Miniaturization of an Osmotic Pressure-Based Glucose Sensor for Continuous Intraperitoneal and Subcutaneous Glucose Monitoring by Means of Nanotechnology
Abstract
:1. Introduction
2. Materials and Methods
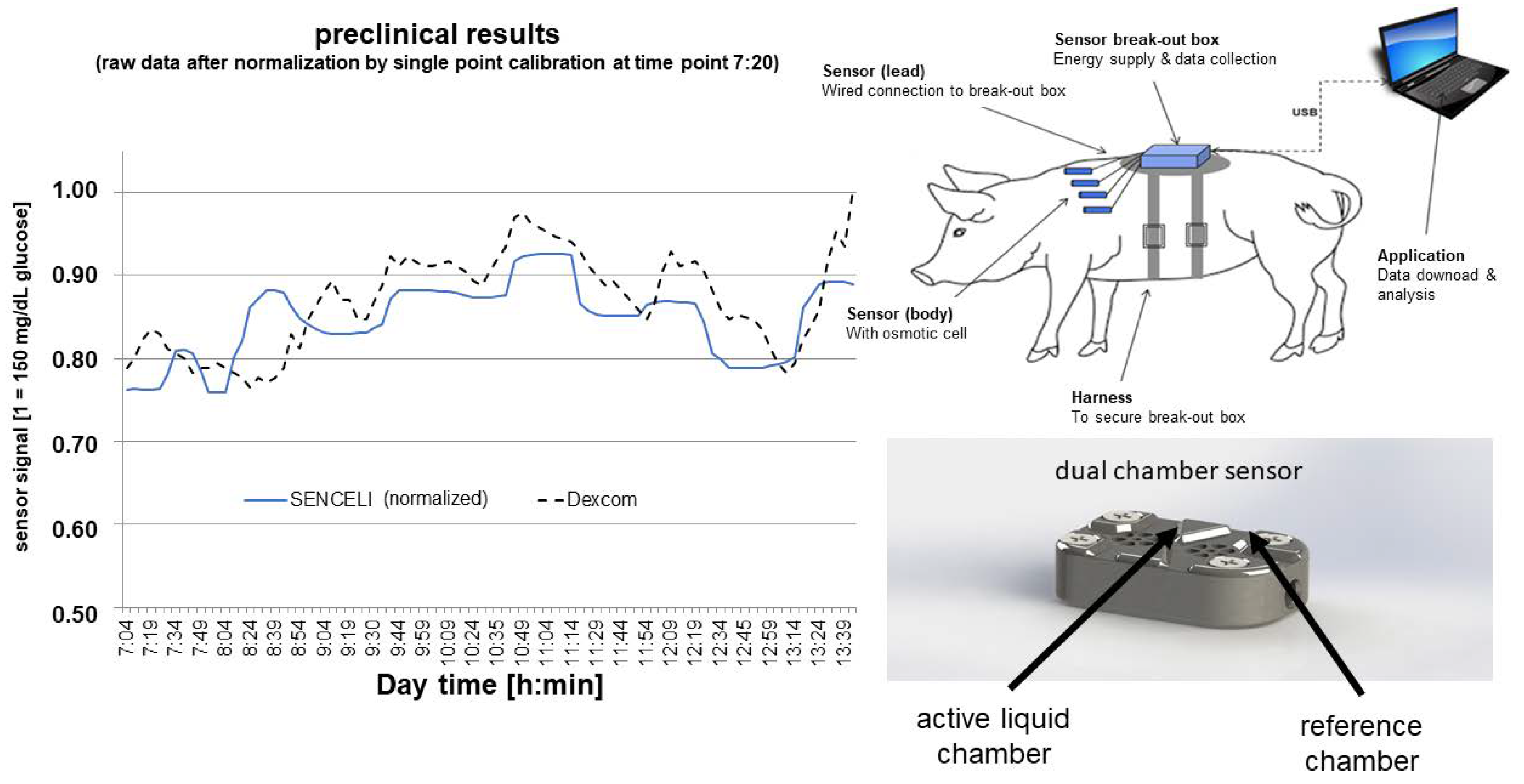
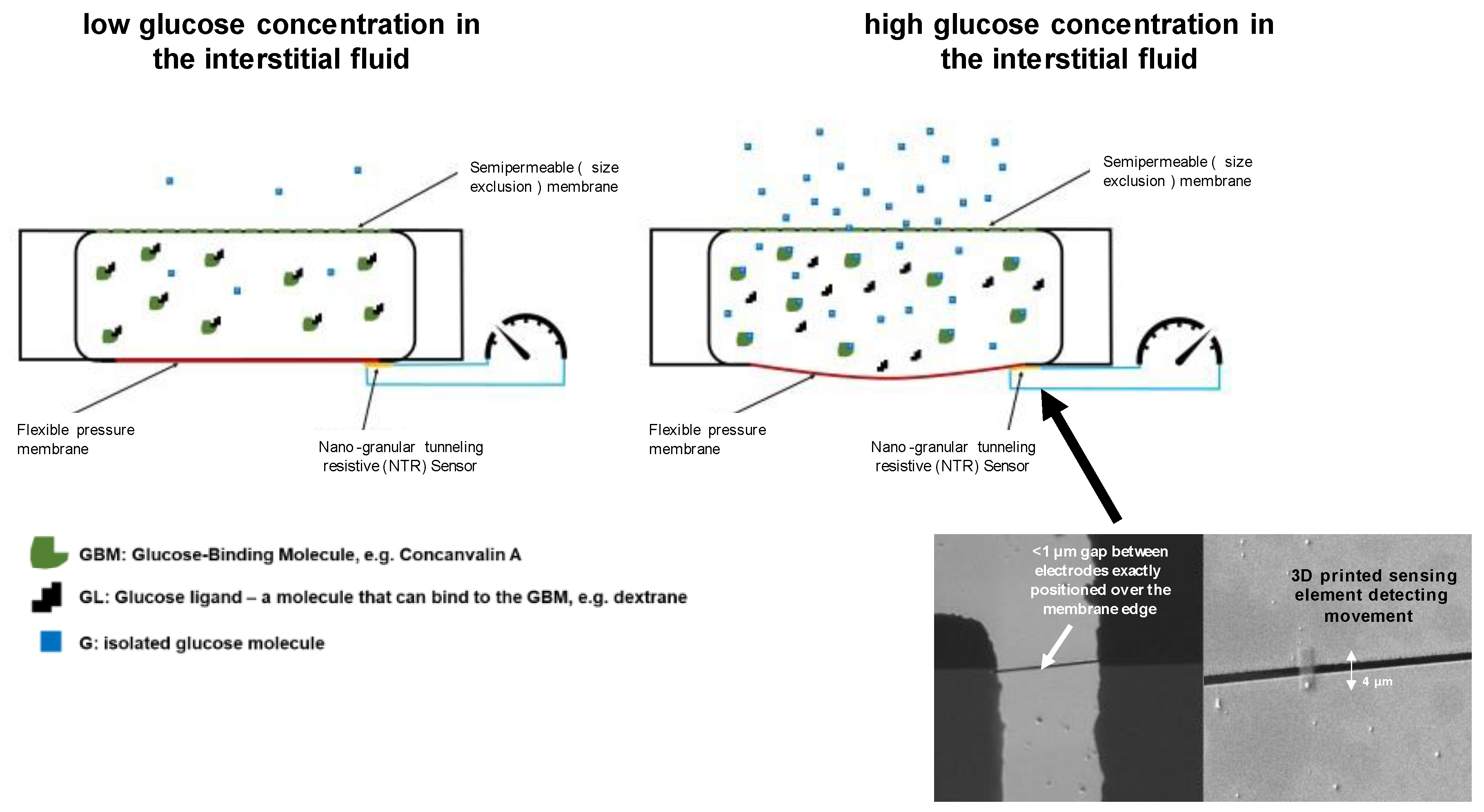
2.1. Measurement Technology
2.2. Nano-Granular Tunneling Resistive (NTR) Sensors
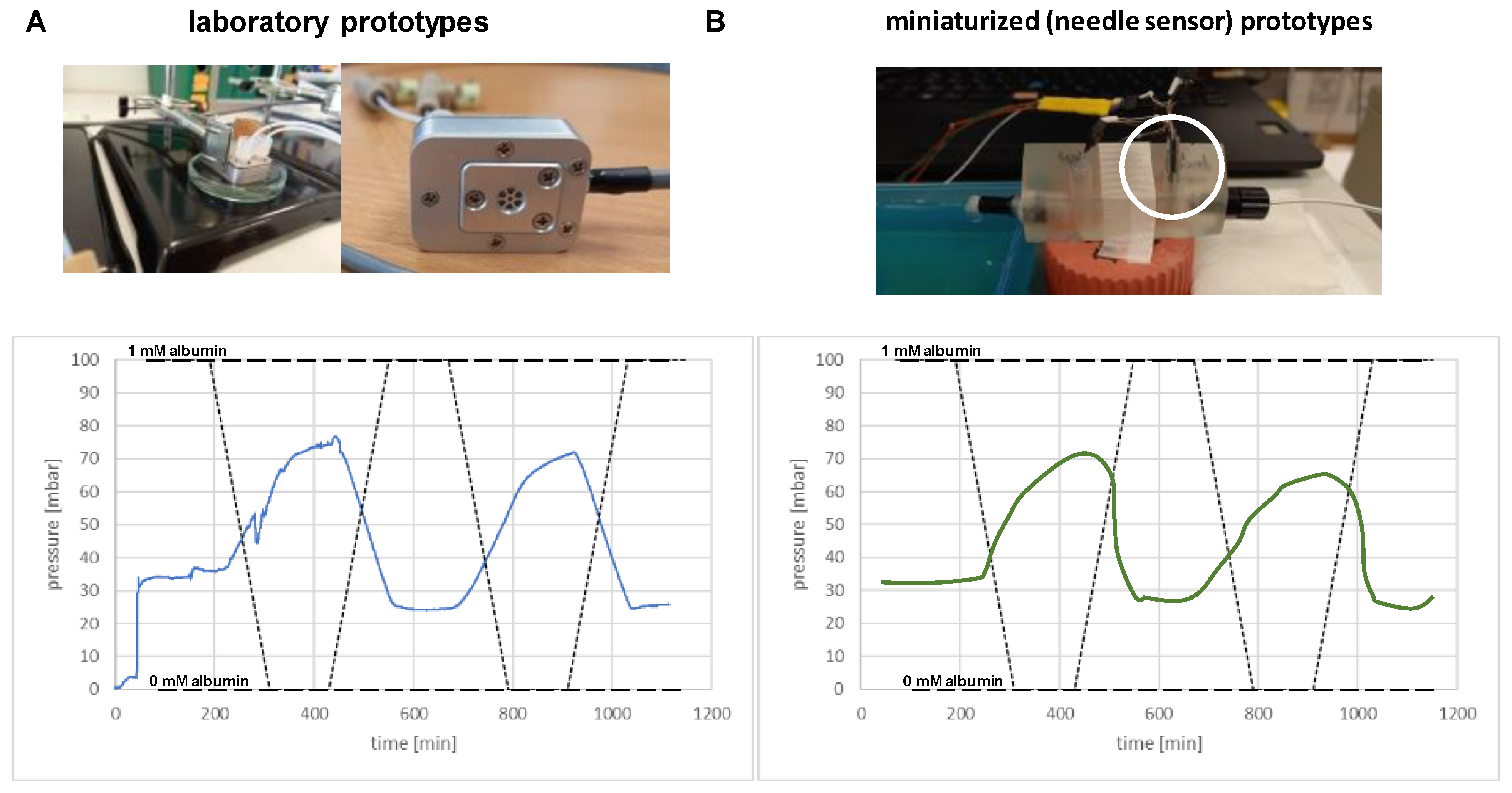
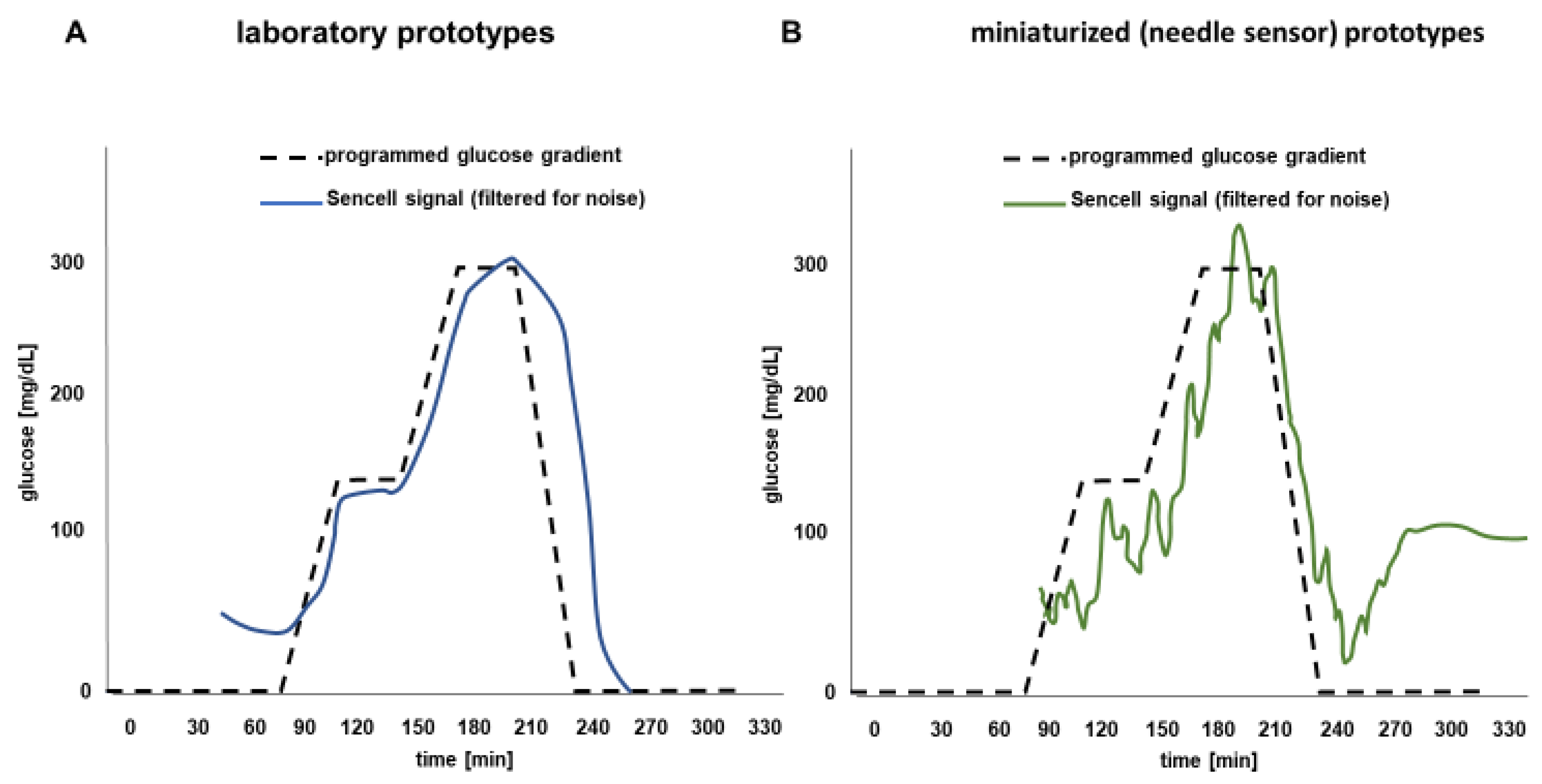
3. Results
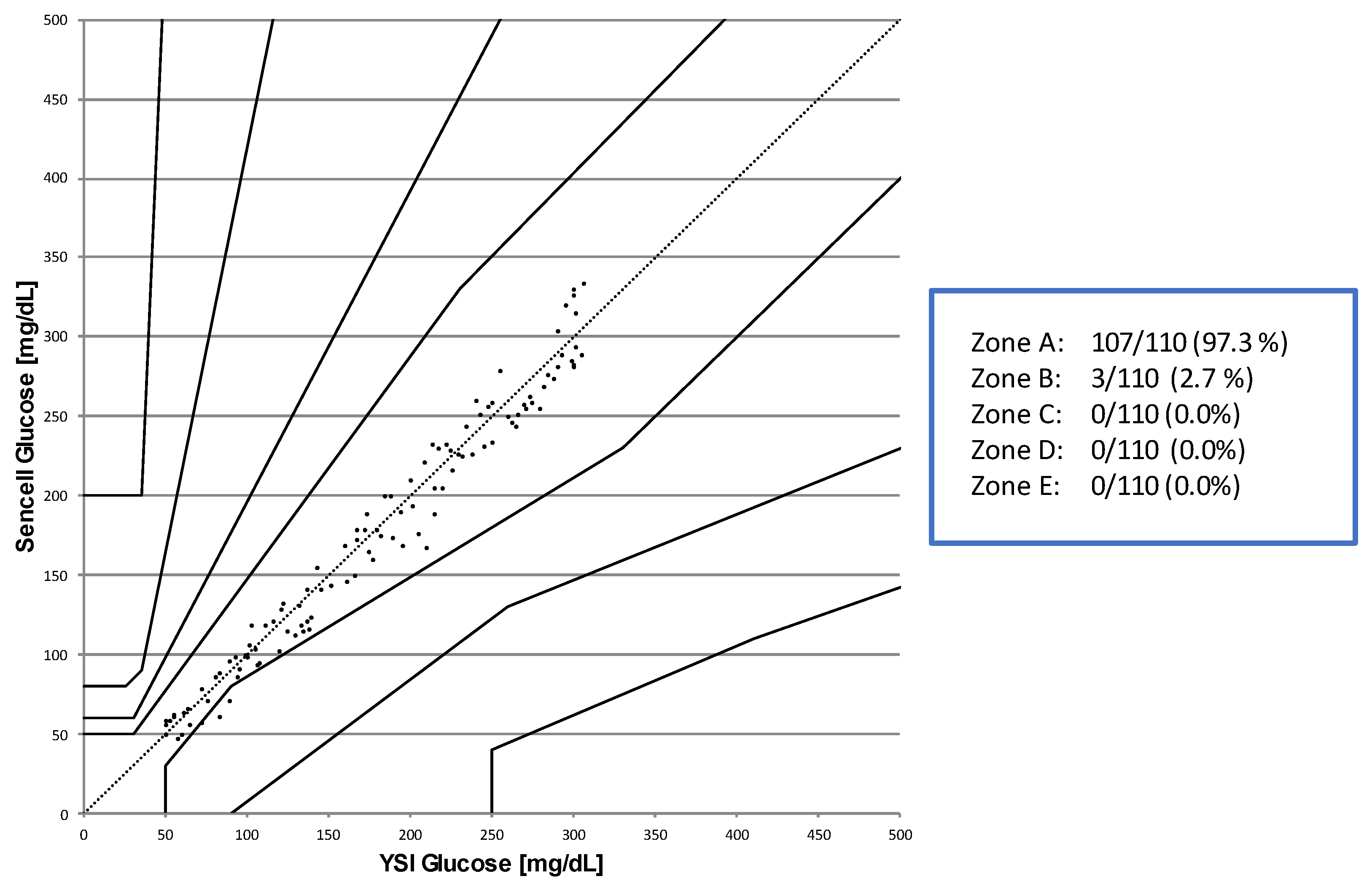
Osmotic Pressure Benchmark Testing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ADA. Standards of Medical Care in Diabetes. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar]
- Zick, R.; Schiwietz, J.; Richter, M.; Pfützner, A. Erste klinische Erfahrungen mit einem Praxistauglichen Glukosesensor (First clinical experience with a glucose sensor suitable for daily routine). Z. Allg. Med. 2000, 76, 255–257. (In German) [Google Scholar]
