Visual and Quantitative Evaluation of Low-Concentration Bismuth in Dual-Contrast Imaging of Iodine and Bismuth Using Clinical Photon-Counting CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bismuth Sample Preparation and Phantom Design
2.2. PCCT Data Acquisition and Reconstruction
2.3. Material Decomposition
2.4. Qualitative and Quantitative Analysis
3. Results
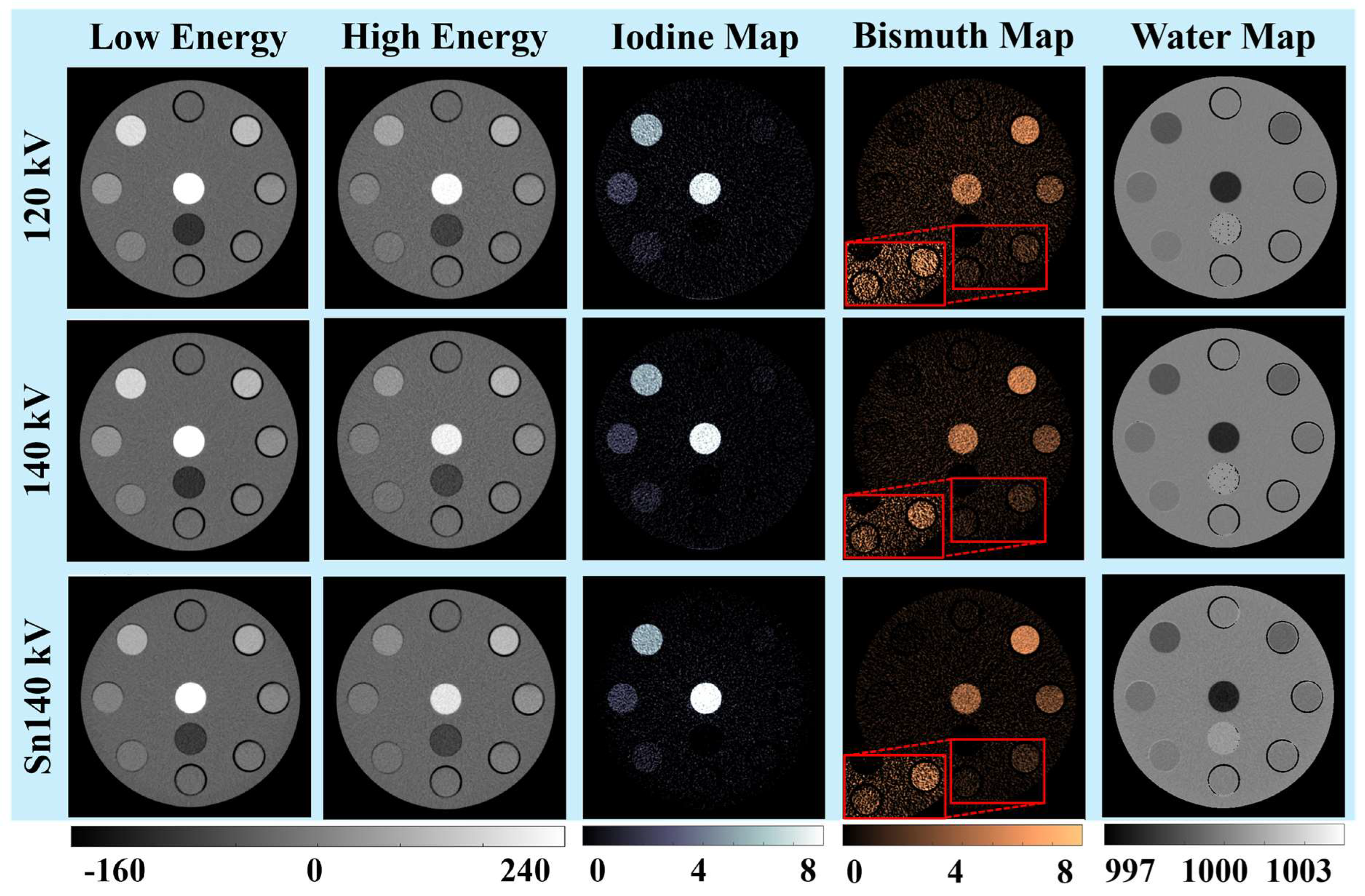
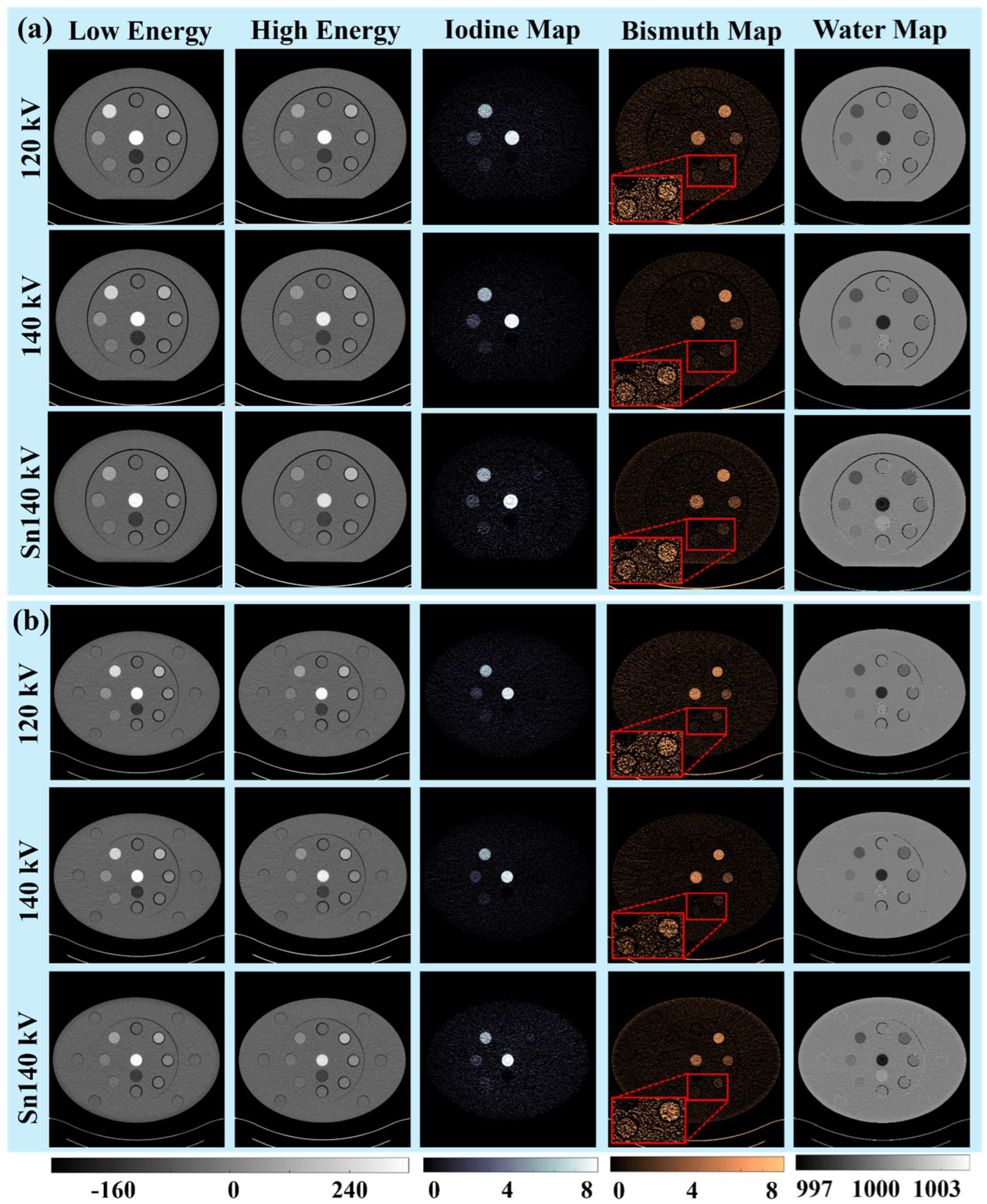
3.1. Visual and Qualitative Evaluations
3.1.1. Iodine and Bismuth Separations
3.1.2. Signals and Noises
3.1.3. Low-Concentration Materials
3.2. Quantitative Evaluations
3.2.1. Quantitative Measurements and Optimal PCCT Tube Potential
3.2.2. Low-Concentration Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, N.G.; Butler, A.P.; Scott, N.J.; Cook, N.J.; Butzer, J.S.; Schleich, N.; Firsching, M.; Grasset, R.; de Ruiter, N.; Campbell, M.; et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur. Radiol. 2010, 20, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119. [Google Scholar] [CrossRef] [PubMed]
- Dangelmaier, J.; Bar-Ness, D.; Daerr, H.; Muenzel, D.; Si-Mohamed, S.; Ehn, S.; Fingerle, A.A.; Kimm, M.A.; Kopp, F.K.; Boussel, L.; et al. Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic repair. Eur. Radiol. 2018, 28, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Cork, T.E.; Lakshmanan, M.N.; Evers, R.; Davies-Venn, C.; Rice, K.A.; Thomas, M.L.; Liu, C.Y.; Kappler, S.; Ulzheimer, S.; et al. Dual-contrast agent photon-counting computed tomography of the heart: Initial experience. Int. J. Cardiovasc. Imaging 2017, 33, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Krauss, B.; Sahbaee, P.; Cork, T.E.; Lakshmanan, M.N.; Bluemke, D.A.; Pourmorteza, A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 2017, 44, 5120–5127. [Google Scholar] [CrossRef] [PubMed]
