Recent Developments and Challenges in Solid-Contact Ion-Selective Electrodes
Abstract
:1. Introduction
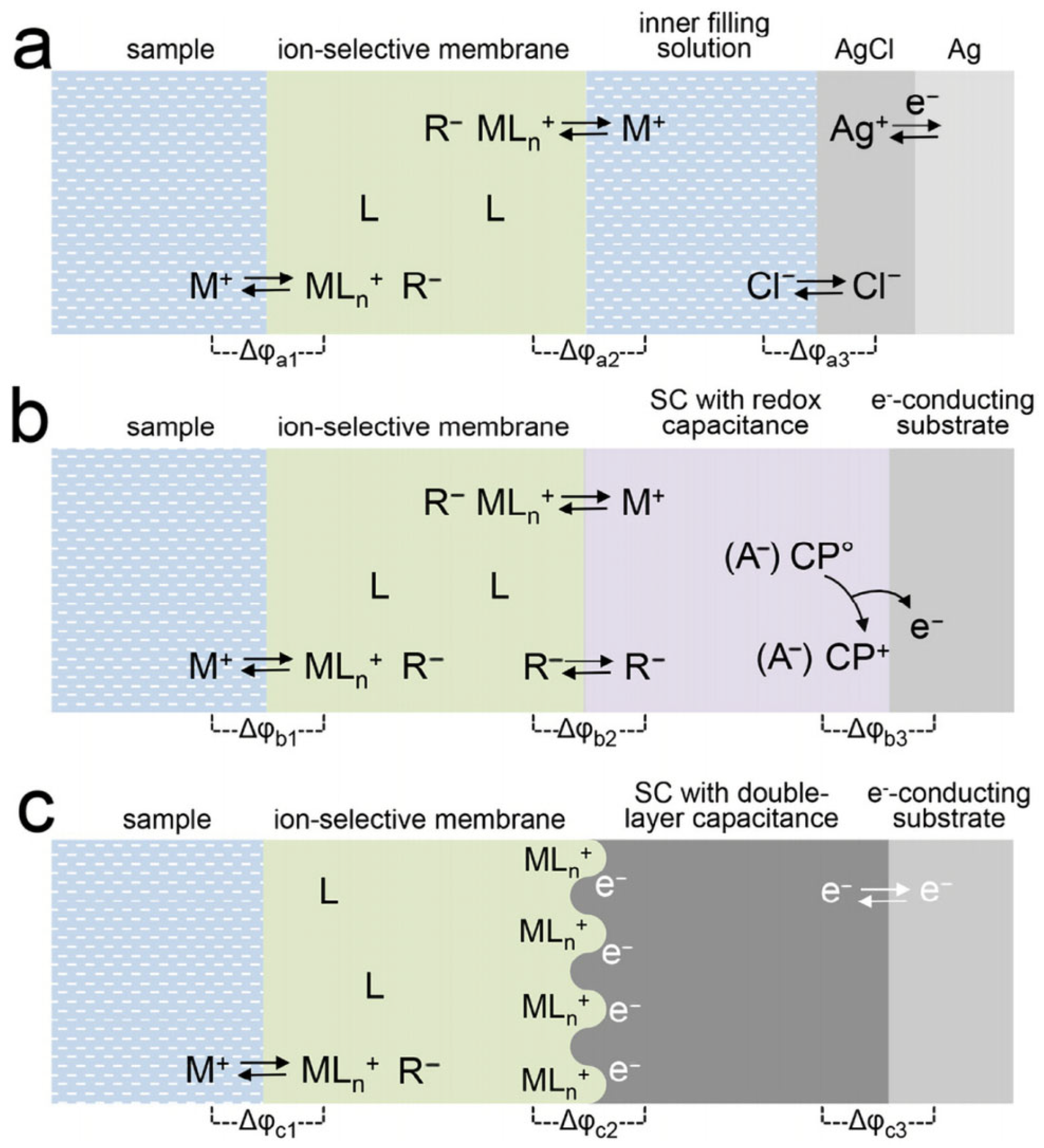
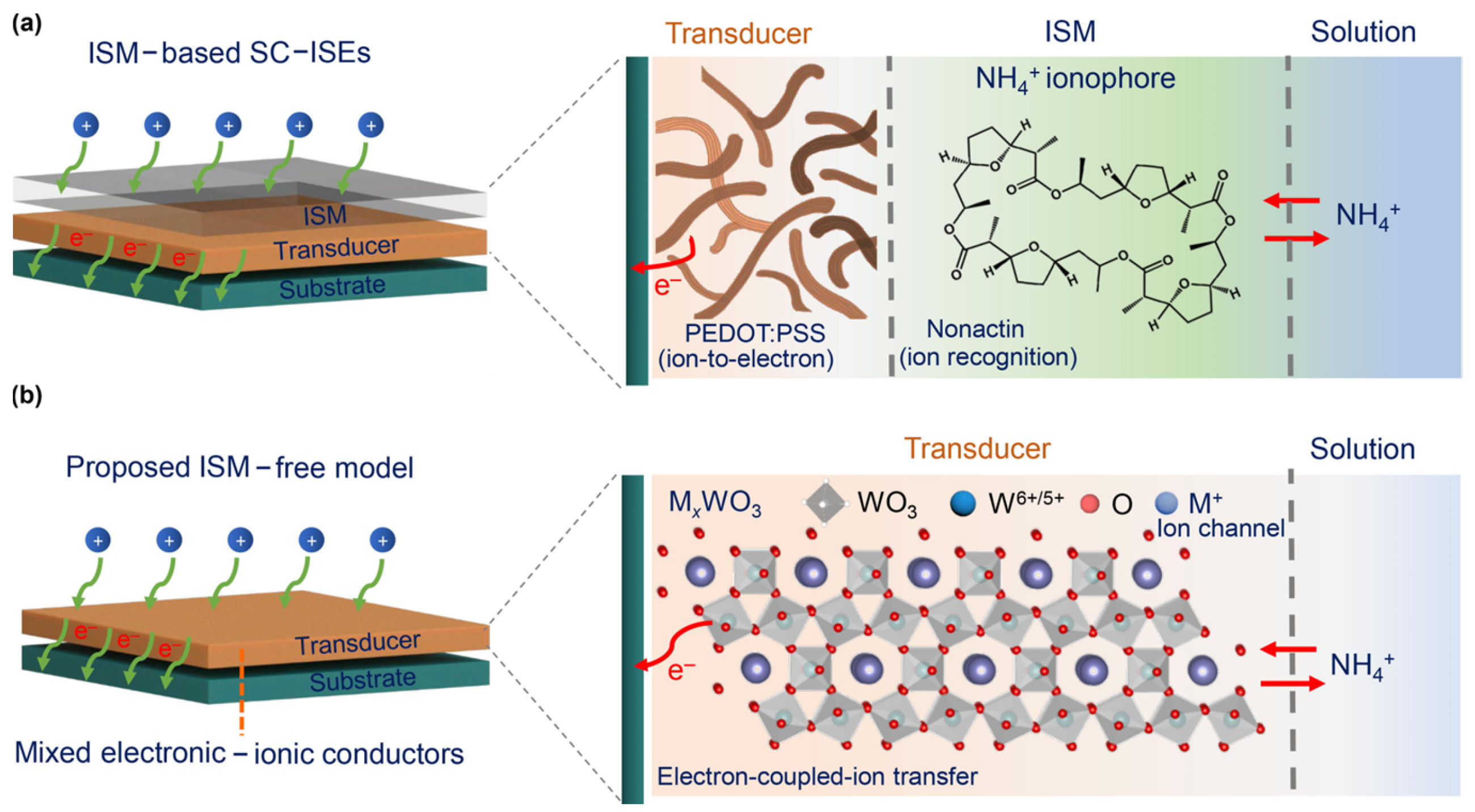
2. Composition and Working Principle of Solid-State Potential Sensors
3. Index of Characteristics
- (1)
- Selectivity: The selectivity of SC-ISEs refers to their ability to detect the target ions while disregarding other interfering ions. In practical measurements, SC-ISEs may also respond to some interfering ions, leading to a deviation of the response slope from the theoretical value and a decrease in sensitivity. An ideal SC-ISE has high selectivity, ensuring accurate information about the presence and activity values of target ions. The selectivity coefficient is usually calculated based on the two-solution method in mixed solutions [26]. A smaller selectivity coefficient indicates better selectivity and stronger anti-interference ability of the SC-ISE.
- (2)
- Reproducibility: Over time, the ionized compounds in the membrane structure enter the aqueous solution, and the stability of the electrode gradually decreases, resulting in a decrease in the slope [27]. Currently, SC-ISEs must be periodically calibrated to meet the requirements of practical testing, and the pre-calibration process is essential, which relates to the accuracy and reproducibility of the test results. However, complex or frequent calibration processes consume a significant amount of time and increase costs, especially for commercial potential sensors, which require users to have certain professional knowledge. It has been found that adding oxidable/reducible active substances with buffering capabilities to the SIM can significantly improve the reproducibility of the initial E° of carbon-based all-solid-state ion-selective electrodes [28]. However, the loss of oxidable/reducible active substances caused by the ion exchange in the ISM may lead to a gradual drift of E°. Therefore, achieving high reproducibility of the standard electrode potential remains a challenge and an important step toward achieving calibration-free measurements.
- (3)