- Di Molfetta, S.; Caruso, I.; Cignarelli, A.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Professional continuous glucose monitoring in patients with diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2023, 25, 1301–1310. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Freckmann, G.; Ehrmann, D.; Faber-Heinemann, G.; Guerra, S.; Waldenmaier, D.; Hermanns, N. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): A multicentre, randomised controlled trial. Lancet 2018, 391, 1367–1377. [Google Scholar] [CrossRef]
- Bolinder, J.; Antuna, R.; Geelhoed-Duijvestijn, P.; Kröger, J.; Weitgasser, R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016, 388, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Riddlesworth, T.D.; Ruedy, K.; Ahmann, A.; Haller, S.; Kruger, D.; McGill, J.B.; Polonsky, W.; Price, D.; Aronoff, S.; et al. DIAMOND Study Group. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann. Intern. Med. 2017, 167, 365–374. [Google Scholar] [CrossRef]
- Lind, M.; Polonsky, W.; Hirsch, I.B. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: The GOLD randomized clinical trial. JAMA 2017, 317, 379–387. [Google Scholar] [CrossRef]
- Aleppo, G.; Ruedy, K.J.; Riddlesworth, T.D.; Kruger, D.F.; Peters, A.L.; Hirsch, I.; Bergenstal, R.M.; Toschi, E.; Ahmann, A.J.; Shah, V.N.; et al. REPLACE-BG Study Group. REPLACE-BG: A Randomized Trial Comparing Continuous Glucose Monitoring With and Without Routine Blood Glucose Monitoring in Adults With Well-Controlled Type 1 Diabetes. Diabetes Care 2017, 40, 538–545. [Google Scholar] [CrossRef]
- Charleer, S.; De Block, C.; Van Huffel, L.; Broos, B.; Fieuws, S.; Nobels, F.; Mathieu, C.; Gillard, P. Quality of Life and Glucose Control After 1 Year of Nationwide Reimbursement of Intermittently Scanned Continuous Glucose Monitoring in Adults Living With Type 1 Diabetes (FUTURE): A Prospective Observational Real-World Cohort Study. Diabetes Care 2020, 43, 389–397. [Google Scholar] [CrossRef]
