Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
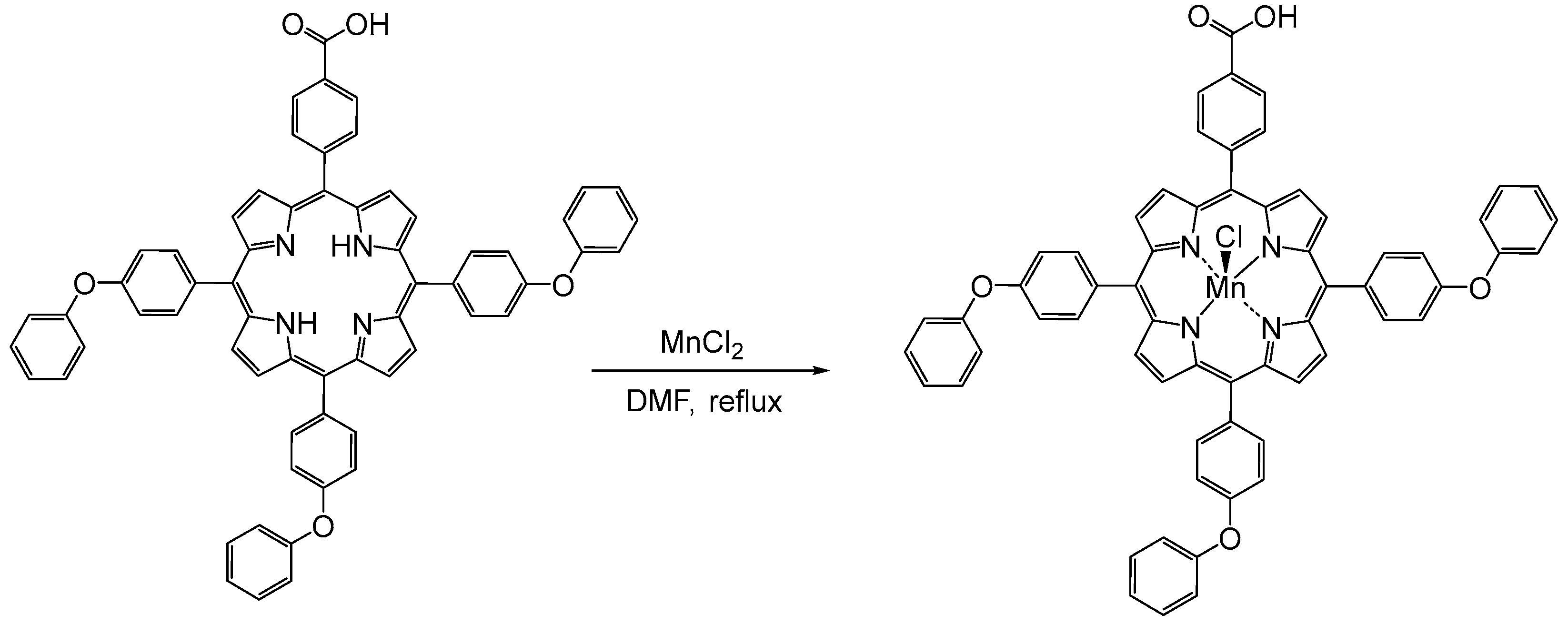
2.2. Manganese Porphyrin (5-(4-Carboxy-phenyl)-10,15,20-tris-(4-phenoxy-phenyl)-porphyrinmanganese(III) Chloride, Mn(III)Cl-COOH-TPOPP, Synthesis
2.3. Triiodide Ions Preparation
2.4. Polymeric Membrane Preparation
2.5. Real Sample Preparation
2.6. Apparatus
3. Results and Discussion
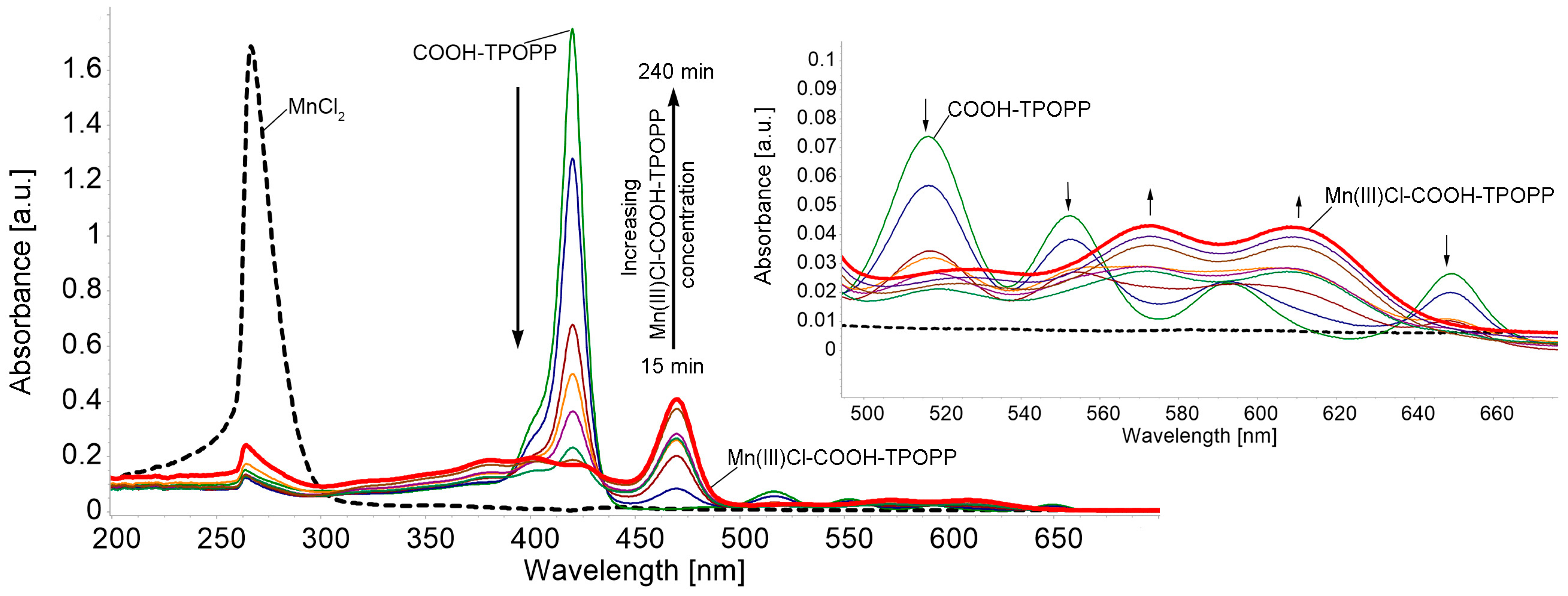
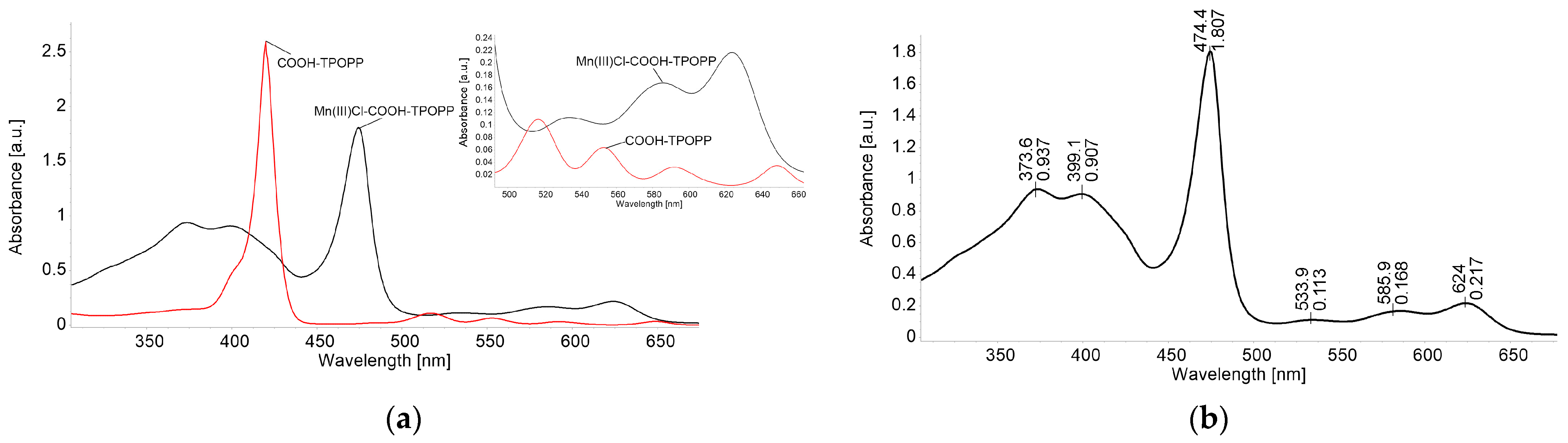
3.1. UV-Vis Monitorization of Mn(III)Cl-COOH-TPOPP Synthesis
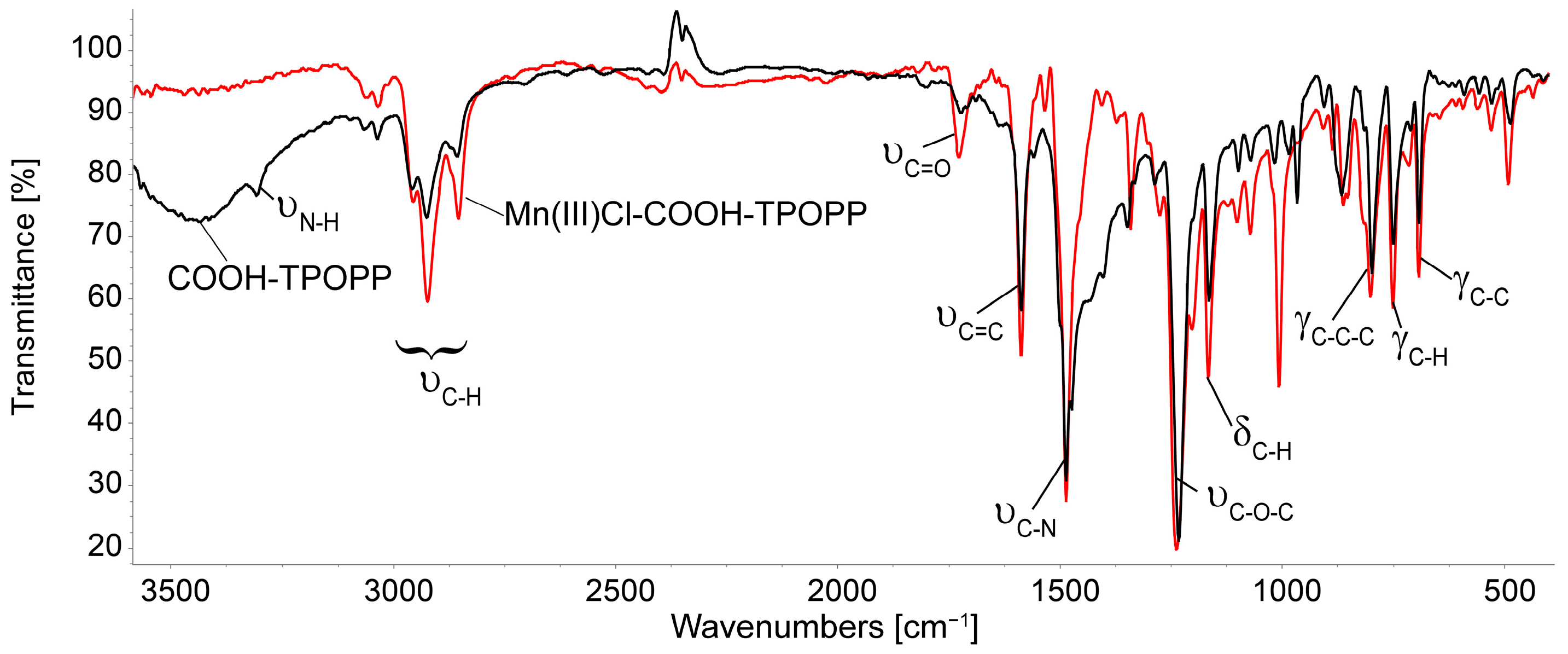
3.2. Comparison of the FT-IR Spectra of COOH-TPOPP and Mn(III)Cl-COOH-TPOPP
3.3. 1H-NMR of Mn(III)Cl-COOH-TPOPP Structure
3.4. Optical Detection of Iodide Anions
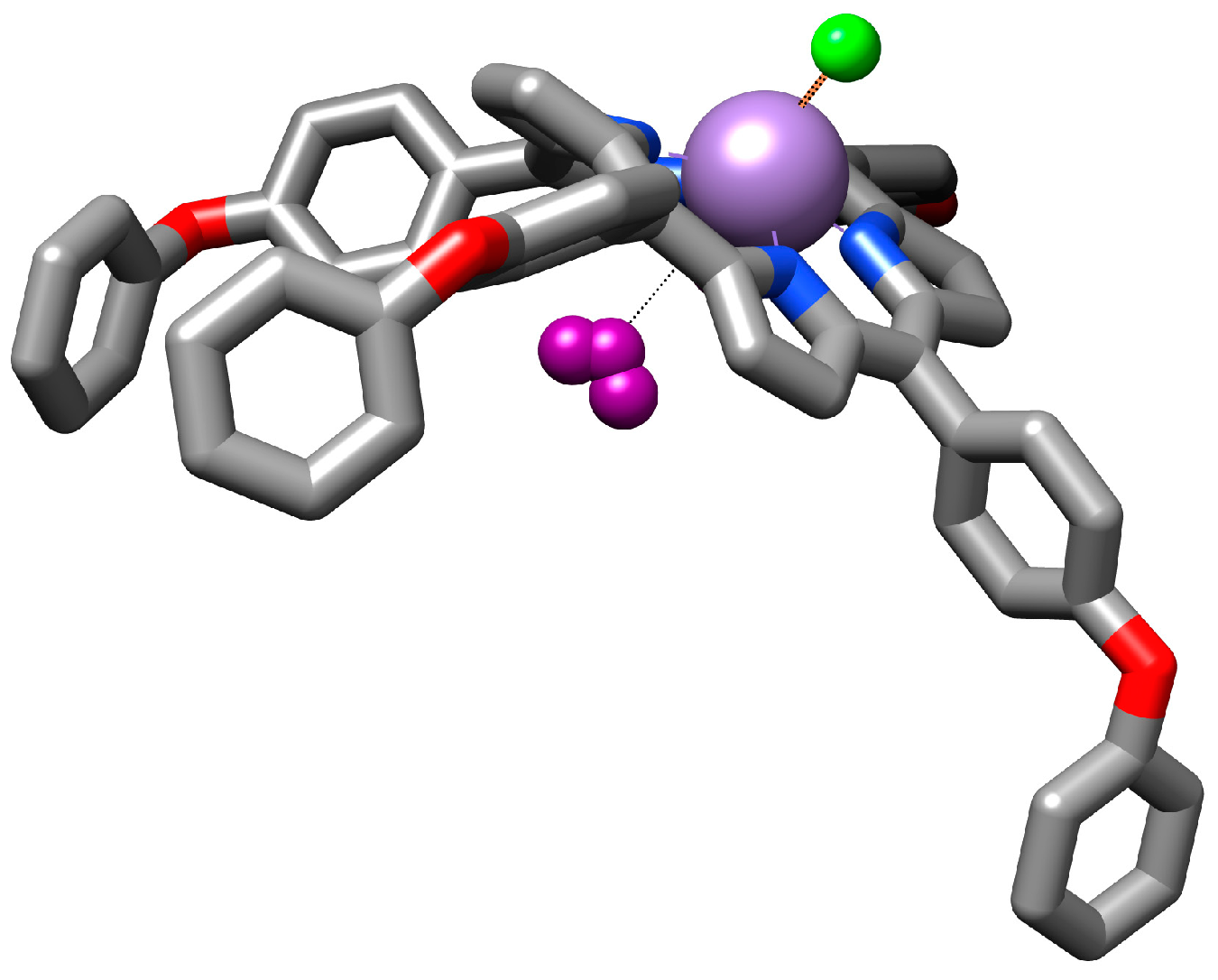
3.4.1. Proposed Mechanism for Triiodide Recognition
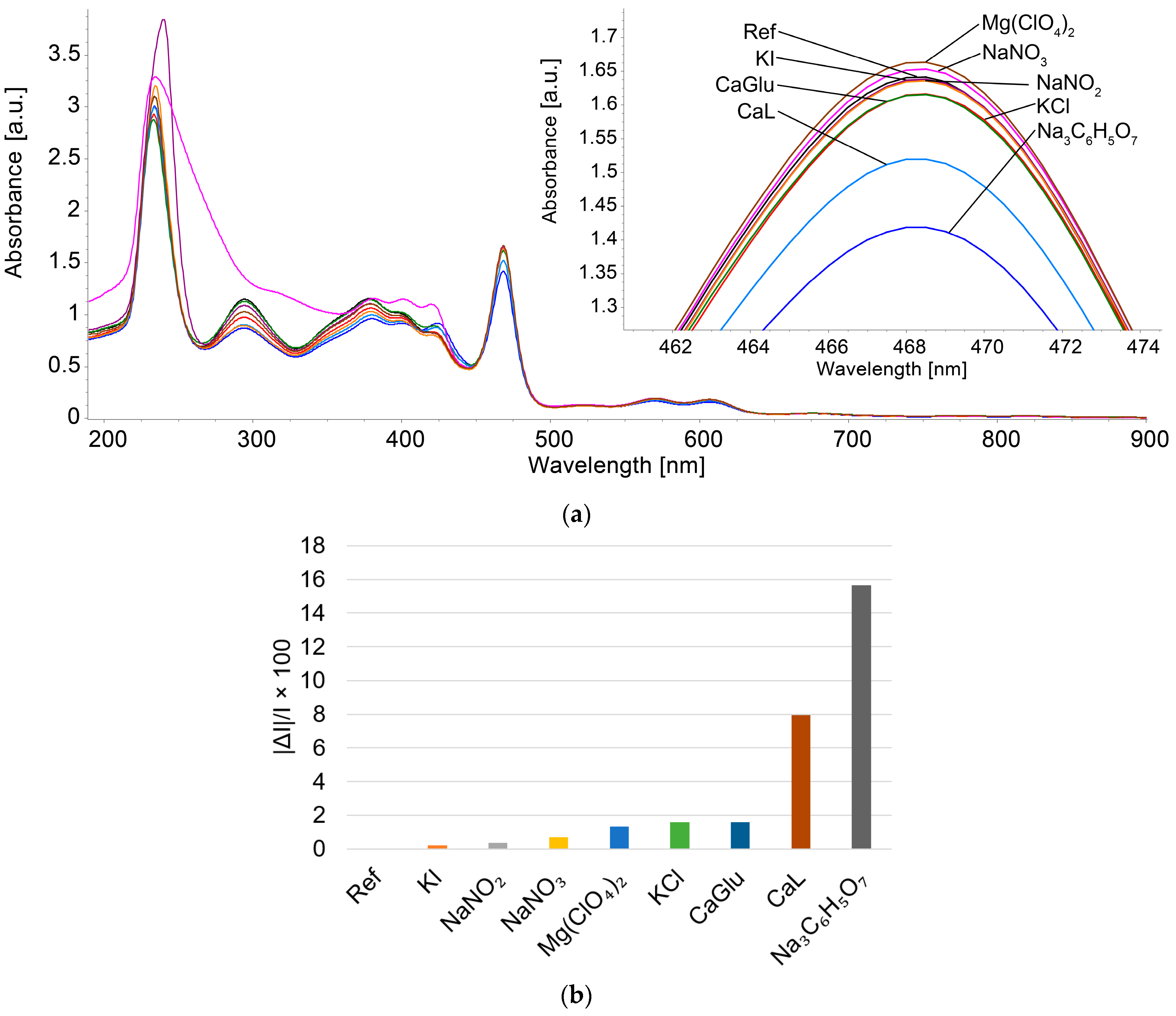
3.4.2. Study of the Influence of Potential Interfering Species on the Spectroscopic Detection of Triiodide
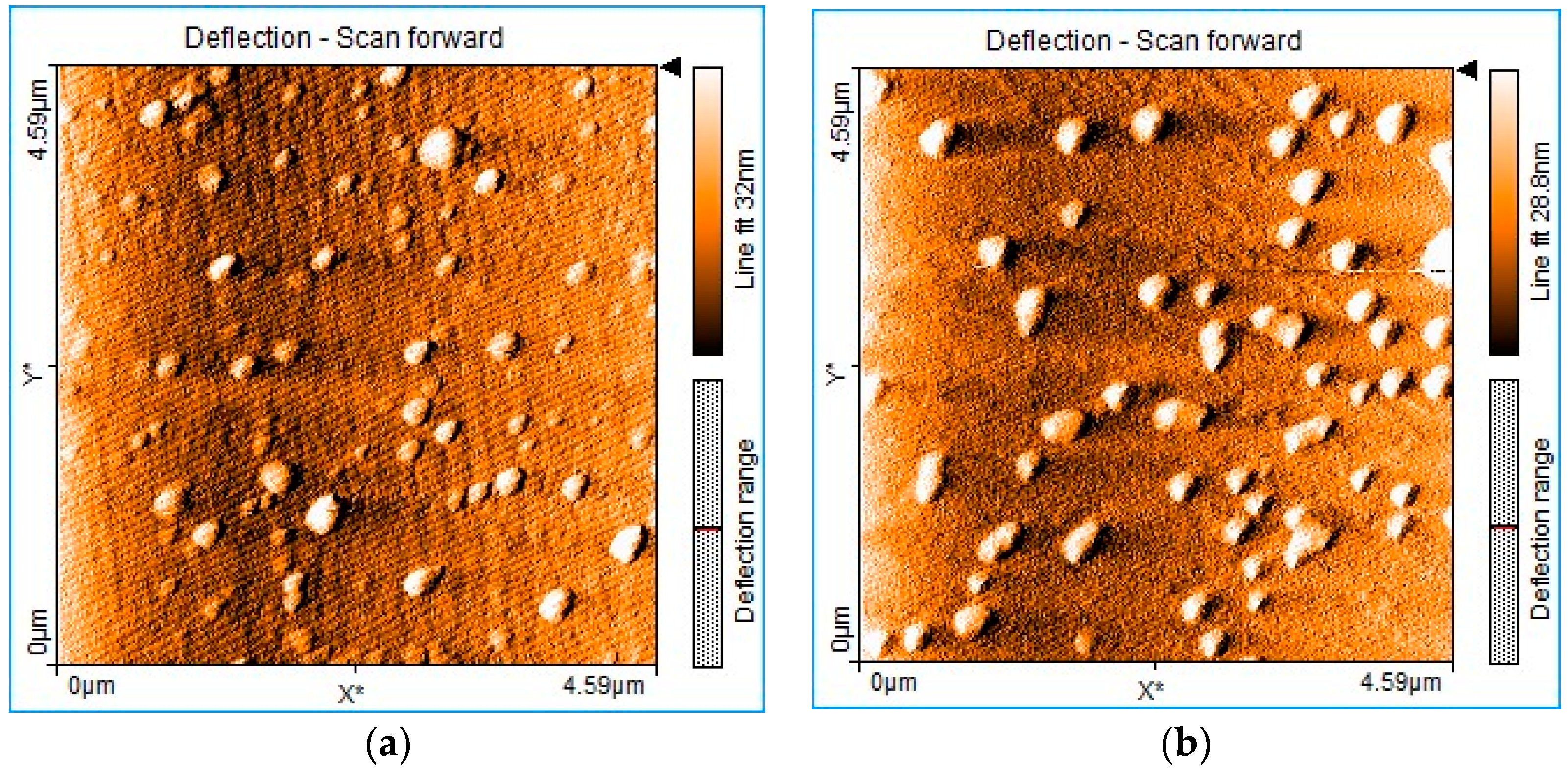
3.5. AFM Investigation of Mn(III)Cl-COOH-TPOPP before and after Treatment with Triiodide Anion
3.6. Potentiometric Detection of Iodide Anions
3.6.1. Proposed Mechanism for the Recognition of Iodide Anions by the Mn(III)Cl-COOH-TPOPP Ionophore
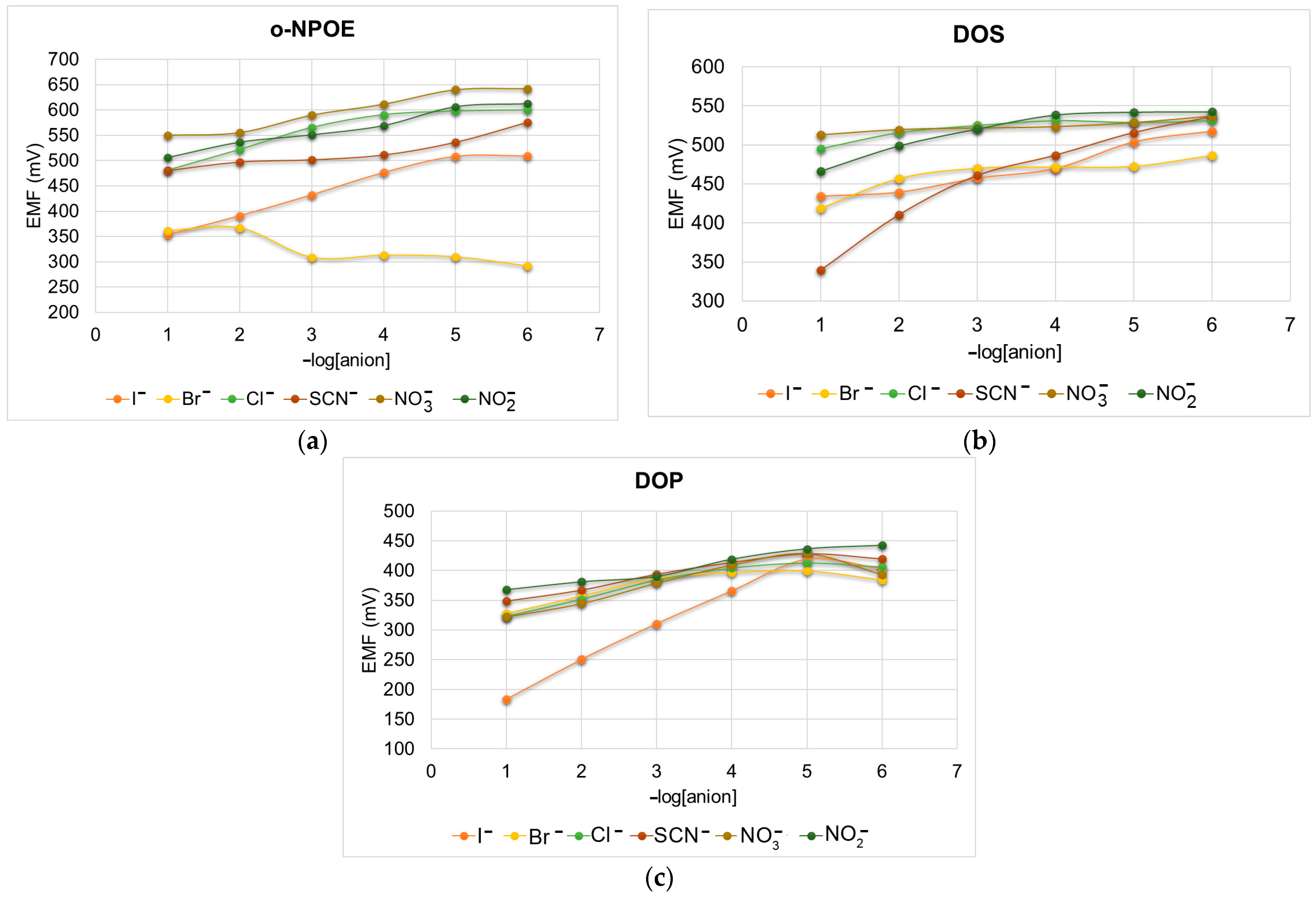
3.6.2. Study of Selectivity Coefficients
3.6.3. Analytical Application
3.6.4. Potentiometric Determination of Iodide Ions Using Different Sensing Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, K.; Skeaff, S. Iodine: Iodine Deficiency Disorders (IDD). Encycl. Food Health 2016, 3, 437–443. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Jia, Q.; Zhang, X.; Jin, X.; Shen, H. Effects of Excessive Iodine Intake on Blood Glucose, Blood Pressure, and Blood Lipids in Adults. Biol. Trace Elem. Res. 2019, 192, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Inoue, K.; Rhee, C.M.; Leung, A.M. Risks of Iodine Excess. Endocr. Rev. 2024, bnae019. [Google Scholar] [CrossRef]