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J.; Rathnayake, S.; Fu, Y.; Gao, D.-W.; Yeh, B.M. Extravasated contrast material in penetrating abdominopelvic trauma: Dual-contrast dual-energy CT for improved diagnosis—Preliminary results in an animal model. Radiology 2013, 268, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J.; Rathnayake, S.; Fu, Y.; Wang, R.; Jones, E.F.; Gao, D.W.; Yeh, B.M. In vivo differentiation of complementary contrast media at dual-energy CT. Radiology 2012, 265, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, S.; Lin, W.C.; Wang, Z.J.; Behr, S.C.; Westphalen, A.C.; Yeh, B.M. Comparison of Positive Oral Contrast Agents for Abdominopelvic CT. AJR Am. J. Roentgenol. 2019, 212, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Rajendran, K.; Fletcher, J.G.; McCollough, C.H.; Yu, L. Simultaneous Dual-contrast Imaging of Small Bowel with Iodine and Bismuth using Photon-counting-detector CT: A Feasibility Animal Study. Investig. Radiol. 2020, 55, 688. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ehman, E.; Fletcher, J.G.; Huprich, J.E.; Hara, A.K.; Silva, A.C.; Farrugia, G.; Limburg, P.; McCollough, C.H. Toward biphasic computed tomography (CT) enteric contrast: Material classification of luminal bismuth and mural iodine in a small-bowel phantom using dual-energy CT. J. Comput. Assist. Tomogr. 2012, 36, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Thivolet, A.; Bonnot, P.E.; Bar-Ness, D.; Kepenekian, V.; Cormode, D.P.; Douek, P.; Rousset, P. Improved Peritoneal Cavity and Abdominal Organ Imaging Using a Biphasic Contrast Agent Protocol and Spectral Photon Counting Computed Tomography K-Edge Imaging. Investig. Radiol. 