Individual Variability in Brain Connectivity Patterns and Driving-Fatigue Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
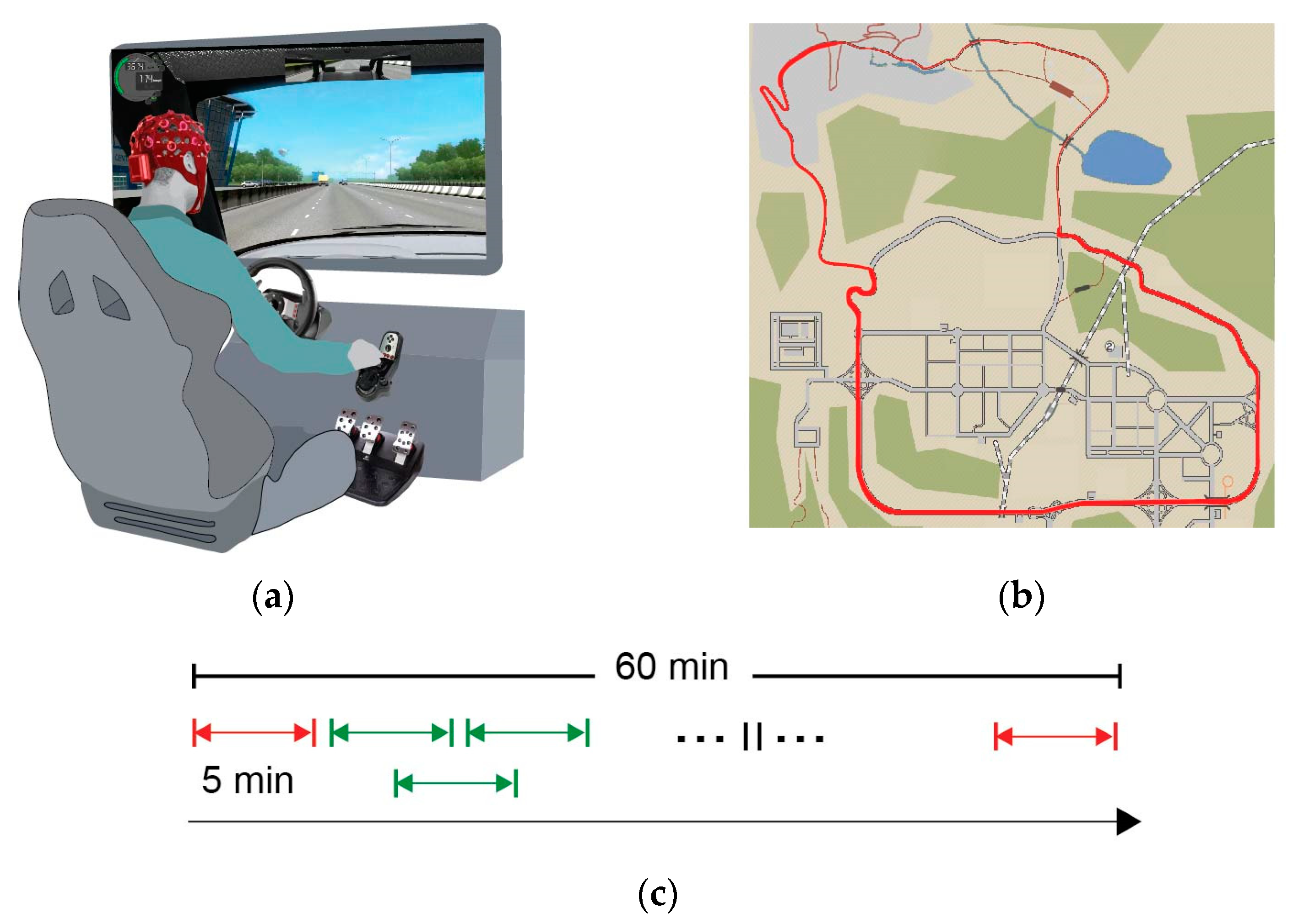
2.2. Experimental Design
2.3. Behavioral Assessment
2.4. EEG Acquisition and Pre-Processing
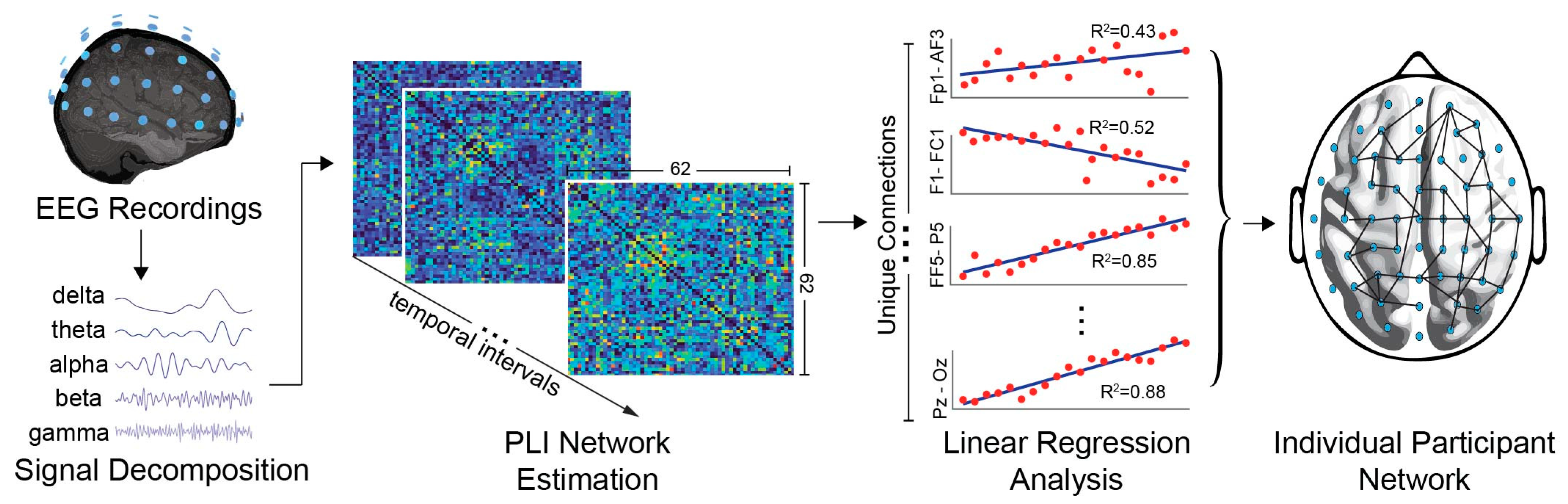
2.5. Functional Connectivity Based on PLI Networks
2.6. Individual and Global Network Analysis
2.7. Network Assessment
3. Results
3.1. Behavioral Results
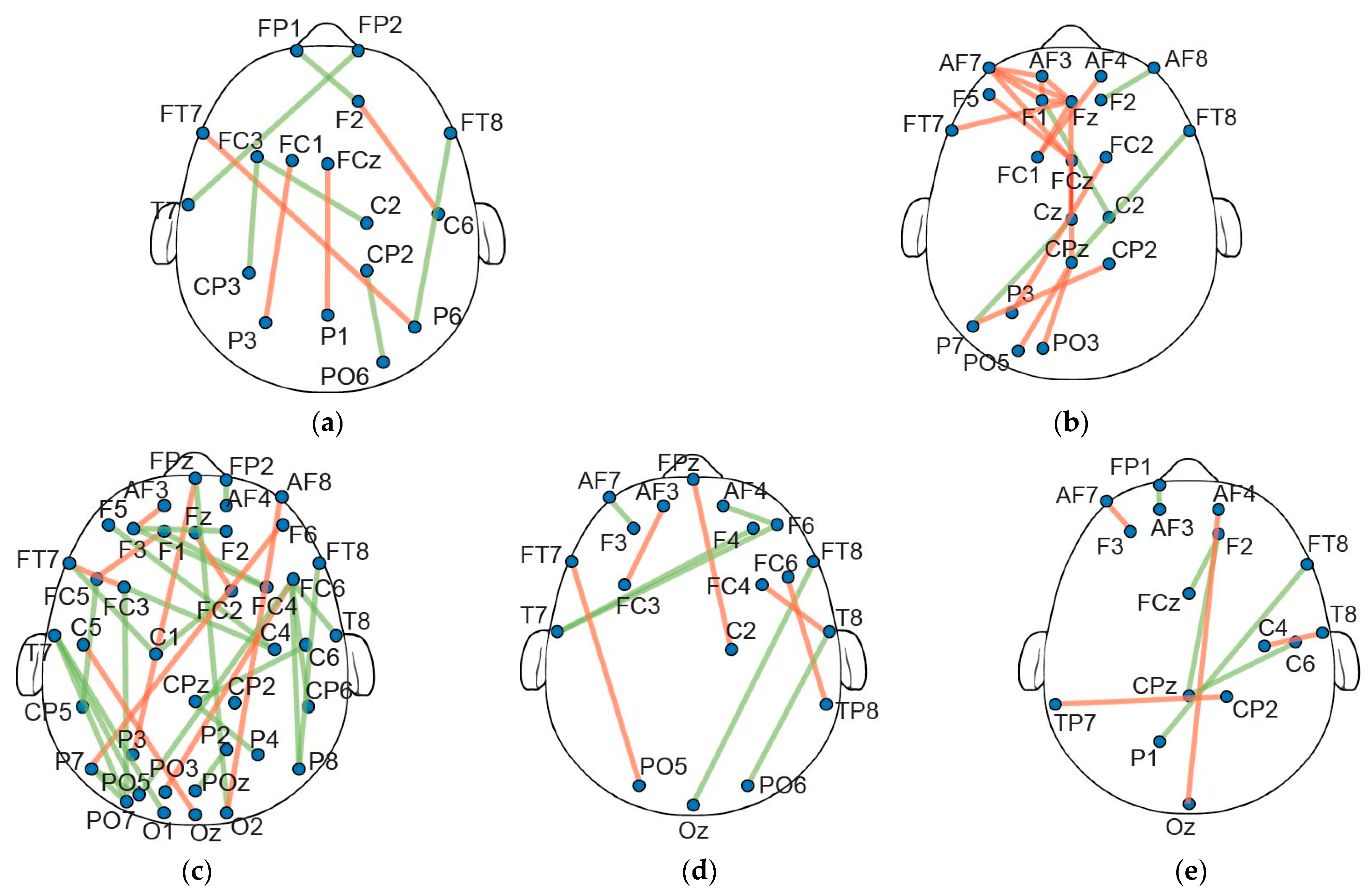
3.2. Individual Participant Network Analysis
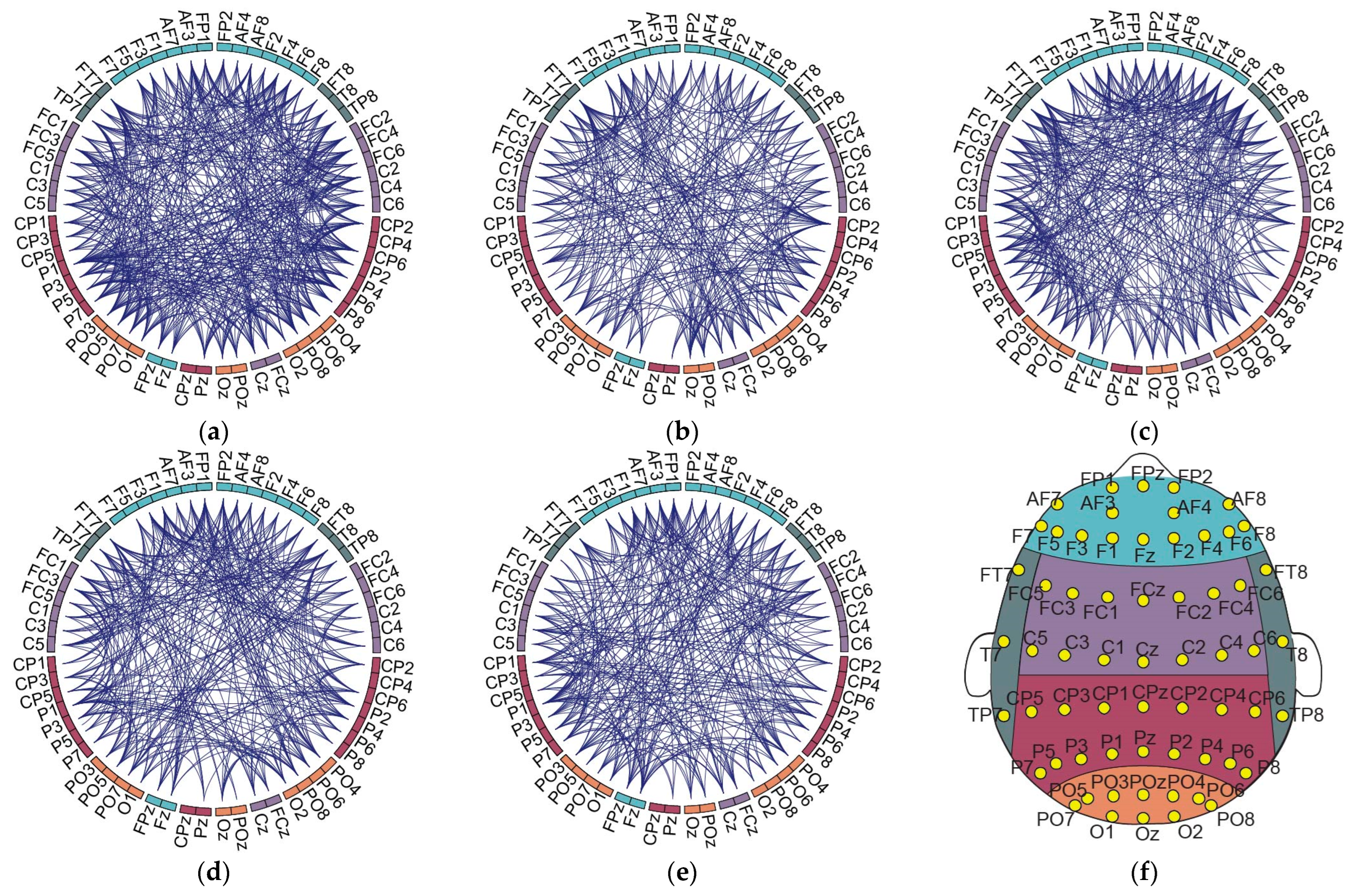
3.3. Association of Individual to the Global Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xi, J.; Chen, H. Modeling and Recognizing Driver Behavior Based on Driving Data: A Survey. Math. Probl. Eng. 2014, 2014, e245641. [Google Scholar] [CrossRef]
- Jackson, M.L.; Kennedy, G.A.; Clarke, C.; Gullo, M.; Swann, P.; Downey, L.A.; Hayley, A.C.; Pierce, R.J.; Howard, M.E. The Utility of Automated Measures of Ocular Metrics for Detecting Driver Drowsiness during Extended Wakefulness. Accid. Anal. Prev. 2016, 87, 127–133. [Google Scholar] [CrossRef]
- Hu, X.; Lodewijks, G. Detecting Fatigue in Car Drivers and Aircraft Pilots by Using Non-Invasive Measures: The Value of Differentiation of Sleepiness and Mental Fatigue. J. Safety Res. 2020, 72, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, K.; Ismail, A.M.H.; Ramasamy, S.; Gnanou, J.V.; Caszo, B.A.; Chen, P.L. Understanding Mental Fatigue and Its Detection: A Comparative Analysis of Assessments and Tools. PeerJ 2023, 11, e15744. [Google Scholar] [CrossRef]
- Wheaton, A.G.; Shults, R.A.; Chapman, D.P.; Ford, E.S.; Croft, J.B. Drowsy Driving and Risk Behaviors—10 States and Puerto Rico, 2011–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 557–562. [Google Scholar]
- Jiao, Y.; Zhang, C.; Chen, X.; Fu, L.; Jiang, C.; Wen, C. Driver Fatigue Detection Using Measures of Heart Rate Variability and Electrodermal Activity. IEEE Trans. Intell. Transp. Syst. 2024, 25, 5510–5524. [Google Scholar] [CrossRef]
- Stancin, I.; Cifrek, M.; Jovic, A. A Review of EEG Signal Features and Their Application in Driver Drowsiness Detection Systems. Sensors 2021, 21, 3786. [Google Scholar] [CrossRef]
- Useche, S.; Cendales, B.; Gómez, V. Measuring Fatigue and Its Associations with Job Stress, Health and Traffic Accidents in Professional Drivers: The Case of BRT Operators. EC Neurol. 2017, 4, 103–118. [Google Scholar]