- Xu, T.; Ren, Z.; Li, S.; Tan, L.; Zhang, W. The relationship of different levels of high iodine and goiter in school children: A meta-analysis. Nutr. Metab. 2021, 18, 46. [Google Scholar] [CrossRef]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Bereda, G. Hyperthyroidism: Definition, causes, pathophysiology and management. J. Biomed. Biol. Sci. 2022, 2, 1–11. [Google Scholar]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Espino-Vázquez, A.N.; Rojas-Castro, F.C.; Fajardo-Yamamoto, L.M. Implications and Practical Applications of the Chemical Speciation of Iodine in the Biological Context. Future Pharmacol. 2022, 2, 377–414. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, A.; Romarís-Hortas, V.; Bermejo-Barrera, P. A review on iodine speciation for environmental, biological and nutrition fields. J. Anal. At. Spectrom. 2011, 26, 2107–2152. [Google Scholar] [CrossRef]
- Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 581–593. [Google Scholar] [CrossRef]
- Jadhav, P.M.; Rode, A.B.; Kótai, L.; Pawar, R.P.; Tekale, S.U. Revisiting applications of molecular iodine in organic synthesis. New J. Chem. 2021, 45, 16389–16425. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.E.G.; Castellanos, B.F.H.; Martínez, E.N.R.; Ortiz, W.A.N.; Mendoza, A.B.; Macías, J.M. Outcomes of foliar iodine application on growth, minerals and antioxidants in tomato plants under salt stress. Folia Hortic. 2022, 34, 27–37. [Google Scholar] [CrossRef]
- Bentley, C.L.; Bond, A.M.; Hollenkamp, A.F.; Mahon, P.J.; Zhang, J. Voltammetric Determination of the Iodide/Iodine Formal Potential and Triiodide Stability Constant in Conventional and Ionic Liquid Media. J. Phys. Chem. C 2015, 119, 22392–22403. [Google Scholar] [CrossRef]