2018, 53, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, L.; Rajendran, K.; Diehn, F.E.; Fletcher, J.G.; McCollough, C.H.; Yu, L. Simultaneous dual-contrast imaging using energy-integrating detector multi-energy CT: An in vivo feasibility study. Med. Phys. 2022, 49, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L. First clinical photon-counting detector CT system: Technical evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Rajendran, K.; McCollough, C.H.; Yu, L. Quantitative accuracy and dose efficiency of dual-contrast imaging using dual-energy CT: A phantom study. Med. Phys. 2020, 47, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tao, S.; Rajendran, K.; McCollough, C.H.; Yu, L. Impact of prior information on material decomposition in dual- and multienergy computed tomography. J. Med. Imaging 2019, 6, 013503. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Rajendran, K.; McCollough, C.H.; Leng, S. Feasibility of multi-contrast imaging on dual-source photon counting detector (PCD) CT: An initial phantom study. Med. Phys. 2019, 46, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Huang, R.; Tao, S.; Michalak, G.J.; McCollough, C.H.; Leng, S. Dual-source photon counting detector CT with a tin filter: A phantom study on iodine quantification performance. Phys. Med. Biol. 2019, 64, 115019. [Google Scholar] [CrossRef] [PubMed]
- Soesbe, T.C.; Xi, Y.; Nasr, K.; Leyendecker, J.R.; Lenkinski, R.E.; Lewis, M.A. Investigating new CT contrast agents: A phantom study exploring quantification and differentiation methods for high-Z elements using dual-energy CT. Eur. Radiol. 2021, 31, 8060–8067. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Niehoff, J.H. Bone Marrow Edema at Photon-Counting CT. Radiology 2024, 310, e231878. [Google Scholar] [CrossRef] [PubMed]



| Scanner Model | PCCT (NAEOTOM Alpha) | |||
|---|---|---|---|---|
| Scan | Tube Voltage (kV) | 120 | 140 | Sn140 |
| Collimation (mm) | 144 × 0.4 | |||
