Label-Free DNA Hybridization Detection Using a Highly Sensitive Fiber Microcavity Biosensor
Abstract
:1. Introduction
2. Materials and Methods
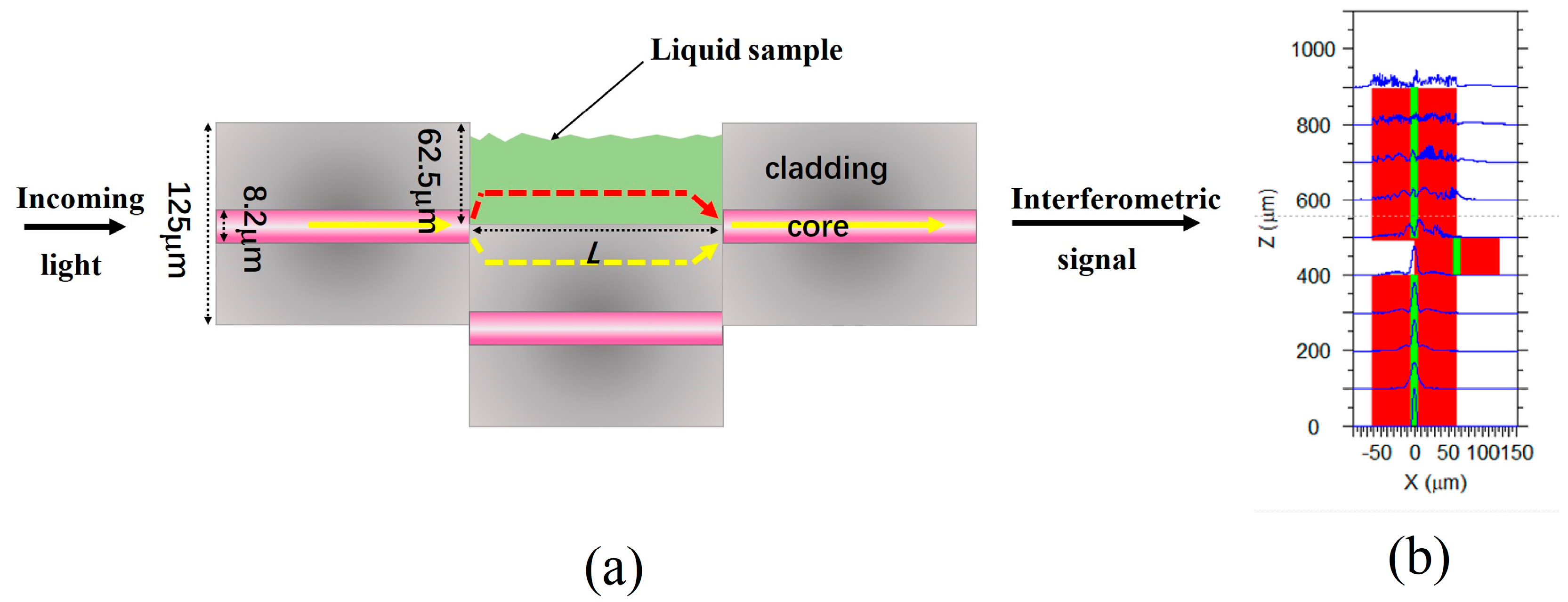
2.1. Sensing Principle and Fabrication of MZI Sensor