- Edis, Z.; Haj Bloukh, S.; Abu Sara, H.; Bhakhoa, H.; Rhyman, L.; Ramasami, P. “Smart” Triiodide Compounds: Does Halogen Bonding Influence Antimicrobial Activities? Pathogens 2019, 8, 182. [Google Scholar] [CrossRef]
- Moulay, S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Naorem, H.; Devi, S.D. Spectrophotometric determination of the formation constant of triiodide ions in aqueous-organic solvent or polymer mixed media both in absence and presence of a surfactant. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.A.; Ramette, R.W.; Mesmer, R.E. Triiodide ion formation equilibrium and activity coefficients in aqueous solution. J. Solut. Chem. 1984, 13, 673–683. [Google Scholar] [CrossRef]
- Sasamura, S.; Ohnuki, T.; Kozai, N.; Amachi, S. Iodate respiration by Azoarcus sp. DN11 and its potential use for removal of radioiodine from contaminated aquifers. Front. Microbiol. 2023, 14, 1162788. [Google Scholar] [CrossRef]
- Kireev, S.V.; Shnyrev, S.L. Study of molecular iodine, iodate ions, iodide ions, and triiodide ions solutions absorption in the UV and visible light spectral bands. Laser Phys. 2015, 25, 075602. [Google Scholar] [CrossRef]
- Choi, J.H.; Ahn, I.H.; Sessler, J.L.; Cho, D.G. Colorimetric iodide detection in water: A new photo-activated indicator system. Supramol. Chem. 2011, 23, 283–286. [Google Scholar] [CrossRef]
- Lipert, R.; Porter, M.; Siperko, L.; Gazda, D.; Rutz, J.; Schultz, J.; Flint, S.; McCoy, J.T. Colorimetric Solid Phase Extraction for the Measurement of Total I (iodine, iodide, and triiodide) in Spacecraft Drinking Water. In Proceedings of the 40th International Conference on Environmental Systems, Barcelona, Spain, 11–15 July 2009; p. 6045. [Google Scholar] [CrossRef]
