Integrated Temperature–Humidity Sensors for a Pouch-Type Battery Using 100% Printing Process
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Composite Materials for Humidity and Temperature Detection
2.2. Fabrication of Integrated Temperature–Humidity Sensor
2.3. Temperature–Humidity Sensors Measurement
3. Results and Discussion
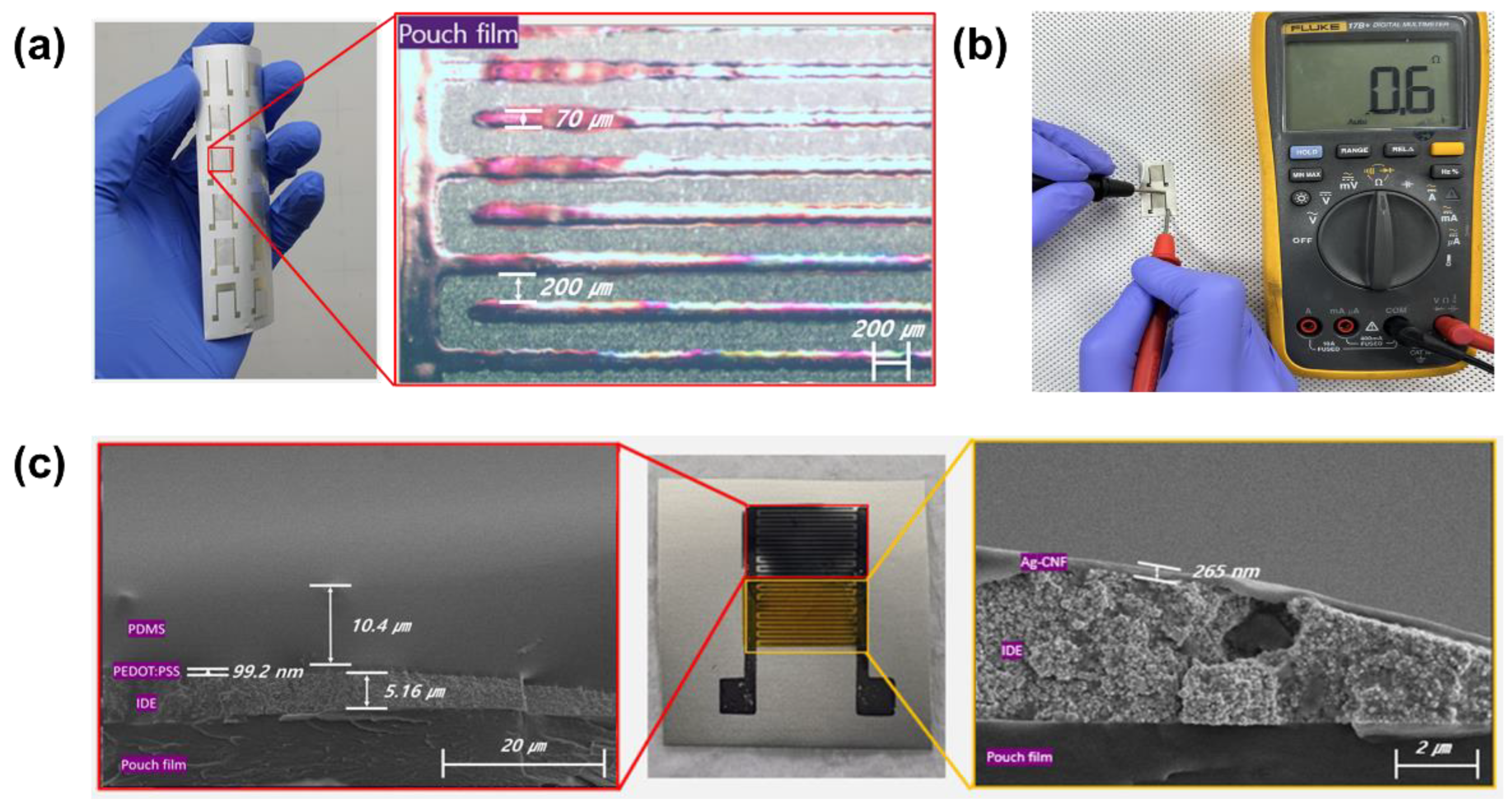
3.1. Fabricated Temperature–Humidity Sensors
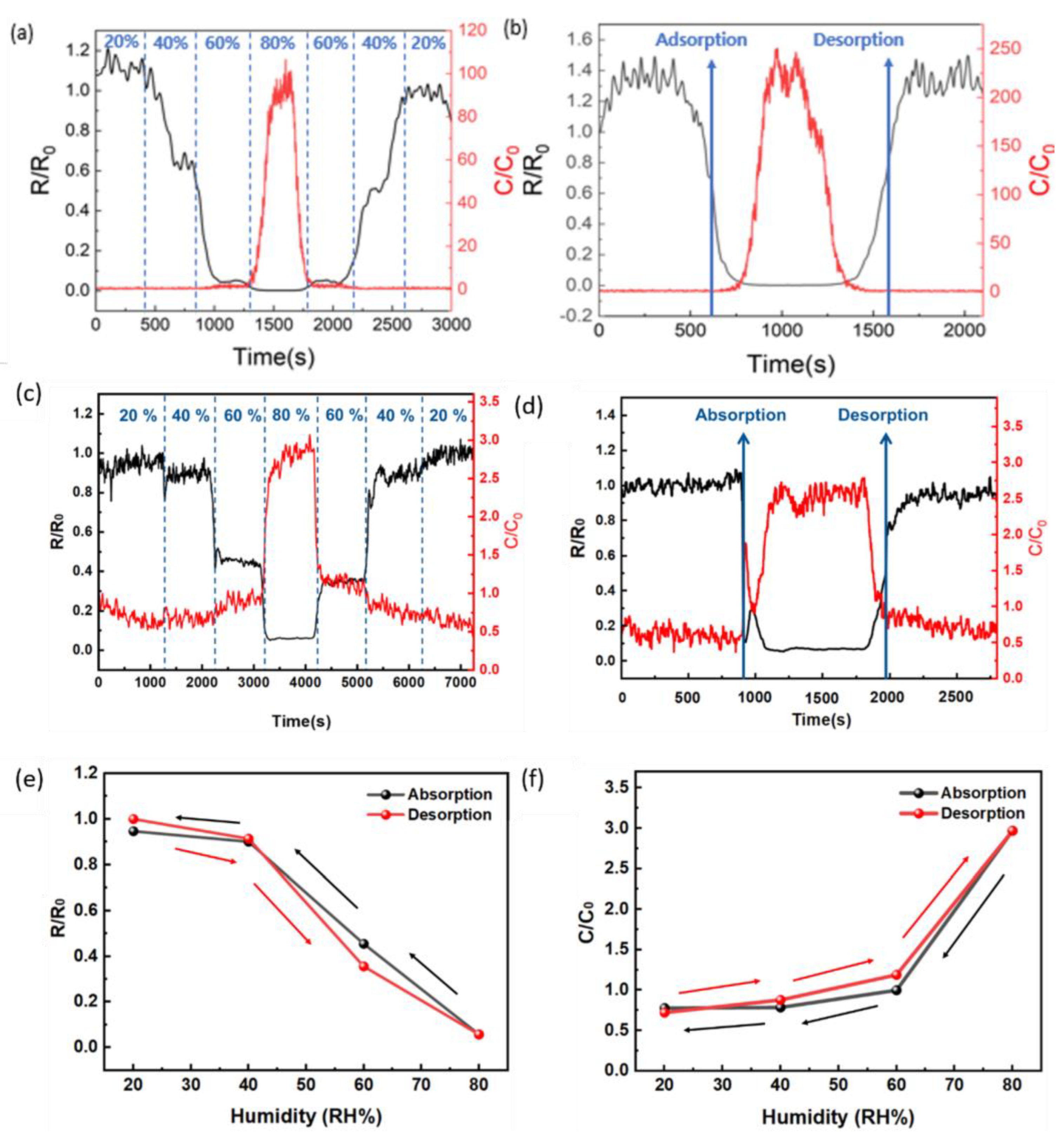
3.2. Performance and Material Analysis of Humidity Sensors
3.3. Performance and Material Analysis of Temperature Sensors
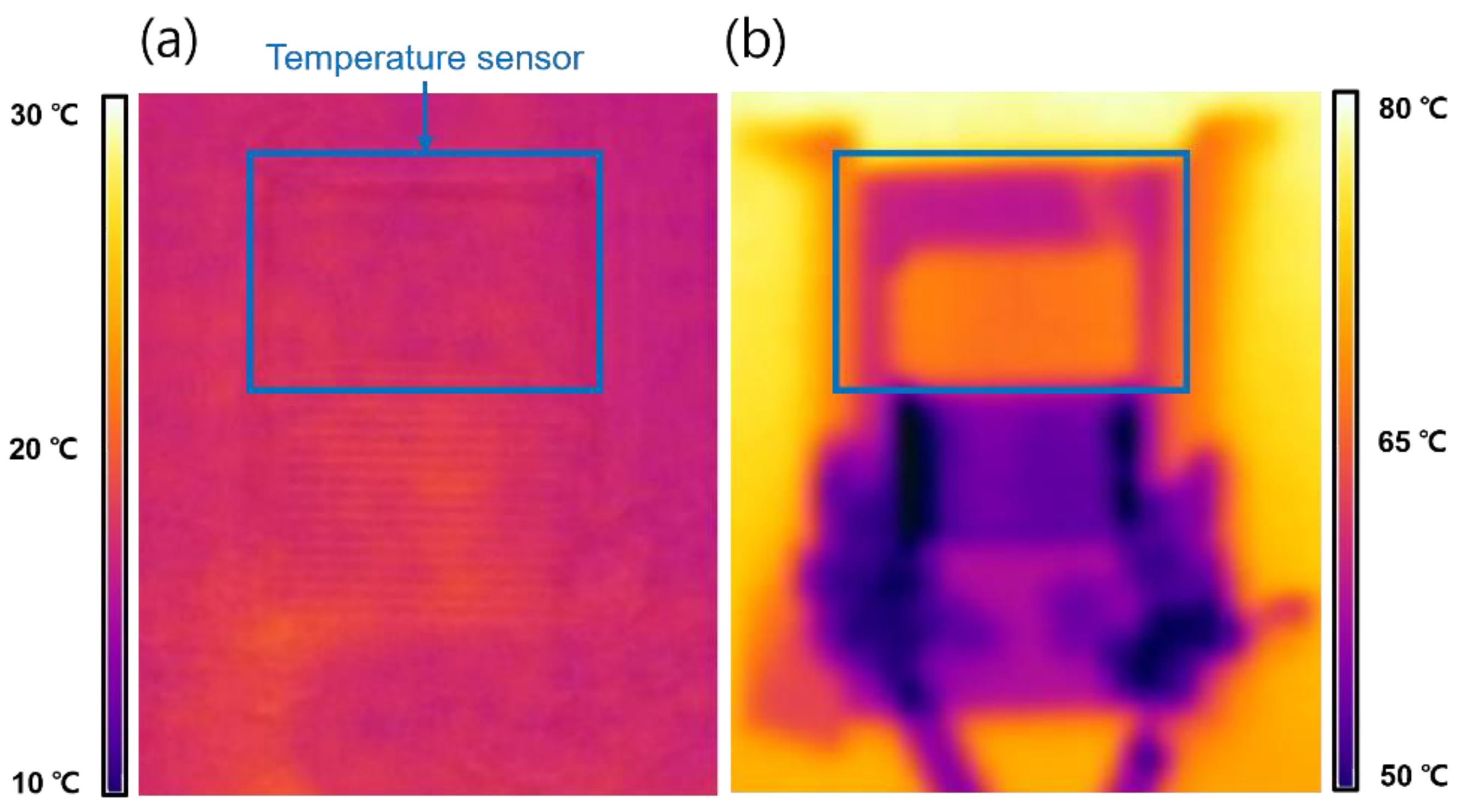
3.4. Performance of Integrated Temperature–Humidity Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament. Available online: www.europarl.europa.eu/RegData/etudes/STUD/2015/569964/IPOL_STU(2015)569964_EN.pdf (accessed on 11 November 2015).
- European Commission. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_3541 (accessed on 14 July 2021).