| Rotation Time (s) | 0.5 | |||
| Pitch | 0.6 | |||
| CTDIvol (mGy) | Small/Medium/Large: 3/8/12 mGy | |||
| Reconstruction | Image Type | Low-/High-Energy Images | ||
| Slice Thickness (mm) | 3.0 * | |||
| Field of View (mm) | Small/Medium/Large: 220/350/420 | |||
| Algorithm | Quantum Iterative Reconstruction (QIR-3) | |||
| Reconstruction Kernel | Qr40 | |||
| Phantom Size | Nominal Concentration (mg/mL) | I (1.0) Bi (0.0) | I (2.0) Bi (0.0) | I (5.0) Bi (0.0) | I (0.0) Bi (0.6) | I (0.0) Bi (1.3) | I (0.0) Bi (2.5) | I (0.0) Bi (5.1) | Range of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Small | 120 kV | 0.87 ± 0.94 0.24 ± 1.19 | 1.90 ± 0.96 0.11 ± 1.20 | 5.31 ± 0.87 −0.37 ± 1.08 | −0.33 ± 0.84 0.87 ± 1.07 | −0.26 ± 0.92 1.47 ± 1.16 | −0.10 ± 0.79 2.58 ± 0.98 | 0.30 ± 0.83 4.74 ± 1.03 | [−0.33, 0.31] [−0.37, 0.24] |
| 140 kV | 0.99 ± 0.73 0.08 ± 0.87 | 1.89 ± 0.72 0.11 ± 0.83 | 5.12 ± 0.71 −0.13 ± 0.83 | −0.32 ± 0.66 0.78 ± 0.78 | −0.18 ± 0.72 1.31 ± 0.82 | −0.06 ± 0.71 2.52 ± 0.81 | 0.25 ± 0.75 4.84 ± 0.88 | [−0.32, 0.25] [−0.21, 0.15] | |
| Sn140 kV | 0.75 ± 0.82 0.14 ± 0.64 | 1.78 ± 0.79 0.09 ± 0.63 | 5.05 ± 0.68 0.00 ± 0.57 | −0.42 ± 0.91 0.69 ± 0.72 | −0.28 ± 0.89 1.27 ± 0.70 | −0.13 ± 0.82 2.52 ± 0.69 | 0.15 ± 0.76 5.00 ± 0.59 | [−0.42, 0.15] [−0.05, 0.14] | |
| Medium | 120 kV | 0.89 ± 1.18 0.05 ± 1.50 | 1.87 ± 1.22 0.10 ± 1.53 | 5.46 ± 1.12 −0.44 ± 1.49 | −0.30 ± 1.22 0.92 ± 1.63 | −0.16 ± 1.07 1.22 ± 1.45 | −0.05 ± 1.12 2.41 ± 0.44 | 0.21 ± 1.15 4.91 ± 1.46 | [−0.30, 0.46] [−0.44, 0.29] |
| 140 kV | 0.94 ± 1.03 −0.04 ± 1.21 | 2.08 ± 1.04 −0.17 ± 1.15 | 5.33 ± 0.96 −0.21 ± 1.10 | −0.25 ± 0.97 0.75 ± 1.18 | 0.04 ± 1.01 0.91 ± 1.21 | 0.01 ± 0.97 2.32 ± 1.11 | 0.19 ± 0.92 4.96 ± 1.06 | [−0.25, 0.33] [−0.35, 0.12] | |