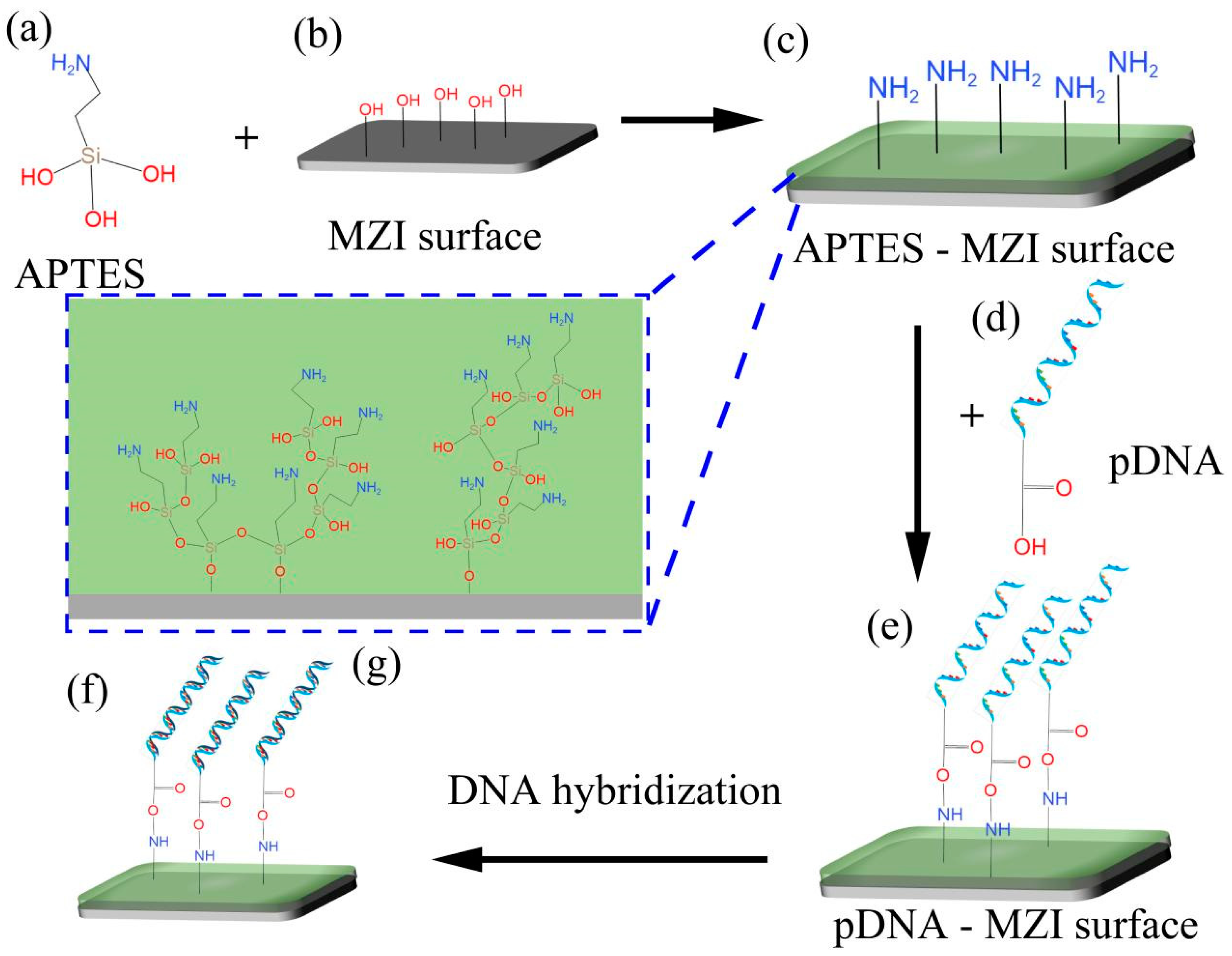
2.2. Materials
- NaOH (0.1 M; Sigma), 3-aminopropyl triethoxysilane (APTES 10% v/v; Cool Chemistry), TE buffer, pure water, and anhydrous ethanol.