- Ruan, H.; Barreras, J.V.; Engstrom, T.; Merla, Y.; Millar, R.; Wu, B. Lithium-ion battery lifetime extension: A review of derating methods. J. Power Sources 2023, 563, 232805. [Google Scholar] [CrossRef]
- Che, Y.; Hu, X.; Lin, X.; Guo, J.; Teodorescu, R. Health prognostics for lithium-ion batteries: Mechanisms, methods, and prospects. Energy Environ. Sci. 2023, 16, 338–371. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, L.; Ge, S.; Wang, C.; Shaffer, C.E.; Rahn, C.D. In situ measurement of radial temperature distributions in cylindrical Li-ion cells. J. Electrochem. Soc. 2014, 161, A1499–A1507. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Walker, W.; Yayathi, S.; Shaw, J.; Ardebili, H. Thermo-electrochemical evaluation of lithium-ion batteries for space applications. J. Power Sources 2015, 298, 217–227. [Google Scholar] [CrossRef]
- Kleiner, J.; Stuckenberger, M.; Komsiyska, L.; Endisch, C. Advanced monitoring and prediction of the thermal state of intelligent battery cells in electric vehicles by physics-based and data-driven modeling. Batteries 2021, 7, 31. [Google Scholar] [CrossRef]
- Xia, G.; Cao, L.; Bi, G. A review on battery thermal management in electric vehicle application. J. Power Sources 2017, 367, 90–105. [Google Scholar] [CrossRef]
- Choudhari, V.G.; Dhoble, D.A.S.; Sathe, T.M. A review on effect of heat generation and various thermal management systems for lithium ion battery used for electric vehicle. J. Energy Storage 2020, 32, 101729. [Google Scholar] [CrossRef]
- Raijmakers, L.H.J.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. A review on various temperature-indication methods for Li-ion batteries. Appl. Energy 2019, 240, 918–945. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; He, H.; Ding, G.; Cui, H.; Liu, L. Future smart battery and management: Advanced sensing from external to embedded multi-dimensional measurement. J. Power Sources 2021, 489, 229462. [Google Scholar] [CrossRef]
- Apparatus of Detecting Thermal Runaway for Electric Vehicle. International Patent Application No. KR20220049142A, 14 October 2020.
- Hyundai Motor Company; Kia Corporation. Apparatus of Detecting Thermal Runaway for Electric Vehicle and Method Thereof. International Patent Application No. 1020200158502, 24 November 2020. [Google Scholar]
- Amphenol Sensing Korea LTD. Battery Temperature Sensor for Vehicles. International Patent Application No. 1021925480000, 11 December 2020. [Google Scholar]
- Amphenol Sensing Korea LTD. Liquid Detection Sensor of Battery Pack for Electric Vehicles. International Patent Application No. 1020512510000, 26 November 2019. [Google Scholar]
- High-Voltage Battery Coolant Leak Detection Method Using an Electric Vehicle Flow Sensor of Electric Vehicle. International Patent Application No. KR102447818B1, 28 September 2022.