- Detection limit and detection range: Both excessively high and low activity of target ions in the test solution can cause the electrode response to deviate from the theoretical value of the Nernst slope. When the concentration of the target ions is too low, interfering ions can enter the selective membrane and interfere with the electrode response, resulting in the limit of detection (LOD). When the activity of the target ions is too high, the ISM will undergo co-extraction with the test solution, resulting in the detection upper limit [29]. According to the definition by IUPAC, the intersection of the extension lines of the horizontal and linear parts represents the activity of the target ions corresponding to the detection upper limit or detection lower limit; the linear part between the detection upper limit and detection lower limit represents the detection range of the electrode.
- (4)
- Response time: The response time refers to the time required for the electrode’s potential to reach 95% of the equilibrium value after immersing from one sample solution to the next. It reflects the speed at which the potential value of SC-ISEs reaches equilibrium.
- (5)
- Lifespan: The lifespan of an electrode refers to the duration of time the electrode can function properly while maintaining its performance indicators. Factors such as aging of the membrane matrix, loss of ion carrier and membrane additives, and external environmental damage to the membrane components can all affect the lifespan of SC-ISEs. Wardak et al. [30] modified polymer membranes with a nanocomposite composed of carbon nanofibers and ionic liquid (1-hexyl-3-methylimidazolium hexafluorophosphate), which significantly enhanced the strength of the ion-selective membrane (ISM), and the electrode retained its properties for four months, with negligible changes in the standard potential (E°) and Nernst slope.
- (6)
- Stability: The stability of the electrode refers to the fluctuation and drift of the electrode’s measured potential during the operation. Ideally, SC should have a non-polarizing interface with a high exchange current density [31]. However, in practical testing, the input current of the measurement amplifier inevitably leads to its charge–discharge, causing polarization of the electrode to varying degrees and resulting in a potential drift. Currently, the stability of the electrode is evaluated using the reverse current timing potential method proposed by the Bobacka group [32].