- Kakkos, I.; Dimitrakopoulos, G.N.; Sun, Y.; Yuan, J.; Matsopoulos, G.K.; Bezerianos, A.; Sun, Y. EEG Fingerprints of Task-Independent Mental Workload Discrimination. IEEE J. Biomed. Health Inform. 2021, 25, 3824–3833. [Google Scholar] [CrossRef]
- Sun, Y.; Lim, J.; Kwok, K.; Bezerianos, A. Functional Cortical Connectivity Analysis of Mental Fatigue Unmasks Hemispheric Asymmetry and Changes in Small-World Networks. Brain Cogn. 2014, 85, 220–230. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Xu, W.; Wu, K.; Liu, Y.; Bezerianos, A.; Sun, Y. Self-Regulation Phenomenon Emerged During Prolonged Fatigue Driving: An EEG Connectivity Study. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2023, 31, 4895–4906. [Google Scholar] [CrossRef]
- Lim, J.; Dinges, D.F. Sleep Deprivation and Vigilant Attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring Neurophysiological Signals in Aircraft Pilots and Car Drivers for the Assessment of Mental Workload, Fatigue and Drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, D.; Lu, B.; Wang, H.; Fu, R. A Novel Semi-Dry Electrode Suitable for Collecting EEG Data for Detecting Driving Fatigue in Long-Period Driving Case. IEEE Sens. J. 2023, 23, 17891–17900. [Google Scholar] [CrossRef]
- Mantellos, G.; Exarchos, T.P.; Dimitrakopoulos, G.N.; Vlamos, P.; Papastamatiou, N.; Karaiskos, K.; Minos, P.; Alexandridis, T.; Axiotopoulos, S.; Tsakiridis, D.; et al. Integrating Wearable Sensors and Machine Learning for the Detection of Critical Events in Industry Workers. In Proceedings of the GeNeDis 2022; Vlamos, P., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 213–222. [Google Scholar]
- Miloulis, S.-T.; Kakkos, I.; Dimitrakopoulos, G.Ν.; Sun, Y.; Karanasiou, I.; Asvestas, P.; Ventouras, E.-C.; Matsopoulos, G. Evaluating Memory and Cognition via a Wearable EEG System: A Preliminary Study. In Proceedings of the Wireless Mobile Communication and Healthcare, Virtual Event, 13–14 November 2021; Ye, J., O’Grady, M.J., Civitarese, G., Yordanova, K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 52–66. [Google Scholar]