- Katerinopoulou, D.; Zalar, P.; Sweelssen, J.; Kiriakidis, G.; Rentrop, C.; Groen, P.; Gelinck, G.H.; Brand, J.v.d.; Smits, E.-C.P. Large-Area All-Printed Temperature Sensing Surfaces Using Novel Composite Thermistor Materials. Adv. Electron. Mater. 2019, 5, 1800605. [Google Scholar] [CrossRef]
- Song, J.; Wei, Y.; Xu, M.; Gao, J.; Luo, L.; Wu, H.; Li, X.; Li, Y.; Wang, X. Highly Sensitive Flexible Temperature Sensor Made Using PEDOT:PSS/PANI. ACS Appl. Polym. Mater. 2022, 4, 766–772. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Marin-Sanchez, A.; Wicklein, B.; Salmeron, J.F.; Castillo, E.; Bobinger, M.; Salinas-Castillo, A. Cellulose nanofibers as substrate for flexible and biodegradable moisture sensors. Compos. Sci. Technol. 2021, 208, 108738. [Google Scholar] [CrossRef]
- Tachibana, S.; Wang, Y.F.; Sekine, T.; Takeda, Y.; Hong, J.; Yoshida, A.; Abe, M.; Miura, R.; Watanabe, Y.; Kumaki, D.; et al. A printed flexible humidity sensor with high sensitivity and fast response using a cellulose nanofiber/carbon black composite. ACS Appl. Mater. Interfaces 2022, 14, 5721–5728. [Google Scholar] [CrossRef] [PubMed]
- Makeen, P.; Ghali, H.A.; Memon, S.; Duan, F. Impacts of electric vehicle fast charging under dynamic temperature and humidity: Experimental and theoretically validated model analyses. Energy 2022, 261, 125335. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, X.; Yuan, S.; Wang, Y.; Xia, Y. Humidity effect on electrochemical performance of Li–O2 batteries. J. Power Sources 2014, 264, 1–7. [Google Scholar] [CrossRef]
- Vuorinen, T.; Niittynen, J.; Kankkunen, T.; Kraft, T.M.; Mäntysalo, M. Inkjet-Printed Graphene/PEDOT:PSS Temperature Sensors on a Skin-Conformable Polyurethane Substrate. Sci. Rep. 2016, 6, 35289. [Google Scholar] [CrossRef]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Hsiao, F.-R.; Liao, Y.-C. Printed micro-sensors for simultaneous temperature and humidity detection. IEEE Sensors J. 2018, 18, 6788–6793. [Google Scholar] [CrossRef]
- Khawaja, Y.; Shankar, N.; Qiqieh, I.; Alzubi, J.; Alzubi, O.; Nallakaruppan, M.K.; Padmanaban, S. Battery management solutions for li-ion batteries based on artificial intelligence. Ain Shams Eng. J. 2023, 14, 102213. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Sekine, T.; Takeda, Y.; Yokosawa, K.; Matsui, H.; Kumaki, D.; Shiba, T.; Nishikawa, T.; Tokito, S. Fully Printed PEDOT:PSS-based Temperature Sensor with High Humidity Stability for Wireless Healthcare Monitoring. Sci. Rep. 2020, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Sha, X.; Jing, D.; Ma, L. Thermal conductivity and rheology of graphene oxide nanofluids and a modified predication model. Appl. Sci. 2022, 12, 3567. [Google Scholar] [CrossRef]