4. Construction Strategies and Applications of SC-ISEs
4.1. Oxidation–Reduction-Type SC-ISEs
4.2. EDL Capacitance-Type SC-ISEs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bobacka, J.; Ivaska, A.; Andrzej, L. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; Blondeau, P.; Andrade, F.J. Distributed electrochemical sensors: Recent advances and barriers to market adoption. Anal. Bioanal. Chem. 2018, 410, 4077–4089. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC-Trend Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Walker, N.L.; Roshkolaeva, A.B.; Chapoval, A.I.; Dick, J.E. Recent advances in potentiometric biosensing. Curr. Opin. Electrochem. 2021, 28, 100735. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Glasscott, M.W.; Vannoy, K.J.; Dick, J.E. Enzyme Kinetics via Open Circuit Potentiometry. Anal. Chem. 2019, 92, 2266–2273. [Google Scholar] [CrossRef]
- Park, J.H.; Zhou, H.; Percival, S.J.; Zhang, B.; Fan, F.-R.F.; Bard, A.J. Open Circuit (Mixed) Potential Changes Upon Contact Between Different Inert Electrodes–Size and Kinetic Effects. Anal. Chem. 2013, 85, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong , L.; Xu , L.; Liu, J.; Wang, W.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y.-J. Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors 2023, 13, 424. [Google Scholar] [CrossRef]
- Mishra, D.; Krause, A.; Sahni, H.S.; Chatterjee, S. Multi-walled carbon nanotube-functional ionophore based composite potentiometric sensor for selective detection of lead in water. Diam. Relat. Mater. 2023, 137, 110156. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly reproducible solid contact ion selective electrodes: Emerging opportunities for potentiometry—A review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Buhlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Developments in the Field of Conducting and Non-conducting Polymer Based Potentiometric Membrane Sensors for Ions Over the Past Decade. Sensors 2008, 8, 2331–2412. [Google Scholar] [CrossRef] [PubMed]
- Grygolowicz-Pawlak, E.; Crespo, G.A.; Ghahraman Afshar, M.; Mistlberger, G.; Bakker, E. Potentiometric Sensors with Ion-Exchange Donnan Exclusion Membranes. Anal. Chem. 2013, 85, 6208–6212. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC-Trend Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Z.; Lin, X.; Ding, J.; Qin, W. In Situ Continuous Measurement of Salinity in Estuarine and Coastal Sediments by All-Solid Potentiometric Sensors. ACS Sens. 2023, 8, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.A.; Macho, M.; Bobacka, J.; Rius, X. Subnanomolar detection limit application of ion-selective electrodes with three-dimensionally ordered macroporous (3DOM) carbon solid contacts. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Yao, Y.; Ying, Y.; Ping, J. Fully Written Flexible Potentiometric Sensor Using Two-Dimensional Nanomaterial-Based Conductive Ink. Anal. Chem. 2018, 90, 13088–13095. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, L.; Gan, S.; Tang, Y.; Qiu, S.; Lyu, Y.; Ma, Y.; Niu, L. Defective vs high-quality graphene for solid-contact ion-selective electrodes: Effects of capacitance and hydrophobicity. Electrochem. Commun. 