. Evaluation of Spatial Gas Temperature and Water Vapor Inhomogeneities in TDLAS in Circular Multipass Absorption Cells Used for the Analysis of Dynamic Tube Flows. Sensors. 2023; 23(9):4345. https://doi.org/10.3390/s23094345
Chicago/Turabian StyleWitt, Felix, Henning Bohlius, and Volker Ebert. 2023. "Evaluation of Spatial Gas Temperature and Water Vapor Inhomogeneities in TDLAS in Circular Multipass Absorption Cells Used for the Analysis of Dynamic Tube Flows" Sensors 23, no. 9: 4345. https://doi.org/10.3390/s23094345