- Chandrawanshi, S.; Patel, K.S. Field determination of iodide in water. Fresenius J. Anal. Chem. 1995, 352, 599–600. [Google Scholar] [CrossRef]
- Maruthupandi, M.; Chandhru, M.; Rani, S.K.; Vasimalai, N. Highly selective detection of iodide in biological, food, and environmental samples using polymer-capped silver nanoparticles: Preparation of a paper-based testing kit for on-site monitoring. ACS Omega 2019, 4, 11372–11379. [Google Scholar] [CrossRef]
- Takeda, A.; Tsukada, H.; Takaku, Y.; Satta, N.; Baba, M.; Shibata, T.; Hasegawa, H.; Unno, Y.; Hisamatsu, S. Determination of Iodide, Iodate and Total Iodine in Natural Water Samples by HPLC with Amperometric and Spectrophotometric Detection, and Off-line UV Irradiation. Anal. Sci. 2016, 32, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Yeon, J.-W.; Kang, Y.; Song, K. Determination of Triiodide Ion Concentration Using UV-Visible Spectrophotometry. Asian J. Chem. 2014, 26, 4084–4086. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Cao, T.; Zhou, Y.; Dong, L.; Liu, L.; Tong, Z. Synthesis of manganese porphyrins/electrochemical reduction of graphene oxide nanocomposite to simultaneously detect dopamine and uric acid under the interference of ascorbic acid. Mater. Chem. Phys. 2023, 307, 128225. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.-F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef] [PubMed]
- Olszowski, P.; Zając, Ł.; Godlewski, S.; Such, B.; Jöhr, R.; Glatzel, T.; Meyer, E.; Szymonski, M. Role of a Carboxyl Group in the Adsorption of Zn Porphyrins on TiO2(011)-2×1 Surface. J. Phys. Chem. C 2015, 119, 21561–21566. [Google Scholar] [CrossRef]