2021, 129, 107091. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B. All-solid-state selective electrodes using carbon black. Talanta 2012, 93, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Bone, C.; Flahaut, E.; Legnani, M.; Boyer, P.T.; Launay, J. Development of a Potentiometric Nitrate Ion Microsensor Improved Using Conductive Polymer Doped with Carbon Nanotubes as a Transducing Layer. Proceedings 2024, 97, 111. [Google Scholar] [CrossRef]
- Abdelaal, S.H.; Azab, N.F.; Hassan, S.A.; Kosasy, A.M. Monitoring of the prohibited 2-phenethylamine in dietary supplements using a t-Butyl calix [8]arene/Ag/CuO composite-based potentiometric sensor. Microchem. J. 2024, 201, 110695. [Google Scholar] [CrossRef]
- Ross, J.W. Calcium-selective electrode with liquid ion exchanger. Science 1967, 156, 1378–1379. [Google Scholar] [CrossRef]
- Bühlmann, P.; Umezawa, Y.; Rondinini, S.; Vertova, A.; Pigliucci, A.; Bertesago, L. Lifetime of ion-selective electrodes based on charged ionophores. Anal. Chem. 2000, 72, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-Selective Electrodes with Colloid-Imprinted Mesoporous Carbon as Solid Contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.P.; Abd El-Rahman, M.K.; Tan, E.K.; Sigurslid, H.H.; Arkan, N.; Lane, J.S.; Whitesides, G.M.; Bühlmann, P. Ionic liquid-based reference electrodes for miniaturized ion sensors: What can go wrong? Sens. Actuators B Chem. 2019, 301, 127112. [Google Scholar] [CrossRef]
- Wardak, C.; Morawska, K.; Paczosa-Bator, B.; Grabarczyk, M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003. [Google Scholar] [CrossRef]
- Vasconcellos, E.C.C.; Evenson, K.M. New Far Infrared Laser Lines Obtained by Optically Pumping 13CD3OD. Int. J. Infrared Millim. Waves 1985, 6, 1157–1167. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
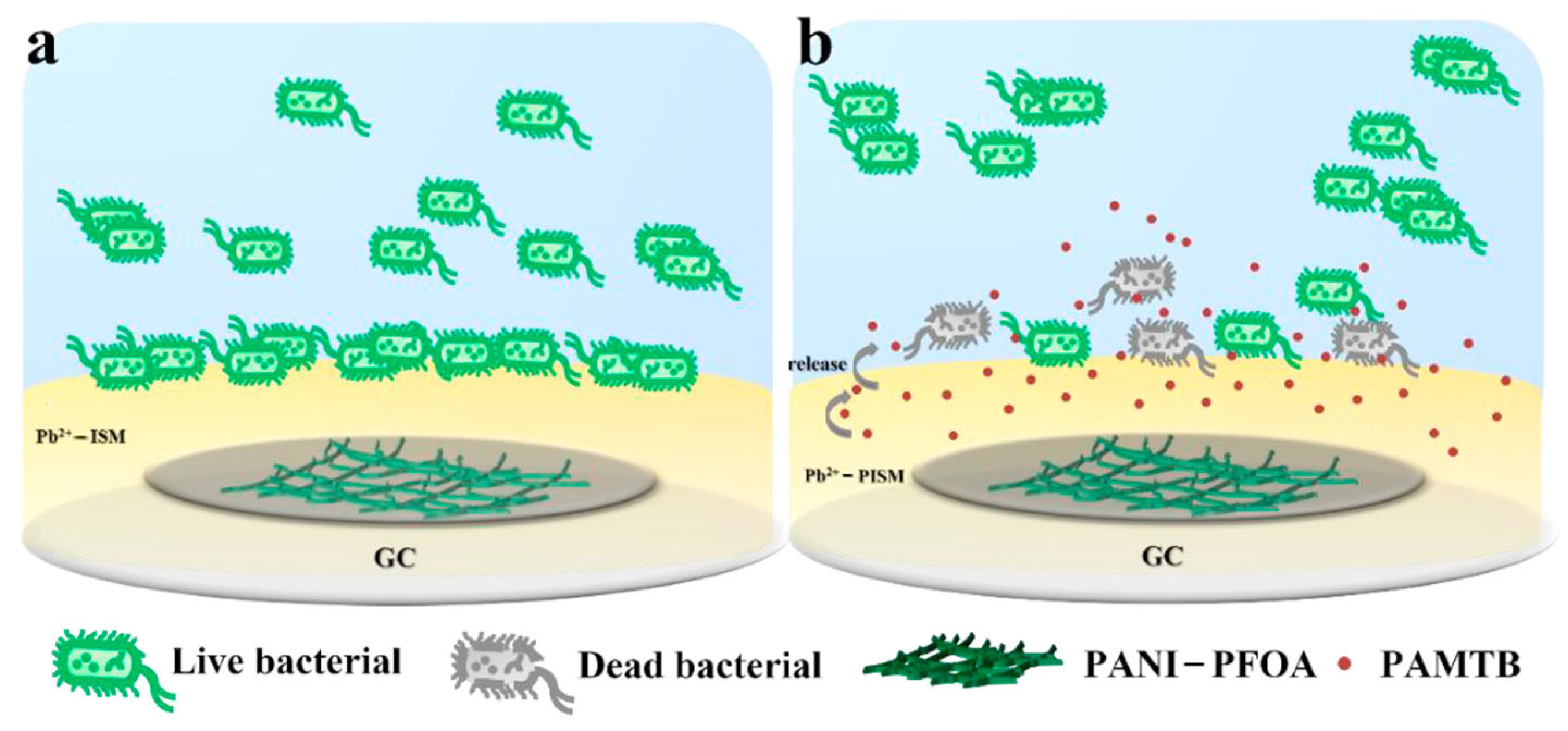
- Qi, L.; Liang, R.; Jiang, T.; Qin, W. Antifouling polymeric membrane ion-selective electrodes. TRAC Trends Anal. Chem. 2022, 150, 116572. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Hou, C.; Fang, S.; Qin, W. Self-Sterilizing Polymeric Membrane Sensors Based on 6-Chloroindole Release for Prevention of Marine Biofouling. Anal. Chem. 2020, 92, 12132–12136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, L.; Li, F.; Zhang, G.; Zhou, W.; Jiang, X. Synthesis of amide derivatives containing capsaicin and their antioxidant and antibacterial activities. J. Food Biochem. 2019, 43, e13061. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Yu, L. Performance assessment of capsaicin derivatives containing amide groups used as active substances for antifouling coatings. Prog. Org. Coat. 2021, 160, 106515. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.; Shan, C.; Xu, L.; Yu, L.; Gao, H. Enhancing the permeance and antifouling properties of thin-film composite nanofiltration membranes modified with hydrophilic capsaicin-mimic moieties. J. Membr. Sci. 2020, 610, 118233. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zeng, X.; Waterhouse, G.I.; Jiang, X.; Zhang, Z.; Yu, L. Antifouling improvement in Pb2+ ion selective electrodes by using an environmentally friendly capsaicin derivative. Talanta 2023, 258, 124436. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Yun, F.; Jiang, X.; Yu, L. Preparation and Evaluation of Gallate Ester Derivatives Used as Promising Antioxidant and Antibacterial Inhibitors. Chem. Biodivers. 2021, 18, e2000913. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Potential stability improvement in Pb2+ ion selective electrodes by applying hydrophobic polyaniline as ion-to-electron transducer. Synth. Met. 2021, 281, 116898. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Gao, X.; Yu, H.; Ma, Z.; Zhang, Y.; Gao, C. Antibiofouling polysulfone ultrafiltration membranes via surface grafting of capsaicin derivatives. Water Sci. Technol. 2019, 79, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Özbek, O.; Berkay Ugur, Ö.; Ören, S.; Burcu Gürdere, M.; Kocabas, S. New solid state contact potentiometric sensor based on a thiosemicarbazone derivative molecule for determination of copper(II) ions in environmental samples. Polyhedron 2024, 252, 116878. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of Electrochemically Reduced Graphene Oxide on Screen-Printed Ion-Selective Electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Bayatsarmadi, B.; Vepsäläinen, M.; Razmjou, A.; Asadnia, M. Highly stable Li+ selective electrode with metal-organic framework as ion-toelectron transduce. Sens. Actuators B Chem. 2022, 350, 130799. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L. MIL series-based MOFs as effective adsorbents for removing hazardous organic pollutants from water. Sep. Purif. Technol. 2023, 322, 124301. [Google Scholar] [CrossRef]
- Mendecki, L.; Mirica, K.A. Conductive Metal–Organic Frameworks as Ion-to-Electron Transducers in Potentiometric Sensors. ACS Appl. Mater. Interfaces 2018, 10, 19248–19257. [Google Scholar] [CrossRef]
- Wang, Y.; Du, K.; Chen, Y.; Li, Y.; He, X. Electrochemical determination of lead based on metal–organic framework MIL-101(Cr) by differential pulse anodic stripping voltammetry. Anal. Methods 2016, 8, 3263–3269. [Google Scholar] [CrossRef]
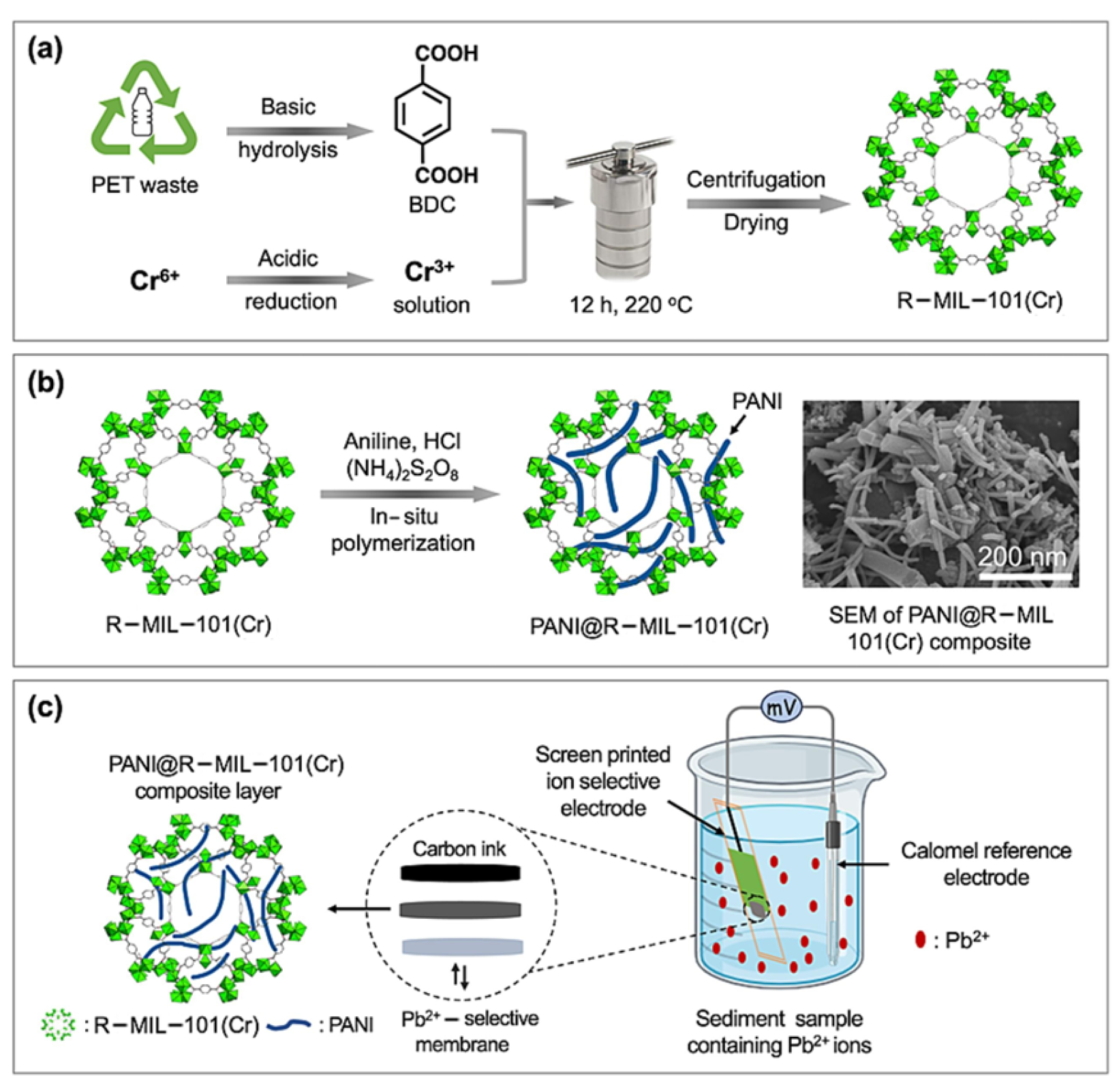
- Keshta, B.E.; Yu, H.; Wang, L.; Uddin, M.A.; El-Attar, H.G.; Keshta, A.E.; Gemeay, A.H.; Hassan, F.; Eid, S.M. Cost-effective synthesis of MIL-101(Cr) from recyclable wastes and composite with polyaniline as an ion-to-electron transducer for potentiometric Pb2+ sensing. Chem. Eng. J. 2024, 485, 150049. [Google Scholar] [CrossRef]
- Rousseau, C.R.; Bühlmann, P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. TrAC-Trend Anal. Chem. 2021, 140, 116277. [Google Scholar] [CrossRef]
- Mu, S.; Yin, T.; Luan, F.; Qin, W. High-redox-capacity solid contact based on ferrocenyl self-assembled monolayer functionalized macroporous gold for an all-solid-state carbonate-selective electrode. Microchem. J. 2024, 197, 109696. [Google Scholar] [CrossRef]