- Liu, W.I.; Malekahmadi, O.; Bagherzadeh, S.A.; Ghashang, M.; Karimipour, A.; Hasani, S.; Tlili, I.; Goodarzi, M. A novel comprehensive experimental study concerned graphene oxide nanoparticles dispersed in water: Synthesise, characterisation, thermal conductivity measurement and present a new approach of RLSF neural network. Int. Commun. Heat Mass Transf. 2019, 109, 104333. [Google Scholar] [CrossRef]
- Kanti, P.K.; Maiya, M.P. Rheology and thermal conductivity of graphene oxide and coal fly ash hybrid nanofluids for various particle mixture ratios for heat transfer applications: Experimental study. Int. Commun. Heat Mass Transf. 2022, 138, 106408. [Google Scholar] [CrossRef]
- Mu, X.; Wu, X.; Zhang, T.; Go, D.B.; Luo, T. Thermal transport in graphene oxide–from ballistic extreme to amorphous limit. Sci. Rep. 2014, 4, 3909. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Jiang, Y.; Islam, M.N.; He, R.; Huang, X.; Cao, P.F.; Advincula, R.C.; Dahotre, N.; Dong, P.; Wu, H.F.; Choi, W. Recent advances in 3D printed sensors: Materials, design, and manufacturing. Adv. Mater. Technol. 2023, 8, 2200492. [Google Scholar] [CrossRef]
- Kim, D.S.; Bae, S.W.; Kim, D.H.; Cho, J.M.; Yu, M.S. Study of extrusion process for pouch film manufacturing. Int. J. Precis. Eng. Manuf. 2017, 18, 1307–1311. [Google Scholar] [CrossRef]
- Yu, M.S.; Kim, D.H.; Cho, J.M.; Bae, S.W.; Kim, D.S. Effect of film properties on formability of aluminum pouch for secondary battery. J. Mech. Sci. Technol. 2017, 31, 2759–2763. [Google Scholar] [CrossRef]
- Fu, L.H.; Gao, Q.L.; Qi, C.; Ma, M.G.; Li, J.F. Microwave-hydrothermal rapid synthesis of cellulose/Ag nanocomposites and their antibacterial activity. Nanomaterials 2018, 8, 978. [Google Scholar] [CrossRef]
- Maleki, A.; Movahed, H.; Ravaghi, P. Magnetic cellulose/Ag as a novel eco-friendly nanobiocomposite to catalyze synthesis of chromene-linked nicotinonitriles. Carbohydr. Polym. 2017, 156, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, D.; Wang, Y.; Shi, J.; Jiang, Z. Fabrication of antimicrobial bacterial cellulose–Ag/AgCl nanocomposite using bacteria as versatile biofactory. J. Nanoparticle Res. 2012, 14, 1084. [Google Scholar] [CrossRef]
- Xu, Y.; Zuo, L.; Lin, T.; Wang, J.; Yue, X. Preparation and characterization of cellulose/Ag nanocomposites. Polym. Compos. 2015, 36, 2220–2229. [Google Scholar] [CrossRef]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Green synthesis of silver nanoparticles using fruit extract of Malus domestica and study of its antimicrobial activity. Dig. J. Nanomater. Biostruct. 2014, 9, 1137–1147. [Google Scholar]