- Bakar, M.B.; Oelgemöller, M.; Senge, M.O. Lead structures for applications in photodynamic therapy. Part 2: Synthetic studies for photo-triggered release systems of bioconjugate porphyrin photosensitizers. Tetrahedron 2009, 65, 7064–7078. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Maggini, S.; Proserpio, D.M.; Ragaini, F.; Gallo, E.; Ranocchiari, M.; Caselli, A. Synthesis and characterization of new tetra-substituted porphyrins with exo-donor carboxylic groups as building blocks for supramolecular architectures: Catalytic and structural studies of their metalated derivatives. J. Porphyr. Phthalocyanines 2010, 14, 804–814. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, G. Novel fluorescent pH sensor based on 5-(4-carboxy-phenyl)-10,15,20-tris(phenyl)-porphyrin. Arab. J. Chem. 2014, 12, 1587–1594. [Google Scholar] [CrossRef]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-Based Nanostructures for Sensing Applications. J. Sens. 2009, 2009, 856053. [Google Scholar] [CrossRef]
- Reeta, P.S.; Kandhadi, J.; Lingamallu, G. One-pot synthesis of β-carboxy tetra aryl porphyrins: Potential applications to dye-sensitized solar cells. Tetrahedron Lett. 2010, 51, 2865–2867. [Google Scholar] [CrossRef]
- Özbek, O.; Isildak, Ö.; Berkel, C. The use of porphyrins in potentiometric sensors as ionophores. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 1–9. [Google Scholar] [CrossRef]
- Vlascici, D.; Pruneanu, S.; Olenic, L.; Pogacean, F.; Ostafe, V.; Chiriac, V.; Pica, E.M.; Bolundut, L.C.; Nica, L.; Fagadar-Cosma, E. Manganese(III) Porphyrin-based Potentiometric Sensors for Diclofenac Assay in Pharmaceutical Preparations. Sensors 2010, 10, 8850–8864. [Google Scholar] [CrossRef]
- Beheshti, S.S.; Sohbat, F.; Amini, M.K. A manganese porphyrin-based sensor for flow-injection potentiometric determination of thiocyanate. J. Porphyr. Phthalocyanines 2010, 14, 158–165. [Google Scholar] [CrossRef]