- In this work, DNA was purchased from Sangon and these sequences are as follows:
- Proble DNA (pDNA):
- 5′-AGGAGGAGACTTAAGTAAAA-3′ (5′ was modified by carboxy (-COOH));
- Complementary DNA (cDNA):
- 5′-TTTTACTTAAGTCTCCTCCT-3′;
- Non-complementary DNA (nonDNA):
- 5′-CTCACGTTAATGCATTTTGGTC-3′;
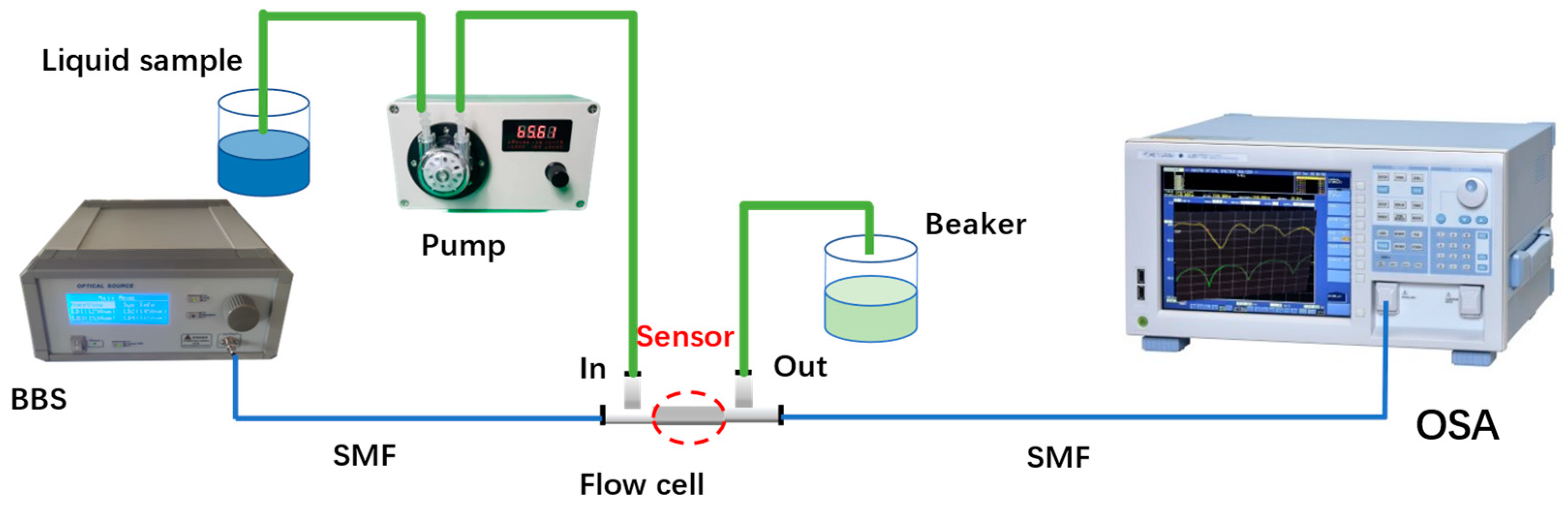
2.3. Experimental System
3. Results and Discussion
3.1. RI Sensitivity Measurement of the MZI Sensor
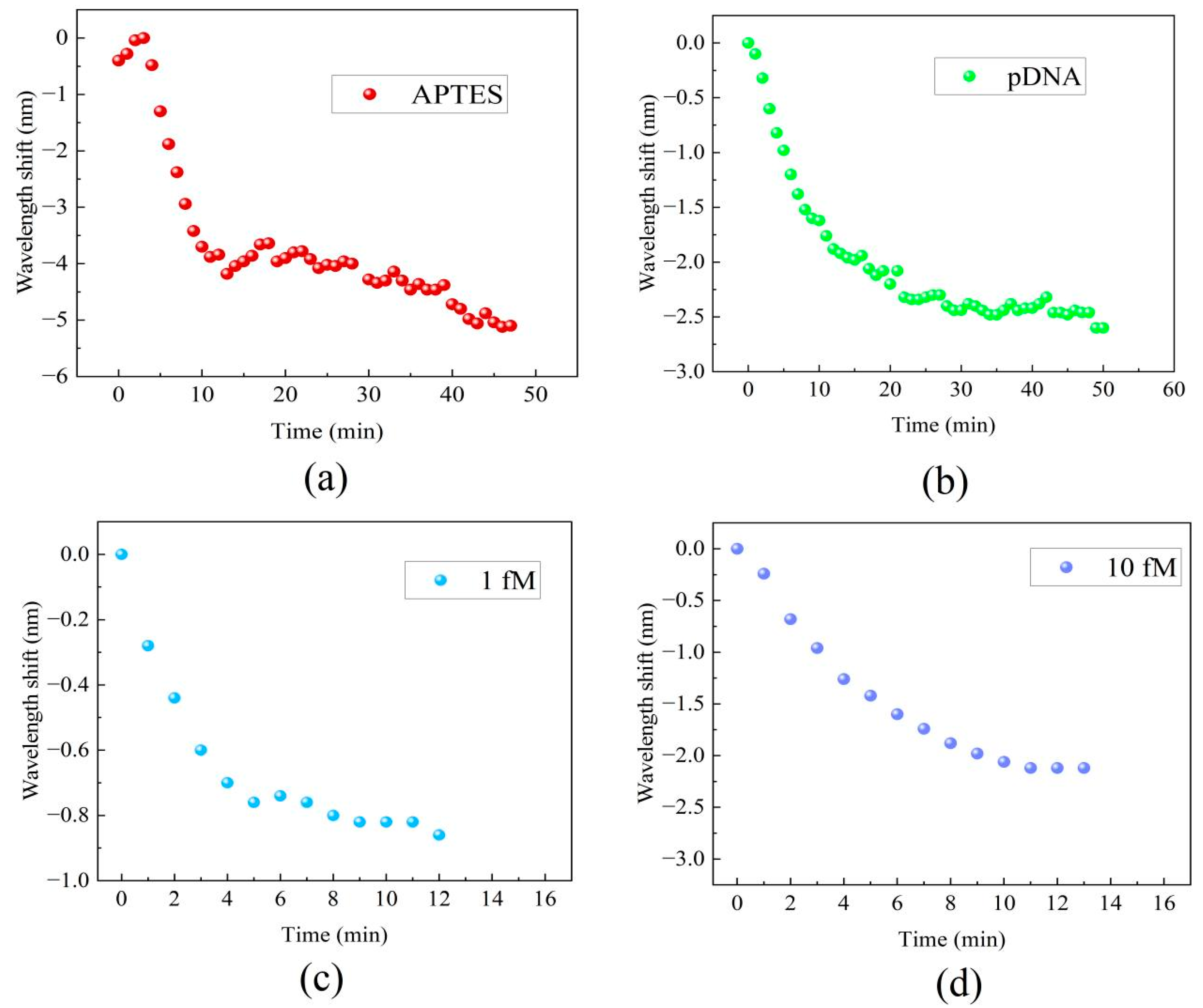
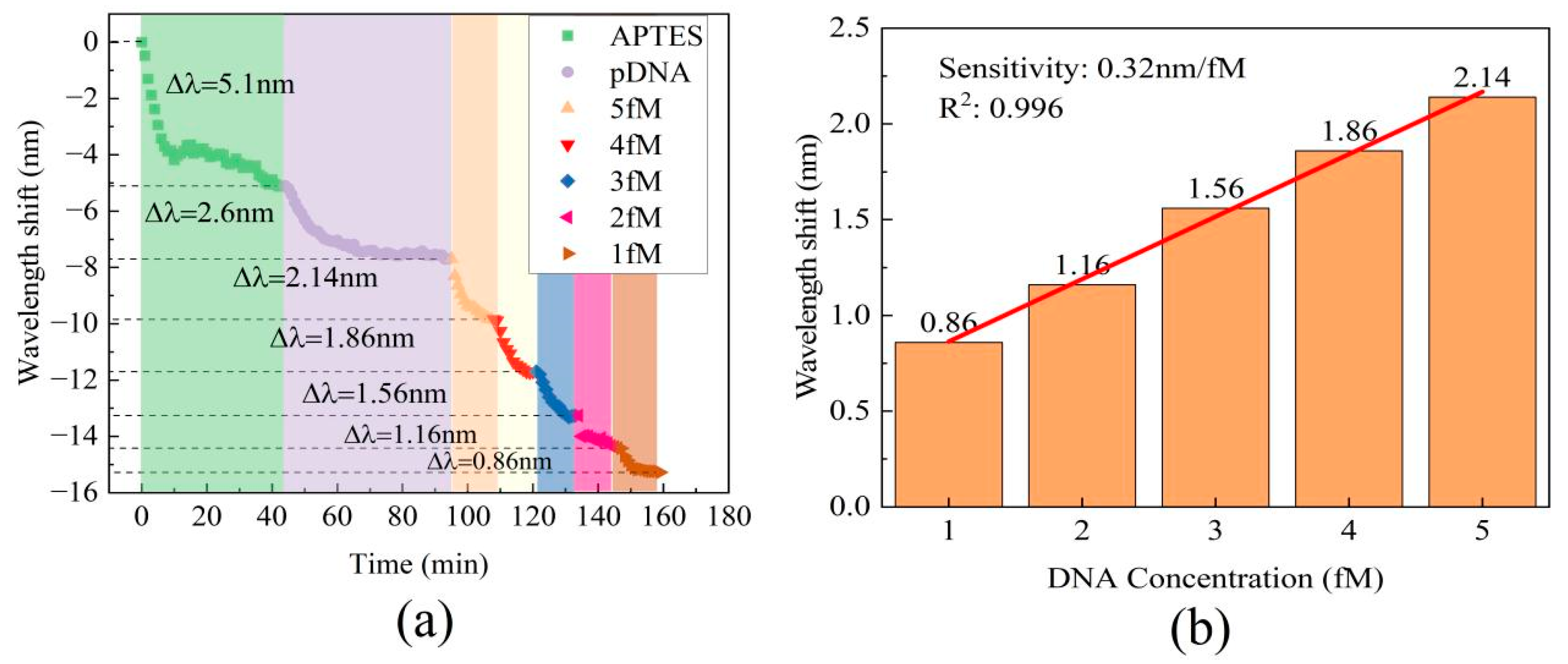
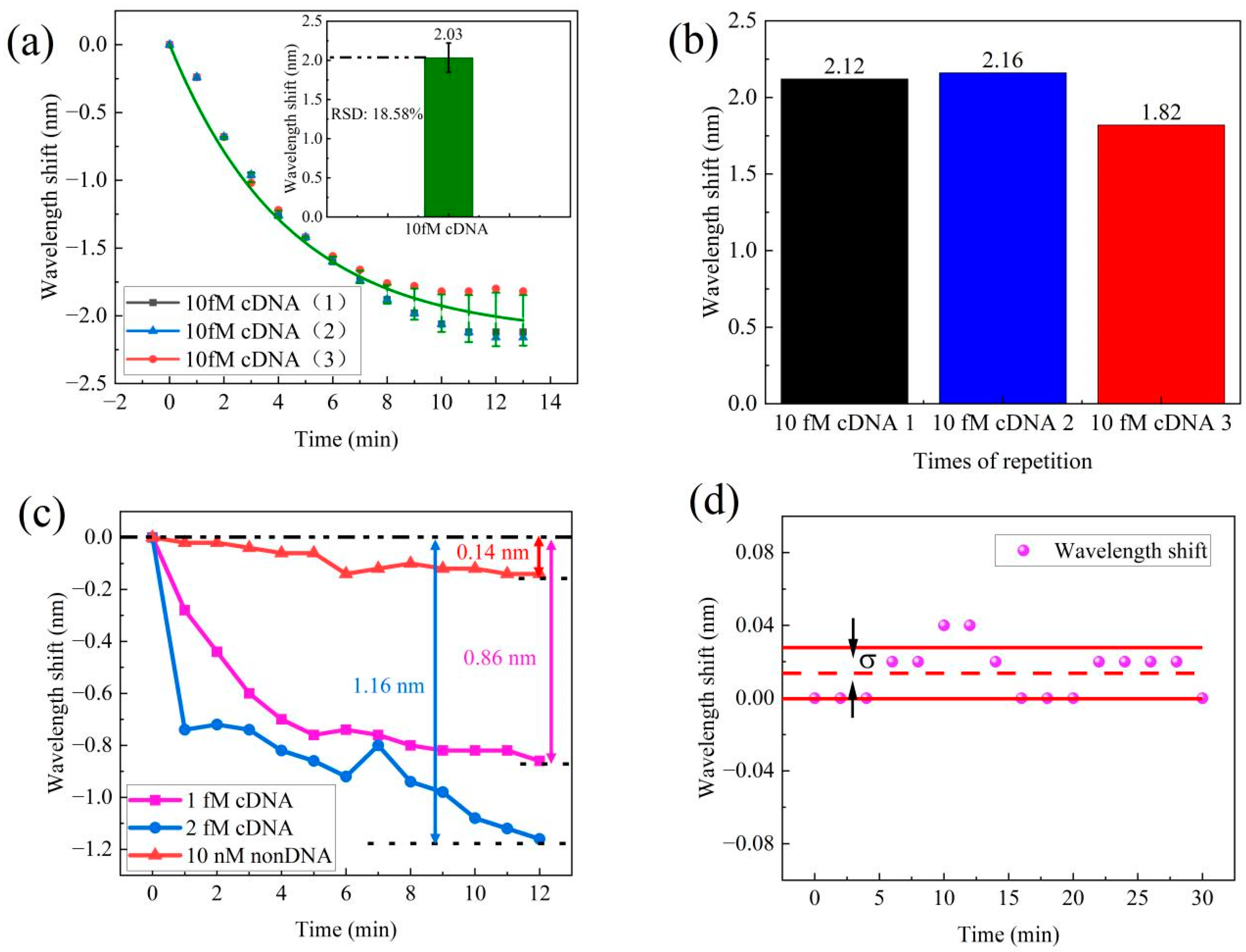
3.2. Results of Complementary DNA Hybridization Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henriksen, T.V.; Drue, S.O.; Frydendahl, A.; Demuth, C.; Rasmussen, M.H.; Reinert, T.; Pedersen, J.S.; Andersen, C.L. Error Characterization and Statistical Modeling Improves Circulating Tumor DNA Detection by Droplet Digital PCR. Clin. Chem. 2022, 68, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Su, T.; Shang, Z.; Jin, D.; Shu, Y.; Xu, Q.; Hu, X. Flexible paper-based Ni-MOF composite/AuNPs/CNTs film electrode for HIV DNA detection. Biosens. Bioelectron. 2021, 184, 113229. [Google Scholar] [CrossRef]
- Furlan, E.M.; Gleeson, D. Improving reliability in environmental DNA detection surveys through enhanced quality control. Mar. Freshw. Res. 2017, 68, 388. [Google Scholar] [CrossRef]
- Riva, F.; Bidard, F.-C.; Houy, A.; Saliou, A.; Madic, J.; Rampanou, A.; Hego, C.; Milder, M.; Cottu, P.; Sablin, M.-P.; et al. Patient-Specific Circulating Tumor DNA Detection during Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin. Chem. 2017, 63, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gong, X.; Xiang, Y.; Yuan, R.; Chai, Y. Quadratic recycling amplification for label-free and sensitive visual detection of HIV DNA. Biosens. Bioelectron. 