- Gelbard-Sagiv, H.; Pardo, S.; Getter, N.; Guendelman, M.; Benninger, F.; Kraus, D.; Shriki, O.; Ben-Sasson, S. Optimizing Electrode Configurations for Wearable EEG Seizure Detection Using Machine Learning. Sensors 2023, 23, 5805. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Wang, H.; Sgarbas, K.; Thakor, N.; Bezerianos, A.; Sun, Y. Functional Connectivity Analysis of Mental Fatigue Reveals Different Network Topological Alterations Between Driving and Vigilance Tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, G.N.; Kakkos, I.; Thakor, N.V.; Bezerianos, A.; Sun, Y. A Mental Fatigue Index Based on Regression Using Mulitband EEG Features with Application in Simulated Driving. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 3220–3223. [Google Scholar]
- Seitzman, B.A.; Gratton, C.; Marek, S.; Raut, R.V.; Dosenbach, N.U.F.; Schlaggar, B.L.; Petersen, S.E.; Greene, D.J. A Set of Functionally-Defined Brain Regions with Improved Representation of the Subcortex and Cerebellum. NeuroImage 2020, 206, 116290. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Alsheddy, A. Driver Fatigue Detection Systems Using Multi-Sensors, Smartphone, and Cloud-Based Computing Platforms: A Comparative Analysis. Sensors 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Sikander, G.; Anwar, S. Driver Fatigue Detection Systems: A Review. IEEE Trans. Intell. Transp. Syst. 2019, 20, 2339–2352. [Google Scholar] [CrossRef]
- Benedetti, G.M.; Morgan, L.A.; Sansevere, A.J.; Harrar, D.B.; Guerriero, R.M.; Wainwright, M.S.; LaRovere, K.L.; Kielian, A.; Ganesan, S.L.; Press, C.A. The Spectrum of Quantitative EEG Utilization Across North America: A Cross-Sectional Survey. Pediatr. Neurol. 2023, 141, 1–8. [Google Scholar] [CrossRef]
- Muldoon, S.F.; Bassett, D.S. Network and Multilayer Network Approaches to Understanding Human Brain Dynamics. Philos. Sci. 2016, 83, 710–720. [Google Scholar] [CrossRef]
- Park, H.-J.; Friston, K. Structural and Functional Brain Networks: From Connections to Cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Anastasiou, A.; Bezerianos, A.; Sun, Y.; Matsopoulos, G.K. Cognitive Reorganization Due to Mental Workload: A Functional Connectivity Analysis Based on Working Memory Paradigms. Appl. Sci. 2023, 13, 2129. [Google Scholar] [CrossRef]
- Giannakopoulou, O.; Kakkos, I.; Dimitrakopoulos, G.N.; Sun, Y.; Matsopoulos, G.K.; Koutsouris, D.D. Time-Dependent Adaptations of Brain Networks in Driving Fatigue. Eng. Proc. 2023, 50, 6. [Google Scholar] [CrossRef]