- Meng, Y. A sustainable approach to fabricating Ag nanoparticles/PVA hybrid nanofiber and its catalytic activity. Nanomaterials 2015, 5, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Bazmandeh, A.Z.; Rezaei, A.; Jafarbigloo, H.R.G.; Javar, A.M.A.; Hassanzadeh, A.; Amirian, A.; Nave, H.H.; Mehrabi, M. Synthesis and Characterization of Biocompatible Silver Nanoparticles using Stachys lavandulifolia Vahl. Extract and their antimicrobial performance study. J. Environ. Treat. Tech. 2020, 8, 284–290. [Google Scholar]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and highly sensitive humidity sensor based on cellulose nanofibers and carbon nanotube composite film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Håkansson, A.; Han, S.; Wang, S.; Lu, J.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X.; Fabiano, S. Effect of (3-glycidyloxypropyl) trimethoxysilane (GOPS) on the electrical properties of PEDOT: PSS films. J. Polym. Sci. B Polym. Phys. 2017, 55, 814–820. [Google Scholar] [CrossRef]
- Chao, L.; Xiaoyan, D.; Shuai, G.; Andrej, C.; Andres, O.; Yakun, H.; Karl, M.; Ning, L.; Christoph, J.B. A Cross-Linked Interconnecting Layer Enabling Reliable and Reproducible Solution-Processing of Organic Tandem Solar Cells. Adv. Energy Mater. 2020, 10, 12. [Google Scholar] [CrossRef]
- Osazuwa, P.O.; Lo, C.Y.; Feng, X.; Nolin, A.; Dhong, C.; Kayser, L.V. Surface Functionalization with (3-Glycidyloxypropyl)trimethoxysilane (GOPS) as an Alternative to Blending for Enhancing the Aqueous Stability and Electronic Performance of PEDOT:PSS Thin Films. ACS Appl. Mater. Interfaces 2023, 15, 54711–54720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 557. [Google Scholar] [CrossRef] [PubMed]






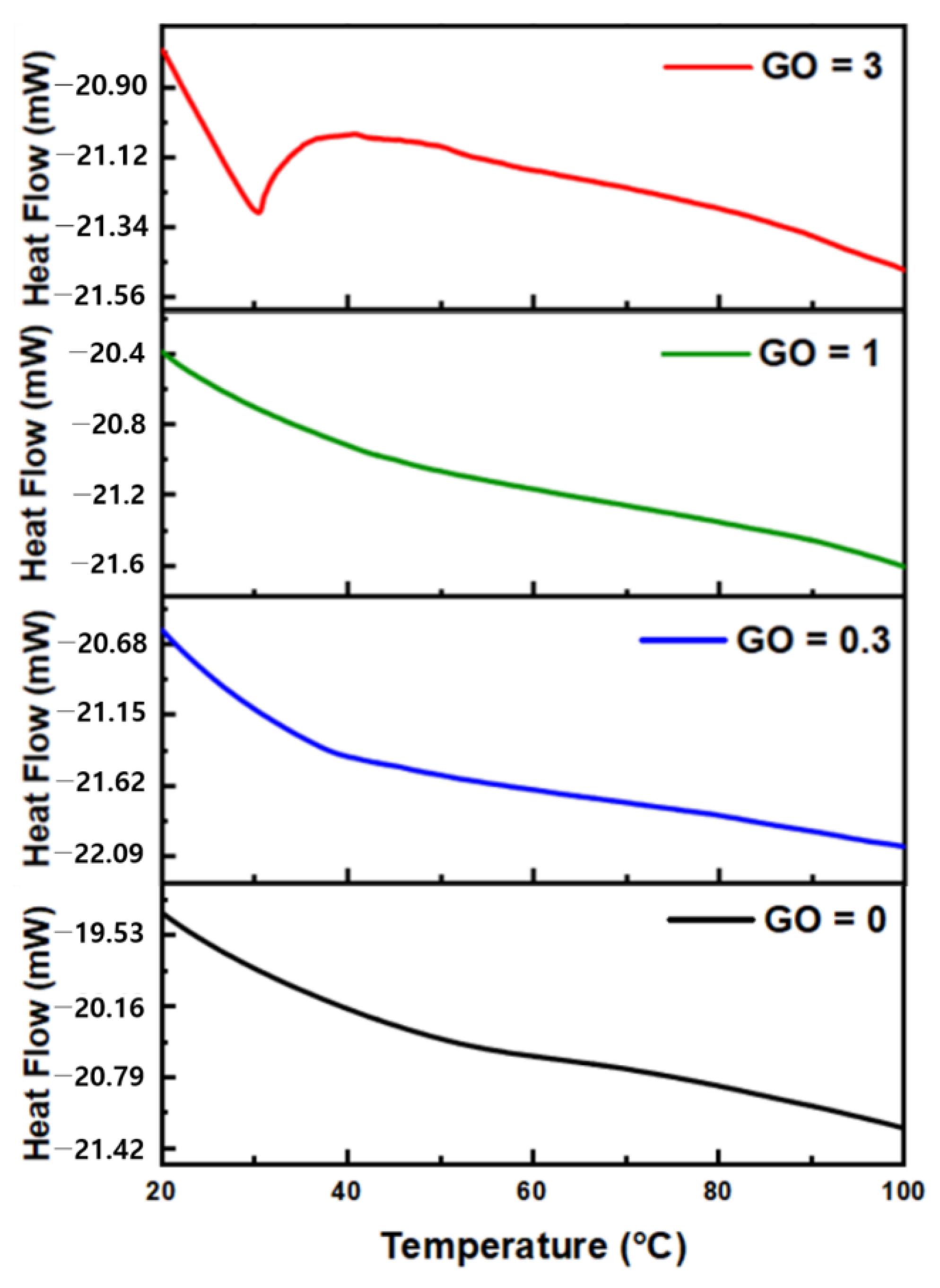


| GO = 0 | GO = 0.3 | GO = 1 | GO = 3 | |
|---|---|---|---|---|
| Response time (s) | 13.6 s | 12.8 s | 12.4 s | 10.6 s |
| Recovery time (s) | 15.6 s | 12.6 s | 11.6 s | 10.4 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, G.; Sim, J.-H.; Won, M.; Jung, M.; Mantry, S.P.; Kim, D.-S. Integrated Temperature–Humidity Sensors for a Pouch-Type Battery Using 100% Printing Process. Sensors 2024, 24, 104. https://doi.org/10.3390/s24010104
Oh G, Sim J-H, Won M, Jung M, Mantry SP, Kim D-S. Integrated Temperature–Humidity Sensors for a Pouch-Type Battery Using 100% Printing Process. Sensors. 2024; 24(1):104. https://doi.org/10.3390/s24010104
Chicago/Turabian StyleOh, Gyeongseok, Jae-Ho Sim, Mijin Won, Minhun Jung, Snigdha Paramita Mantry, and Dong-Soo Kim. 2024. "Integrated Temperature–Humidity Sensors for a Pouch-Type Battery Using 100% Printing Process" Sensors 24, no. 1: 104. https://doi.org/10.3390/s24010104