2014, 55, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, E.E.; Torquato, F.; Frøslev, T.G.; Moore, A.B.M.; Sørensen, J.M.; Range, P.; Ben-Hamadou, R.; Bach, S.S.; Møller, P.R.; Thomsen, P.F. Using vertebrate environmental DNA from seawater in biomonitoring of marine habitats. Conserv. Biol. 2020, 34, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Antonopoulou, S.; Mantzana, P.; Georgiou, P.-C.; Vourli, S.; Protonotariou, E.; Vagiakou, E.; Skoura, L.; Pournaras, S.; Meletiadis, J. Can bronchial secretion cultures identify the etiologic agent of COVID-19-associated pulmonary aspergillosis in ICU patients? Comparison with a species-specific Aspergillus PCR in serum. Med. Mycol. 2023, 61, myac094. [Google Scholar] [CrossRef]
- Foo, P.C.; Nurul Najian, A.B.; Muhamad, N.A.; Ahamad, M.; Mohamed, M.; Yean Yean, C.; Lim, B.H. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: A comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 2020, 20, 34. [Google Scholar] [CrossRef]
- Zou, H.; Li, T.; Zhang, J.; Shao, H.; Kageyama, K.; Feng, W. Rapid detection of Colletotrichum siamense from infected tea plants using filter-disc DNA extraction and loop-mediated isothermal amplification. Plant Dis. 2023. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Shi, Y.; Wu, J.; Wu, J.; Chen, H. Selective endpoint visualized detection of Vibrio parahaemolyticus with CRISPR/Cas12a assisted PCR using thermal cycler for on-site application. Talanta 2020, 214, 120818. [Google Scholar] [CrossRef]