- Wen, Y.; Peng, S.; Wang, Z.; Hao, J.; Qin, T.; Lu, S.; Zhang, J.; He, D.; Fan, X.; Cao, G. Facile synthesis of ultrathin NiCo2S4 nano-petals inspired by blooming buds for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 7144–7152. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Qin, W. All-Solid-State Polymeric Membrane Ion-Selective Electrodes Based on NiCo2S4 as a Solid Contact. Anal. Chem. 2022, 94, 3574–3580. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Li, J.; Wang, M.; Cui, H. Ultra-high rate capability of the synergistically built dual nanostructure of NiCo2S4/nickel foam as an electrode in supercapacitors. Nanoscale 2020, 12, 22330–22339. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Toubar, S.S.; Ashour, A.A.; El-Eryan, R.T. A Portable Solid-Contact Potentiometric Sensor Based on Zeolite-Carbon Paste for Assay of Prucalopride Succinate in Tablet Dosage Form: Green Profile Assessment. Measurement 2022, 204, 112071. [Google Scholar] [CrossRef]
- Radić, J.; Perović, D.; Gričar, E.; Kolar, M. Potentiometric Determination of Maprotiline Hydrochloride in Pharmaceutical and Biological Matrices Using a Novel Modified Carbon Paste Electrode. Sensors 2022, 22, 9201. [Google Scholar] [CrossRef] [PubMed]
- Narasimhachar, R.; Basavaraj, B.; Vijaykumar, B.T.; Sannakki, B. Studies on the electrical properties of polyaniline with cadmiumoxide nanocomposites. Mater. Today Proc. 2023, 92, 1676–1680. [Google Scholar] [CrossRef]
- Foronda, J.R.F.; Aryaswara, L.G.; Santos, G.N.C.; Raghu, S.N.V.; Muflikhun, M.A. Broad-class volatile organic compounds (VOCs) detection via polyaniline/zinc oxide (PANI/ZnO) composite materials as gas sensor application. Heliyon 2023, 9, e13544. [Google Scholar] [CrossRef]
- Niemiec, B.; Piech, R.; Paczosa-Bator, B. All-Solid-State Carbon Black Paste Electrodes Modified by Poly(3-octylthiophene-2,5-diyl) and Transition Metal Oxides for Determination of Nitrate Ions. Molecules 2023, 28, 4313. [Google Scholar] [CrossRef] [PubMed]
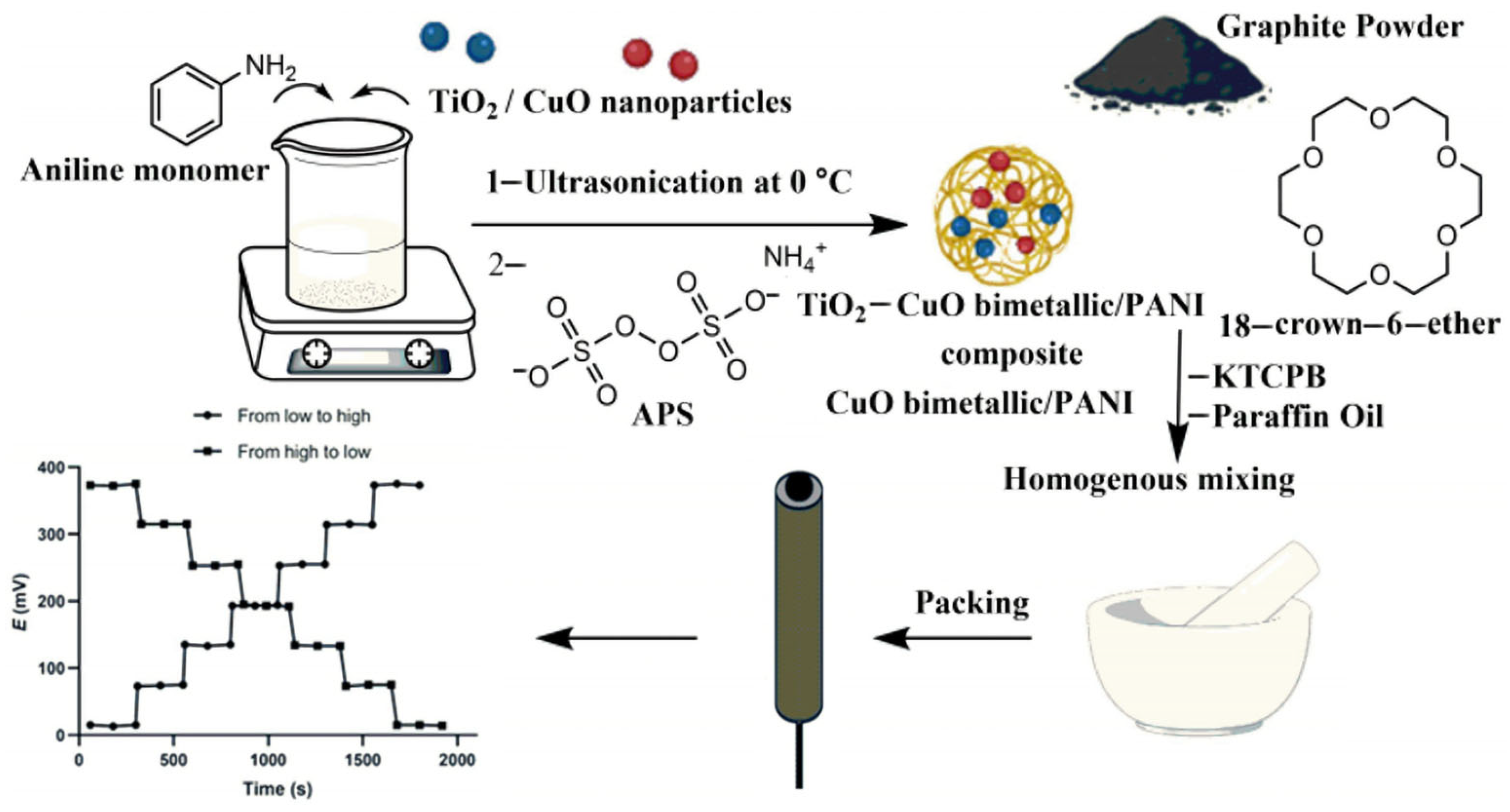
- Abdallah, N.A.; Ahmed, S.A.; Almaghrabi, M.; Alahmadi, Y.M. Utilization of a TiO2–CuO Bimetallic/Polyaniline Nanocomposite as a Transducer in a Solid Contact Potentiometric Sensor for the Determination of Vildagliptin. Polymers 2023, 15, 3991. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Sensors. Anal. Chem. 2008, 80, 4499–4517. [Google Scholar] [CrossRef]
- Joon, N.K.; He, N.; Ruzgas, T.; Bobacka, J.; Lisak, G. PVC-Based Ion-Selective Electrodes with a Silicone Rubber Outer Coating with Improved Analytical Performance. Anal. Chem. 2019, 91, 10524–10531. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhong, L.; Zhang, Y.; Mo, X.; Bao, Y.; Ma, Y.; Wang, W.; Han, D.; Gan, S.; Niu, L. A mixed electronic-ionic conductor-based bifunctional sensing layer beyond ionophores for sweat electrolyte monitoring. Sci. Bull. 2023, 68, 3181–3191. [Google Scholar] [CrossRef]