- Charleer, S.; Mathieu, C.; Nobels, F.; De Block, C.; Radermecker, R.P.; Hermans, M.P.; Taes, Y.; Vercammen, C.; T’Sjoen, G.; Crenier, L.; et al. RESCUE Trial Investigators. Effect of Continuous Glucose Monitoring on Glycemic Control, Acute Admissions, and Quality of Life: A Real-World Study. J. Clin. Endocrinol. Metab. 2018, 103, 1224–1232. [Google Scholar] [CrossRef]
- Fokkert, M.; van Dijk, P.; Edens, M.; Barents, E.; Mollema, J.; Slingerland, R.; Gans, R.; Bilo, H. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care. 2019, 7, e000809. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.; Reznik, Y. Personal continuous glucose monitoring (CGM) in diabetes management: Review of the literature and implementation for practical use. Diabetes Res. Clin. Pract. 2012, 96, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.R. The Safety of Nonadjunctive Use of Continuous Glucose Monitors for Insulin Dosing: Still Not Resolved. J. Diabetes Sci. Technol. 2017, 11, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L. Interferences with CGM Systems: Practical Relevance? J. Diabetes Sci. Technol. 2022, 16, 271–274. [Google Scholar] [CrossRef]
- Pfützner, A.; Jensch, H.; Cardinal, C.; Srikanthamoorthy, G.; Riehn, E.; Thomé, N. Laboratory Protocol and Pilot Results for Dynamic Interference Testing of Continuous Glucose Monitoring Sensors. J. Diabetes Sci. Technol. 2022, 19322968221095573. [Google Scholar] [CrossRef]
- Johannessen, E.; Krushinitskaya, O.; Sokolov, A.; Philipp, H.; Hoogerwerf, A.; Hinderling, C.; Kautio, K.; Lenkkeri, J.; Strömmer, E.; Kondratyev, V.; et al. Toward an injectable continuous osmotic glucose sensor. J. Diabetes Sci. Technol. 2010, 4, 882–892. [Google Scholar] [CrossRef]
- Dukic, M.; Winhold, M.; Schwalb, C.H.; Adams, J.D.; Stavrov, V.; Huth, M.; Fantner, G.E. Direct-write nanoscale printing of nanogranular tunnelling strain sensors for sub-micrometre cantilevers. Nat. Commun. 2016, 7, 12487. [Google Scholar] [CrossRef]
- Huth, M.; Porrati, F.; Barth, S. Living up to its potential—Direct-write nanofabrication with focused electron beams. J. Appl. Phys. 2021, 130, 170901. [Google Scholar] [CrossRef]
- Parkes, J.L.; Slatin, S.L.; Pardo, S.; Ginsberg, B.H. A new consensus error grid to evaluate the clinical significance of inaccuracy in the measurement of blood glucose. Diabetes Care 2000, 23, 1143–1148. [Google Scholar] [CrossRef]
- Pfützner, A.; Klonoff, D.C.; Pardo, S.; Parkes, J.L. Technical aspects of the Parkes Error Grid. J. Diabetes Sci. Technol. 2013, 7, 1275–1281. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/blood-glucose-monitoring-test-systems-prescription-point-care-use (accessed on 19 March 2023).