- Tompson, S.H.; Falk, E.B.; Vettel, J.M.; Bassett, D.S. Network Approaches to Understand Individual Differences in Brain Connectivity: Opportunities for Personality Neuroscience. Personal. Neurosci. 2018, 1, e5. [Google Scholar] [CrossRef]
- Stam, C.; Daffertshofer, A. Phase Lag Index: Assessment of Functional Connectivity from Multi Channel EEG and MEG with Diminished Bias from Common Sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef]
- James, G.A.; Hazaroglu, O.; Bush, K.A. A Human Brain Atlas Derived via N-Cut Parcellation of Resting-State and Task-Based fMRI Data. Magn. Reson. Imaging 2016, 34, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional Connectome Fingerprinting: Identifying Individuals Using Patterns of Brain Connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Tavor, I.; Parker Jones, O.; Mars, R.B.; Smith, S.M.; Behrens, T.E.; Jbabdi, S. Task-Free MRI Predicts Individual Differences in Brain Activity during Task Performance. Science 2016, 352, 216–220. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Kakkos, I.; Matsopoulos, G.K.; Yuan, J.; Suckling, J.; Xu, L.; Cao, S.; Chen, W.; Hu, X.; et al. Inferring the Individual Psychopathologic Deficits With Structural Connectivity in a Longitudinal Cohort of Schizophrenia. IEEE J. Biomed. Health Inform. 2022, 26, 2536–2546. [Google Scholar] [CrossRef]
- Peng, Z.; Rong, J.; Wu, Y.; Zhou, C.; Yuan, Y.; Shao, X. Exploring the Different Patterns for Generation Process of Driving Fatigue Based on Individual Driving Behavior Parameters. Transp. Res. Rec. 2021, 2675, 408–421. [Google Scholar] [CrossRef]
- Lim, J.; Wu, W.-C.; Wang, J.; Detre, J.A.; Dinges, D.F.; Rao, H. Imaging Brain Fatigue from Sustained Mental Workload: An ASL Perfusion Study of the Time-on-Task Effect. NeuroImage 2010, 49, 3426–3435. [Google Scholar] [CrossRef]
- Love, S.; Truelove, V.; Rowland, B.; Kannis-Dymand, L. Metacognition and Self-Regulation on the Road: A Qualitative Approach to Driver Attention and Distraction. Appl. Cogn. Psychol. 2022, 36, 1312–1324. [Google Scholar] [CrossRef]
- Sun, Y.; Lim, J.; Meng, J.; Kwok, K.; Thakor, N.; Bezerianos, A. Discriminative Analysis of Brain Functional Connectivity Patterns for Mental Fatigue Classification. Ann. Biomed. Eng. 2014, 42, 2084–2094. [Google Scholar] [CrossRef]