- Goncalves, H.M.R.; Moreira, L.; Pereira, L.; Jorge, P.; Gouveia, C.; Martins-Lopes, P.; Fernandes, J.R.A. Biosensor for label-free DNA quantification based on functionalized LPGs. Biosens. Bioelectron. 2016, 84, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Z.; Lin, X.; Nian, W.; Zheng, X.; Wu, J. Electrochemical Biosensors for Circulating Tumor DNA Detection. Biosensors 2022, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic Acid Detection Using CRISPR/Cas Biosensing Technologies. ACS Synth. Biol. 2020, 9, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, M.; Jiang, S.; Du, L.; Cheng, Y.; Li, P.; Wang, C. Design and fabrication of Gr/Ag-coated tilted grating sensor for ultra-sensitive detection of DNA hybridization. Sens. Actuators B-Chem. 2022, 359, 131587. [Google Scholar] [CrossRef]
- Leung, A.; Shankar, P.M.; Mutharasan, R. Label-free detection of DNA hybridization using gold-coated tapered fiber optic biosensors (TFOBS) in a flow cell at 1310 nm and 1550 nm. Sens. Actuators B-Chem. 2008, 131, 640–645. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-n.; Zheng, W.; Li, X.; Zhao, Y. Optical fiber SPR biosensor based on gold nanoparticle amplification for DNA hybridization detection. Talanta 2022, 247, 123599. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Manicardi, A.; Candiani, A.; Giannetti, S.; Cucinotta, A.; Spoto, G.; Konstantaki, M.; Pissadakis, S.; Selleri, S.; Corradini, R. Detection of unamplified genomic DNA by a PNA-based microstructured optical fiber (MOF) Bragg-grating optofluidic system. Biosens. Bioelectron. 2015, 63, 248–254. [Google Scholar] [CrossRef]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.-O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Chee, H.Y.; Rashid, S.A.; Ahmad, M.Z.; Abu Bakar, M.H.; Mahdi, M.A.; Yaacob, M.H. Carbon quantum dots functionalized tapered optical fiber for highly sensitive and specific detection of Leptospira DNA. Opt. Laser Technol. 