- Santos, E.M.G.; Couto, C.M.C.M.; Araújo, A.N.; Montenegro, M.C.B.S.M.; Reis, B.F. Determination of gibberellic acid by sequential injection analysis using a potentiometric detector based on Mn(III)-porphyrin with improved characteristics. J. Braz. Chem. Soc. 2004, 15, 701–707. [Google Scholar] [CrossRef]
- Zhang, D.; Lan, W.; Zhou, Z.; Yang, L.; Liu, Q.; Bian, Y.; Jiang, J. Manganese(III) Porphyrin-Based Magnetic Materials. Top. Curr. Chem. 2019, 377, 18. [Google Scholar] [CrossRef] [PubMed]
- Gkini, K.; Balis, N.; Papadakis, M.; Verykios, A.; Scoulicidou, M.-C.; Drivas, C.; Kennou, S.; Golomb, M.; Walsh, A.; Coutsolelos, A.G.; et al. Manganese Porphyrin Interface Engineering in Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 7353–7363. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, G.; Zhang, Z.; Yong, T.; Yang, X.; Gan, L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics 2021, 11, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.-W.; Dong, M.-M.; Zhang, R.-Q.; Wang, C.-K.; Fu, X.-X. An ultra-sensitive gas sensor based on a two-dimensional manganese porphyrin monolayer. Phys. Chem. Chem. Phys. 2021, 23, 11852–11862. [Google Scholar] [CrossRef] [PubMed]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and electrochemical-mediated detection of ascorbic acid using manganese porphyrin and its gold hybrids. Comptes Rendus Chim. 2018, 21, 327–338. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef]
- Lahaye, D.; Muthukumaran, K.; Hung, C.-H.; Gryko, D.; Rebouças, J.S.; Spasojević, I.; Batinic’-Haberle, I.; Lindsey, J.S. Design and synthesis of manganese porphyrins with tailored lipophilicity: Investigation of redox properties and superoxide dismutase activity. Bioorg. Med. Chem. 2007, 15, 7066–7086. [Google Scholar] [CrossRef]