- Helton, W. Validation of a Short Stress State Questionnaire. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2004, 48, 1238–1242. [Google Scholar] [CrossRef]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the International 10-20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S. High-Frequency Brain Activity and Muscle Artifacts in MEG/EEG: A Review and Recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, I.; Dimitrakopoulos, G.N.; Gao, L.; Zhang, Y.; Qi, P.; Matsopoulos, G.K.; Thakor, N.; Bezerianos, A.; Sun, Y. Mental Workload Drives Different Reorganizations of Functional Cortical Connectivity Between 2D and 3D Simulated Flight Experiments. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2019, 27, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Delorme, A.; Makeig, S. Eeglab—An Open Source Matlab Toolbox for Electrophysiological Research. Biomed. Technol. 2013, 58 (Suppl. S1). [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Wang, Q.; Hua, C. Exploring the Fatigue Affecting Electroencephalography Based Functional Brain Networks during Real Driving in Young Males. Neuropsychologia 2019, 129, 200–211. [Google Scholar] [CrossRef]
- Maulud, D.; Abdulazeez, A.M. A Review on Linear Regression Comprehensive in Machine Learning. J. Appl. Sci. Technol. Trends 2020, 1, 140–147. [Google Scholar] [CrossRef]
- Goldhammer, F.; Naumann, J.; Stelter, A.; Tóth, K.; Rölke, H.; Klieme, E. The Time on Task Effect in Reading and Problem Solving Is Moderated by Task Difficulty and Skill: Insights from a Computer-Based Large-Scale Assessment. J. Educ. Psychol. 2014, 106, 608–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Zhou, Y.; Xu, C.; Liao, Y. Recognising Drivers’ Mental Fatigue Based on EEG Multi-Dimensional Feature Selection and Fusion. Biomed. Signal Process. Control 2023, 79, 104237. [Google Scholar] [CrossRef]
- Soong, T.T. Fundamentals of Probability and Statistics for Engineers, 1st ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-86813-3. [Google Scholar]
- Helton, W.S.; Russell, P.N. Working Memory Load and the Vigilance Decrement. Exp. Brain Res. 2011, 212, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Quevenco, F.-C.; Kwok, K. EEG Alpha Activity Is Associated with Individual Differences in Post-Break Improvement. NeuroImage 2013, 76, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Routray, A. Effect of Sleep Deprivation on Functional Connectivity of EEG Channels. IEEE Trans. Syst. Man Cybern. Syst. 2013, 43, 666–672. [Google Scholar] [CrossRef]