- Tanisaki, S. Crystal structure of monoclinic tungsten trioxide at room temperature. J. Phys. Soc. Jpn. 1960, 15, 573–581. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Z.; Liang, Q.; Ye, R.; Guo, S.; Zeng, X.; Hu, J.; Li, A. Ultrasensitive electrochemical biosensor for microRNA-377 detection based on MXene-Au nanocomposite and G-quadruplex nano-amplification strategy. Electrochim. Acta 2022, 428, 140945. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Chen, Z.; Zhao, D.; Zhang, X.; Zhuo, J.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Ti3C2Tx MXene for electrode materials of supercapacitors. J. Mater. Chem. A 2021, 9, 11501–11529. [Google Scholar] [CrossRef]
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Liu, S.; Yin, T.; Li, Z.; Wang, J. Potentiometric/time resolved chronopotentiometric sensing for an all-solid-state ion-selective electrode based on MXene/MWCNTs as solid contact. Sens. Actuators B Chem. 2024, 405, 135269. [Google Scholar] [CrossRef]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Magdy, R.; Hemdan, A.; Fares, N.V.; Mahmoud, A.M.; Marzouk, H.M.; Farouk, M. Sustainable graphene nanoparticle-enhanced in-line potentiometric ion selective sensor for testing of Perindopril in human plasma. Microchem. J. 2024, 198, 110108. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ragab, M.T.; Ramadan, N.K.; El-Ragehy, N.A.; El-Zeany, B.A. Design of Solid-contact Ion-selective Electrode with Graphene Transducer Layer for the Determination of Flavoxate Hydrochloride in Dosage Form and in Spiked Human Plasma. Electroanalysis 2020, 32, 2803–2811. [Google Scholar] [CrossRef]
- Xu, J.; Jia, F.; Li, F.; An, Q.; Gan, S.; Zhang, Q.; Ivaska, A.; Niu, L. Simple and Efficient Synthesis of Gold Nanoclusters and Their Performance as Solid Contact of Ion Selective Electrode. Electrochim. Acta 2016, 222, 1007–1012. [Google Scholar] [CrossRef]
- Li l Yin, T.; Qin, W. An effective solid contact for an all-solid-state polymeric membrane Cd2+-selective electrode: Three-dimensional porous graphene-mesoporous platinum nanoparticle composite. Sens. Actuator B Chem. 2017, 239, 438–446. [Google Scholar] [CrossRef]
- Zeng, X.; Qin, W. A solid-contact Ca2+-selective electrode based on an inorganic redox buffer of Ag@AgCl/1-tetradecyl-3-methylimidazolium chloride as ion-to-electron transducer. Talanta 2020, 209, 120570. [Google Scholar] [CrossRef] [PubMed]
- Duy Nguyen, L.; Mau Dang, C.; Chanh Duc Doan, T. Highly stable ammonium ion-selective electrodes based on one-pot synthesized gold nanoparticle-reduced graphene oxide as ion-to-electron transducers. Microchem. J. 2023, 190, 108717. [Google Scholar] [CrossRef]
- Chu, T.; Chu, J.; Gao, B.; He, B. Modern evolution of paper-based analytical devices for wearable use: From disorder to order. Analyst 2020, 145, 5388–5399. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Li, T.; Ji, Y.; Li, R. Molecularly imprinted polymer-enhanced biomimetic paper-based analytical devices: A review. Anal. Chem. 2021, 1148, 238196. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Fathy, M.A. All-solid-state paper-based potentiometric combined sensor modified with reduced graphene oxide (rGO) and molecularly imprinted polymer for monitoring losartan drug in pharmaceuticals and biological samples. Talanta 2023, 253, 123907. [Google Scholar] [CrossRef]