2023, 157, 108696. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Zhou, X.; Zhang, Y.; Zhao, Y.; Linh Viet, N.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C. In-situ DNA detection with an interferometric-type optical sensor based on tapered exposed core microstructured optical fiber. Sens. Actuators B-Chem. 2022, 351, 130942. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Zhou, X.; Gong, P.; Zhang, Y.; Zhao, Y.; Nguyen, L.V.; Ebendorff-Heidepriem, H.; Warren-Smith, S.C. Plug-in label-free optical fiber DNA hybridization sensor based on C-type fiber Vernier effect. Sens. Actuators B-Chem. 2022, 354, 131212. [Google Scholar] [CrossRef]
- Janik, M.; Hamidi, S.V.; Koba, M.; Perreault, J.; Walsh, R.; Bock, W.J.; Smietana, M. Real-time isothermal DNA amplification monitoring in picoliter volumes using an optical fiber sensor. Lab A Chip 2021, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Jiang, J.; Yao, L.; Dai, Z.; Liu, Z.; Qu, H.; Hu, X. Ultrasensitive cascaded in-line Fabry-Perot refractometers based on a C-shaped fiber and the Vernier effect. Opt. Express 2022, 30, 27704–27714. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.-W.; Rao, Y.-J.; Xu, L.-C.; Zhu, T.; Wu, D.; Yao, J. In-fiber Mach–Zehnder interferometer formed by large lateral offset fusion splicing for gases refractive index measurement with high sensitivity. Sens. Actuators B Chem. 2011, 160, 1198–1202. [Google Scholar] [CrossRef]
- Śmietana, M.; Janik, M.; Koba, M.; Bock, W.J. Transition between bulk and surface refractive index sensitivity of micro-cavity in-line Mach-Zehnder interferometer induced by thin film deposition. Opt. Express 2017, 25, 26118–26123. [Google Scholar] [CrossRef] [PubMed]