- Dewitte, S.; Bruyneel, S.; Geyskens, K. Self-Regulating Enhances Self-Regulation in Subsequent Consumer Decisions Involving Similar Response Conflicts. J. Consum. Res. 2009, 36, 394–405. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, M.; Yang, Y.; Gao, J.; Rao, N.; Lin, P. The Reorganization of Human Brain Networks Modulated by Driving Mental Fatigue. IEEE J. Biomed. Health Inform. 2017, 21, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Puspasari, M.A.; Iridiastadi, H.; Sutalaksana, I.Z.; Sjafruddin, A. Effect of Driving Duration on EEG Fluctuations. Int. J. Technol. 2017, 8, 1089–1096. [Google Scholar] [CrossRef]
- Harvy, J.; Bezerianos, A.; Li, J. Reliability of EEG Measures in Driving Fatigue. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2022, 30, 2743–2753. [Google Scholar] [CrossRef]
- Otto, T.; Zijlstra, F.R.H.; Goebel, R. Feeling the Force: Changes in a Left-Lateralized Network of Brain Areas under Simulated Workday Conditions Are Reflected in Subjective Mental Effort Investment. PLoS ONE 2018, 13, e0198204. [Google Scholar] [CrossRef] [PubMed]
- Jap, B.T.; Lal, S.; Fischer, P. Inter-Hemispheric Electroencephalography Coherence Analysis: Assessing Brain Activity during Monotonous Driving. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2010, 76, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, K.; Khosravi, M.R.; Attar, H.; Almatarneh, S. EEG-Based Driving Fatigue Recognition Using Hybrid Deep Transfer Learning Approach. In Proceedings of the 2022 International Engineering Conference on Electrical, Energy, and Artificial Intelligence (EICEEAI), Zarqa, Jordan, 21 November 2022; pp. 1–6. [Google Scholar]
- Awais, M.; Badruddin, N.; Drieberg, M. EEG Brain Connectivity Analysis to Detect Driver Drowsiness Using Coherence: 15th International Conference on Frontiers of Information Technology, FIT 2017. In Proceedings of the 2017 International Conference on Frontiers of Information Technology, FIT 2017, 2017 International Conference on Frontiers of Information Technology, FIT 2017, Islamabad, Pakistan, 18–20 December 2017; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017; pp. 110–114, ISBN 978-1-5386-3567-4. [Google Scholar]