- Jafarizadeh, B.; Chowdhury, A.H.; Khakpour, I.; Pala, N.; Wang, C. Design Rules for a Wearable Micro-Fabricated Piezo-Resistive Pressure Sensor. Micromachines 2022, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Son, H.; Park, S.; Lee, J.; Jang, J.; Lee, M.; Choi, H.J. Fabrication of Piezo-Resistance Composites Containing Thermoplastic Polyurethane/Hybrid Filler Using 3D Printing. Sensors 2021, 21, 6813. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 1, 182. [Google Scholar] [CrossRef]
- Juan, C.G.; Poleton, B.; Quendo, C.; Garcia-Martinez, H.; Avila-Navarro, E.; Bronchalo, E.; Sabater-Navarro, J.M. Study of Qu-Based Resonant Microwave Sensors and Design of 3-D-Printed Devices Dedicated to Glucose Monitoring. IEEE Trans. Instrum. Meas. 2021, 70, 8005716. [Google Scholar] [CrossRef]
- Pfützner, A.; Strobl, S.; Sachsenheimer, D.; Lier, A.; Ramljak, S.; Demircik, F. Evaluation of the Non-Invasive Glucose Monitoring Device GlucoTrack® in Patients with Type 2 Diabetes and Subjects with Prediabetes. J. Diabetes Treat. 2019, 1, 1070. [Google Scholar]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Joseph, J.I. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2021, 15, 167–173, Erratum in J. Diabetes Sci. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kropff, J.; Choudhary, P.; Neupane, S.; Barnard, K.; Bain, S.C.; Kapitza, C.; Forst, T.; Link, M.; Dehennis, A.; DeVries, J.H. Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care. 2017, 40, 63–68. [Google Scholar] [CrossRef]
- Christiansen, M.P.; Klaff, L.J.; Bailey, T.S.; Brazg, R.; Carlson, G.; Tweden, K.S. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: The PRECISION study. Diabetes Technol. Ther. 2019, 21, 231–237. [Google Scholar] [CrossRef]
- Aronson, R.; Abitbol, A.; Tweden, K.S. First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes. Metab. 2019, 21, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Jafri, R.Z.; Balliro, C.A.; El-Khatib, F.; Maheno, M.M.; Hillard, M.A.; O’Donovan, A.; Selagamsetty, R.; Zheng, H.; Damiano, E.R.; Russell, S.J. A Three-Way Accuracy Comparison of the Dexcom G5, Abbott Freestyle Libre Pro, and Senseonics Eversense Continuous Glucose Monitoring Devices in a Home-Use Study of Subjects with Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 846–852. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfützner, A.; Tencer, B.; Stamm, B.; Mehta, M.; Sharma, P.; Gilyazev, R.; Jensch, H.; Thomé, N.; Huth, M. Miniaturization of an Osmotic Pressure-Based Glucose Sensor for Continuous Intraperitoneal and Subcutaneous Glucose Monitoring by Means of Nanotechnology. Sensors 2023, 23, 4541. https://doi.org/10.3390/s23094541
Pfützner A, Tencer B, Stamm B, Mehta M, Sharma P, Gilyazev R, Jensch H, Thomé N, Huth M. Miniaturization of an Osmotic Pressure-Based Glucose Sensor for Continuous Intraperitoneal and Subcutaneous Glucose Monitoring by Means of Nanotechnology. Sensors. 2023; 23(9):4541. https://doi.org/10.3390/s23094541
Chicago/Turabian StylePfützner, Andreas, Barbora Tencer, Boris Stamm, Mandar Mehta, Preeti Sharma, Rustam Gilyazev, Hendrick Jensch, Nicole Thomé, and Michael Huth. 2023. "Miniaturization of an Osmotic Pressure-Based Glucose Sensor for Continuous Intraperitoneal and Subcutaneous Glucose Monitoring by Means of Nanotechnology" Sensors 23, no. 9: 4541. https://doi.org/10.3390/s23094541