- Egorov, V.V.; Zdrachek, E.A.; Nazarov, V.A. Improved Separate Solution Method for Determination of Low Selectivity Coefficients. Anal. Chem. 2014, 86, 3693–3696. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Elkhovikova, A.A.; Lomova, T.N. Coordination of Manganese(III) Porphyrins with Pyridine as a Model of Formation of Donor–Acceptor Dyads with Fullerene Acceptors. Russ. J. Inorg. Chem. 2024. [Google Scholar] [CrossRef]
- Bharati, S.L.; Sarma, C.; Hazarika, P.J.; Chaurasia, P.K.; Anand, N.; Yadava, S. Novel Mn(III) Porphyrins and Prospects of Their Application in Catalysis. Russ. J. Inorg. Chem. 2019, 64, 335–341. [Google Scholar] [CrossRef]
- Boucher, L.J. Manganese porphyrin complexes. Coord. Chem. Rev. 1972, 7, 289–329. [Google Scholar] [CrossRef]
- Galinato, M.G.I.; Brocious, E.P.; Paulat, F.; Martin, S.; Skodack, J.; Harland, J.B.; Lehnert, N. Elucidating the Electronic Structure of High-Spin [MnIII(TPP)Cl] Using Magnetic Circular Dichroism Spectroscopy. Inorg. Chem. 2020, 59, 2144–2162. [Google Scholar] [CrossRef]
- Che, C.M.; Yu, W.Y. Ruthenium-oxo and-tosylimido porphyrin complexes for epoxidation and aziridination of alkenes. Pure Appl. Chem. 1999, 71, 281–288. [Google Scholar] [CrossRef]
- Li, X.; Nomura, K.; Guedes, A.; Goto, T.; Sekino, T.; Fujitsuka, M.; Osakada, Y. Enhanced Photocatalytic Activity of Porphyrin Nanodisks Prepared by Exfoliation of Metalloporphyrin-Based Covalent Organic Frameworks. ACS Omega 2022, 7, 7172–7178. [Google Scholar] [CrossRef] [PubMed]
- Kubovics, M.; Careta, O.; Vallcorba, O.; Romo-Islas, G.; Rodríguez, L.; Ayllón, J.A.; Domingo, C.; Nogués, C.; López-Periago, A.M. Supercritical CO2 Synthesis of Porous Metalloporphyrin Frameworks: Application in Photodynamic Therapy. Chem. Mater. 2023, 35, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Suwarno, A.C.; Yulizar, Y.; Haerudin, H.; Kurniawaty, I.; Apriandanu, D.O.B. Investigation of metalloporphyrin in maltenes phase of crude oil Duri. J. Phys. Conf. Ser. 2020, 1442, 012049. [Google Scholar] [CrossRef]