| Sn140 kV | 0.76 ± 1.70 0.14 ± 1.25 | 1.77 ± 1.50 0.06 ± 1.04 | 5.17 ± 1.44 −0.10 ± 1.06 | −0.26 ± 1.53 0.69 ± 1.12 | −0.28 ± 1.64 1.15 ± 1.16 | −0.04 ± 1.64 2.40 ± 1.21 | 0.49 ± 1.53 4.94 ± 1.08 | [−0.28, 0.49] [−0.13, 0.14] | |
| Large | 120 kV | 0.88 ± 1.08 0.44 ± 1.38 | 1.78 ± 1.16 0.01 ± 1.53 | 5.39 ± 1.17 −0.28 ± 1.50 | 0.05 ± 1.16 0.9 ± 1.48 | −0.20 ± 1.21 1.33 ± 1.67 | −0.19 ± 1.04 2.58 ± 1.31 | −0.05 ± 1.08 5.20 ± 1.42 | [−0.22, 0.39] [−0.34, 0.15] |
| 140 kV | 0.76 ± 0.96 0.05 ± 1.16 | 1.49 ± 1.02 0.29 ± 1.22 | 5.13 ± 0.98 −0.08 ± 1.21 | 0.01 ± 0.95 0.30 ± 1.14 | −0.01 ± 0.88 1.05 ± 1.11 | −0.21 ± 0.92 2.54 ± 1.13 | 0.09 ± 0.93 5.04 ± 1.07 | [−0.51, 0.13] [−0.33, 0.29] | |
| Sn140 kV | 0.59 ± 1.84 0.22 ± 1.26 | 1.44 ± 1.67 0.18 ± 1.13 | 5.20 ± 1.86 −0.13 ± 1.28 | −0.10 ± 1.76 0.40 ± 1.22 | −0.32 ± 1.75 1.30 ± 1.20 | −0.15 ± 1.75 2.48 ± 1.21 | 0.42 ± 2.04 4.93 ± 1.39 | [−0.56, 0.42] [−0.24, 0.22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataei, A.; Vasan, V.; Soesbe, T.C.; Brewington, C.C.; Zhou, Z.; Yu, L.; Hallam, K.A.; Ren, L. Visual and Quantitative Evaluation of Low-Concentration Bismuth in Dual-Contrast Imaging of Iodine and Bismuth Using Clinical Photon-Counting CT. Sensors 2024, 24, 3567. https://doi.org/10.3390/s24113567
Ataei A, Vasan V, Soesbe TC, Brewington CC, Zhou Z, Yu L, Hallam KA, Ren L. Visual and Quantitative Evaluation of Low-Concentration Bismuth in Dual-Contrast Imaging of Iodine and Bismuth Using Clinical Photon-Counting CT. Sensors. 2024; 24(11):3567. https://doi.org/10.3390/s24113567
Chicago/Turabian StyleAtaei, Afrouz, Vasantha Vasan, Todd C. Soesbe, Cecelia C. Brewington, Zhongxing Zhou, Lifeng Yu, Kristina A. Hallam, and Liqiang Ren. 2024. "Visual and Quantitative Evaluation of Low-Concentration Bismuth in Dual-Contrast Imaging of Iodine and Bismuth Using Clinical Photon-Counting CT" Sensors 24, no. 11: 3567. https://doi.org/10.3390/s24113567