- Yao, L.; Baker, J.L.; Schiff, N.D.; Purpura, K.P.; Shoaran, M. Predicting Task Performance from Biomarkers of Mental Fatigue in Global Brain Activity. J. Neural Eng. 2021, 18, 036001. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Guo, J.; Hanson, N.J.; Wang, K.; Chai, J.; Guo, F. The Effects of Mental Fatigue on Fine Motor Performance in Humans and Its Neural Network Connectivity Mechanism: A Dart Throwing Study. Cereb. Cortex 2024, 34, bhae085. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, G.; Wang, S.; Wan, F.; Sun, Y.; Wang, H.; Bezerianos, A.; Li, C.; Sun, Y. Toward Practical Driving Fatigue Detection Using Three Frontal EEG Channels: A Proof-of-Concept Study. Physiol. Meas. 2021, 42, 044003. [Google Scholar] [CrossRef] [PubMed]
- Kong, V.C.; Marshall, A.; Chan, H.B. Cone Beam Computed Tomography: The Challenges and Strategies in Its Application for Dose Accumulation. J. Med. Imaging Radiat. Sci. 2016, 47, 92–97. [Google Scholar] [CrossRef]
- Nobukawa, S.; Wagatsuma, N.; Inagaki, K. Gamma Band Functional Connectivity Enhanced by Driving Experience. In Proceedings of the 2021 IEEE 3rd Global Conference on Life Sciences and Technologies (LifeTech), Nara, Japan, 9–11 March 2021; pp. 379–381. [Google Scholar]
- Kong, W.; Zhou, Z.; Jiang, B.; Babiloni, F.; Borghini, G. Assessment of Driving Fatigue Based on Intra/Inter-Region Phase Synchronization. Neurocomputing 2017, 219, 474–482. [Google Scholar] [CrossRef]
- Huang, K.-C.; Chuang, C.-H.; Wang, Y.-K.; Hsieh, C.-Y.; King, J.-T.; Lin, C.-T. The Effects of Different Fatigue Levels on Brain-Behavior Relationships in Driving. Brain Behav. 2019, 9, e01379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ahn, S.; Jang, H.; Jun, S.C.; Kim, J.G. Utilization of a Combined EEG/NIRS System to Predict Driver Drowsiness. Sci. Rep. 2017, 7, 43933. [Google Scholar] [CrossRef]
- Gordon, E.M.; Nelson, S.M. Three Types of Individual Variation in Brain Networks Revealed by Single-Subject Functional Connectivity Analyses. Curr. Opin. Behav. Sci. 2021, 40, 79–86. [Google Scholar] [CrossRef]
- Naselaris, T.; Allen, E.; Kay, K. Extensive Sampling for Complete Models of Individual Brains. Curr. Opin. Behav. Sci. 2021, 40, 45–51. [Google Scholar] [CrossRef]
- Saxby, D.; Matthews, G.; Hitchcock, E.M.; Warm, J.S. Fatigue States Are Multidimensional: Evidence from Studies of Simulated Driving. 2007. Available online: https://www.nads-sc.uiowa.edu/dscna/2007/papers/Section%202A%20-%20Design%20and%20Analysis%20I/Saxby.pdf (accessed on 23 April 2024).