- Ottiger, P.; Pfaffen, C.; Leist, R.; Leutwyler, S.; Bachorz, R.A.; Klopper, W. Strong N-H···π Hydrogen Bonding in Amide-Benzene Interactions. J. Phys. Chem. B 2009, 113, 2937–2943. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
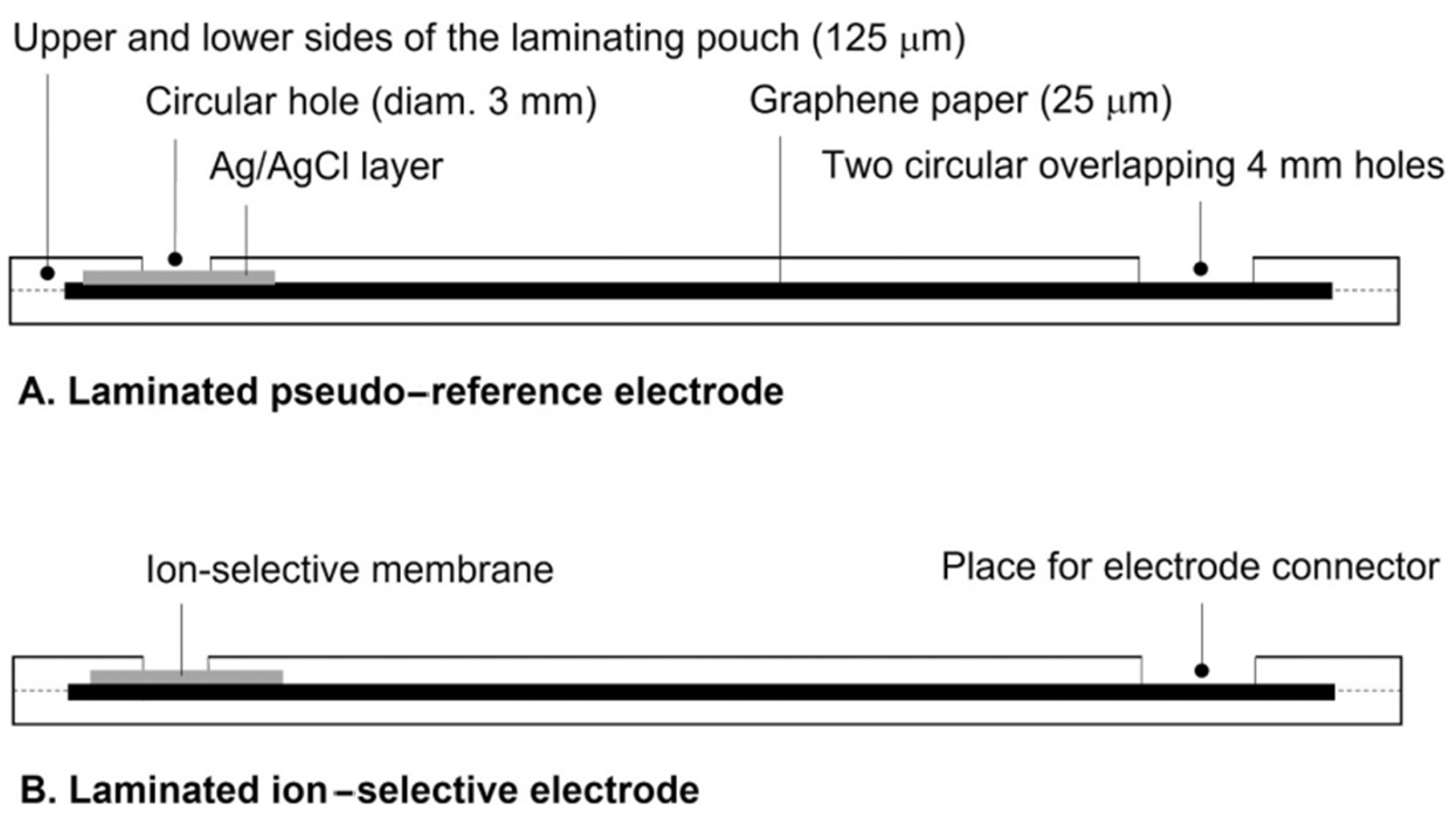
- Mandjoukov, B.; Lindfors, T. Planar, low-cost, flexible, and fully laminated graphene paper pseudo-reference and potassium-selective electrodes. Sens. Actuators B Chem. 2024, 403, 135190. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Mahendran, V.; Philip, J. Sensing of Biologically Important Cations Such as Na+, K+, Ca2+, Cu2+, and Fe3+ Using Magnetic Nanoemulsions. Langmuir 2013, 29, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Teekayupak, K.; Lomae, A.; Agir, I.; Chuaypen, N.; Dissayabutra, T.; Henry, C.S.; Chailapakul, O.; Ozer, T.; Ruecha, N. Large-scale fabrication of ion-selective electrodes for simultaneous detection of Na+, K+, and Ca2+ in biofluids using a smartphone-based potentiometric sensing platform. Microchim. Acta 2023, 190, 237. [Google Scholar] [CrossRef]


| The Type of SC-ISEs | Sample Matrix | Ion | Range (mol/L) | Response Slope (mV/Decade) | LOD (mol/L) | Ref |
|---|---|---|---|---|---|---|
| Oxidation–Reduction | Seawater | Pb2+ | 1.0 × 10−3 to 1.0 × 10−6 | 28.5 ± 0.8 | 1.9 × 10−7 | [38] |
| Sediment of Lake | Pb2+ | 1.0 × 10−7 to 1.0 × 10−2 | 29.89 | 1 × 10−7 | [48] | |
| Seawater | CO32− | 2.6 × 10−5 to 5.3 × 10−4 | 28.3 ± 0.4 | 1.1 × 10−5 | [50] | |
| Solution (CaCl2) | Ca2+ | 1.0 × 10−6 to 2.9 × 10−2 | 27.5 ± 0.2 | 5.0 × 10−7 | [52] | |
| Soil | NO3− | 1.0 × 10−7 to 1.0 × 10−1 | 58.85 | 2.5 × 10−6 | [58] | |
| Human Plasma | Vildagliptin | 1.0 × 10−8 to 1.0 × 10−2 | 60.04 ± 1.4 | 4.5 × 10−9 | [59] | |
| Human Biofluids | NH4+ | 1.0 × 10−5 to 1.0 × 10−1 | 55.7 | 10−4.8 | [62] | |
| EDL Capacitance | Solution (CaCl2) | Ca2+ | 1.0 × 10−5 to 1.0 × 10−1 | 25.8 ± 0.5 | 7.9 × 10−7 | [70] |
| Human Plasma | PER | 5.5 × 10−8 to 1.4 × 10−7 | 58.89 | 3.2 × 10−9 | [72] | |
| Lake Water | NH4+ | 1.0 × 10−5 to 1.0 × 10−2 | 56.94 ± 1.57 | 3.80 × 10−6 | [77] | |
| Human Urine | LOS-K | 8. × 10−5 to 6.9 × 10−2 | 58.2 ± 0.3 | 2.7 ± 0.3 × 10−7 | [80] | |
| Solution (KNO3) | K+ | 10−5.5 to 10−1 | 57.4 ± 0.3 | 6 × 10−7 | [83] | |
| Human Urine | Ca2+ | 10−4 to 10−1 | 30.1 | 10−5 | [86] | |
| Na+ | 10−4 to 10−1 | 42.9 | 10−5 | |||
| K+ | 10−3 to 10−1 | 38.03 | 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Tian, Y.; Gao, W.; Xu, G. Recent Developments and Challenges in Solid-Contact Ion-Selective Electrodes. Sensors 2024, 24, 4289. https://doi.org/10.3390/s24134289
Gao L, Tian Y, Gao W, Xu G. Recent Developments and Challenges in Solid-Contact Ion-Selective Electrodes. Sensors. 2024; 24(13):4289. https://doi.org/10.3390/s24134289
Chicago/Turabian StyleGao, Lili, Ye Tian, Wenyue Gao, and Guobao Xu. 2024. "Recent Developments and Challenges in Solid-Contact Ion-Selective Electrodes" Sensors 24, no. 13: 4289. https://doi.org/10.3390/s24134289
APA StyleGao, L., Tian, Y., Gao, W., & Xu, G. (2024). Recent Developments and Challenges in Solid-Contact Ion-Selective Electrodes. Sensors, 24(13), 4289. https://doi.org/10.3390/s24134289