- Parandin, F.; Heidari, F.; Rahimi, Z.; Olyaee, S. Two-Dimensional photonic crystal Biosensors: A review. Opt. Laser Technol. 2021, 144, 107397. [Google Scholar] [CrossRef]
- Zito, G.; Sanità, G.; Guilcapi Alulema, B.; Lara Yépez, S.N.; Lanzio, V.; Riminucci, F.; Cabrini, S.; Moccia, M.; Avitabile, C.; Lamberti, A.; et al. Label-free DNA biosensing by topological light confinement. Nanophotonics 2021, 10, 4279–4287. [Google Scholar] [CrossRef]
- Romano, S.; Zito, G.; Torino, S.; Calafiore, G.; Penzo, E.; Coppola, G.; Cabrini, S.; Rendina, I.; Mocella, V. Label-free sensing of ultralow-weight molecules with all-dielectric metasurfaces supporting bound states in the continuum. Photonics Res. 2018, 6, 726–733. [Google Scholar] [CrossRef]
- O’Mahony, T.F.; Morris, M.A. Hydroxylation methods for mesoporous silica and their impact on surface functionalisation. Microporous Mesoporous Mater. 2021, 317, 110989. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Chee, H.Y.; Ahmad, M.Z.; Mahdi, M.A.; Abu Bakar, M.H.; Yaacob, M.H. Sensitive Leptospira DNA detection using tapered optical fiber sensor. J. Biophotonics 2018, 11, e201700363. [Google Scholar] [CrossRef]
- Zhuravlev, L. Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir 1987, 3, 316–318. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Li, H.; Han, D.; Li, X.; Wang, F.; Gao, J.; Geng, C.; Zhang, Z.; Cui, C.; et al. Aminosilane Molecular Layer Enables Successive Capture-Diffusion-Deposition of Ions toward Reversible Zinc Electrochemistry. ACS Nano 2023, 17, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular Structure of 3-Aminopropyltriethoxysilane Layers Formed on Silanol-Terminated Silicon Surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Zhang, Y.-n.; Zhao, Y.; Lv, R.-Q. A review for optical sensors based on photonic crystal cavities. Sens. Actuators A Phys. 2015, 233, 374–389. [Google Scholar] [CrossRef]