- Gratton, C.; Laumann, T.O.; Nielsen, A.N.; Greene, D.J.; Gordon, E.M.; Gilmore, A.W.; Nelson, S.M.; Coalson, R.S.; Snyder, A.Z.; Schlaggar, B.L.; et al. Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 2018, 98, 439–452.e5. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Tervo-Clemmens, B.; Calabro, F.J.; Montez, D.F.; Kay, B.P.; Hatoum, A.S.; Donohue, M.R.; Foran, W.; Miller, R.L.; Hendrickson, T.J.; et al. Reproducible Brain-Wide Association Studies Require Thousands of Individuals. Nature 2022, 603, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Aidman, E.; Chadunow, C.; Johnson, K.; Reece, J. Real-Time Driver Drowsiness Feedback Improves Driver Alertness and Self-Reported Driving Performance. Accid. Anal. Prev. 2015, 81, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Ru, H.; Gao, L.; Zhou, T.; Tian, Y.; Thakor, N.V.; Bezerianos, A.; Li, J.; Sun, Y. Neural Mechanisms of Mental Fatigue Revisited: A New Insight from Brain Connectome. Engineering 2018, 5, 276–286. [Google Scholar] [CrossRef]
- Gartstein, M.A.; Hancock, G.R.; Potapova, N.V.; Calkins, S.D.; Bell, M.A. Modeling Development of Frontal Electroencephalogram (EEG) Asymmetry: Sex Differences and Links with Temperament. Dev. Sci. 2020, 23, e12891. [Google Scholar] [CrossRef]

| Frequency Band | Number of Connections |
|---|---|
| delta | 440 |
| theta | 287 |
| alpha | 361 |
| beta | 313 |
| gamma | 275 |
| Frequency Band | Mean Percentage 1 (%) | Min Percentage (%) | Max Percentage (%) |
|---|---|---|---|
| delta | 17.5 ± 6 | 10.00 | 29.09 |
| theta | 18.3 ± 7 | 9.76 | 32.06 |
| alpha | 21.5 ± 11 | 7.8 | 47.37 |
| beta | 18.4 ± 6 | 10.54 | 35.14 |
| gamma | 16.3 ± 7 | 7.80 | 36.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulou, O.; Kakkos, I.; Dimitrakopoulos, G.N.; Tarousi, M.; Sun, Y.; Bezerianos, A.; Koutsouris, D.D.; Matsopoulos, G.K. Individual Variability in Brain Connectivity Patterns and Driving-Fatigue Dynamics. Sensors 2024, 24, 3894. https://doi.org/10.3390/s24123894
Giannakopoulou O, Kakkos I, Dimitrakopoulos GN, Tarousi M, Sun Y, Bezerianos A, Koutsouris DD, Matsopoulos GK. Individual Variability in Brain Connectivity Patterns and Driving-Fatigue Dynamics. Sensors. 2024; 24(12):3894. https://doi.org/10.3390/s24123894
Chicago/Turabian StyleGiannakopoulou, Olympia, Ioannis Kakkos, Georgios N. Dimitrakopoulos, Marilena Tarousi, Yu Sun, Anastasios Bezerianos, Dimitrios D. Koutsouris, and George K. Matsopoulos. 2024. "Individual Variability in Brain Connectivity Patterns and Driving-Fatigue Dynamics" Sensors 24, no. 12: 3894. https://doi.org/10.3390/s24123894