- Song, B.; Zhang, H.; Liu, B.; Lin, W.; Wu, J. Label-free in-situ real-time DNA hybridization kinetics detection employing microfiber-assisted Mach-Zehnder interferometer. Biosens. Bioelectron. 2016, 81, 151–158. [Google Scholar] [CrossRef]

| Structure | RI Sensitivity | LOD | Ref. |
|---|---|---|---|
| Tapered optical fiber | 2235 nm/RIU | 1 fM | [19] |
| ECF + MZI | 1771 nm/RIU | 0.31 nM | [20] |
| C-type fiber + SMF | 10,791 nm/RIU | 67.5 nM | [21] |
| Microfiber-assisted MZI | −11,571 nm/RIU | 0.0001 pmol/μL | [37] |
| SMF + MZI | −17,905 nm/RIU | 48.9 aM | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, G.; Yu, X.; Fan, Y.; Chen, X.; Liu, S. Label-Free DNA Hybridization Detection Using a Highly Sensitive Fiber Microcavity Biosensor. Sensors 2024, 24, 278. https://doi.org/10.3390/s24010278
Wu Y, Wang G, Yu X, Fan Y, Chen X, Liu S. Label-Free DNA Hybridization Detection Using a Highly Sensitive Fiber Microcavity Biosensor. Sensors. 2024; 24(1):278. https://doi.org/10.3390/s24010278
Chicago/Turabian StyleWu, Yao, Guiyu Wang, Xiujuan Yu, Yuanji Fan, Xuefeng Chen, and Shengchun Liu. 2024. "Label-Free DNA Hybridization Detection Using a Highly Sensitive Fiber Microcavity Biosensor" Sensors 24, no. 1: 278. https://doi.org/10.3390/s24010278
APA StyleWu, Y., Wang, G., Yu, X., Fan, Y., Chen, X., & Liu, S. (2024). Label-Free DNA Hybridization Detection Using a Highly Sensitive Fiber Microcavity Biosensor. Sensors, 24(1), 278. https://doi.org/10.3390/s24010278