- Sil, D.; Bhowmik, S.; Khan, F.S.T.; Rath, S.P. Experimental and Theoretical Investigation of a Series of Novel Dimanganese(III) μ-Hydroxo Bisporphyrins: Magneto–Structural Correlation and Effect of Metal Spin on Porphyrin Core Deformation. Inorg. Chem. 2016, 55, 3239–3251. [Google Scholar] [CrossRef]
- Bertini, I.; Luchinat, C.; Parigi, G.; Pierattelli, R. NMR Spectroscopy of Paramagnetic Metalloproteins. ChemBioChem 2005, 6, 1536–1549. [Google Scholar] [CrossRef]
- Swartjes, A.; White, P.B.; Bruekers, J.P.J.; Elemans, J.A.A.W.; Nolte, R.J.M. Paramagnetic relaxation enhancement NMR as a tool to probe guest binding and exchange in metallohosts. Nat. Commun. 2022, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, E.; Gárate-Morales, J.L.; Sandoval-Lira, J.; Hernández-Pérez, J.M.; Aguilar-Sánchez, R. Porphyrin Supramolecular Arrays Formed by Weakly Interacting Meso-Functional Groups on Au(111). Molecules 2019, 24, 3326. [Google Scholar] [CrossRef]
- Ishizuka, T.; Grover, N.; Kingsbury, C.J.; Kotani, H.; Senge, M.O.; Kojima, T. Nonplanar porphyrins: Synthesis, properties, and unique functionalities. Chem. Soc. Rev. 2022, 51, 7560–7630. [Google Scholar] [CrossRef]
- Antony, A.; Mitra, J. Refractive index-assisted UV/Vis spectrophotometry to overcome spectral interference by impurities. Anal. Chim. Acta 2021, 1149, 238186. [Google Scholar] [CrossRef]
- Kumar, S. Recent Developments of Biobased Plasticizers and Their Effect on Mechanical and Thermal Properties of Poly (vinyl chloride): A Review. Ind. Eng. Chem. Res. 2019, 58, 11659–11672. [Google Scholar] [CrossRef]
- Vlascici, D.; Lascu, A.; Fratilescu, I.; Anghel, D.; Epuran, C.; Birdeanu, M.; Chiriac, V.; Fagadar-Cosma, E. Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate. Chemosensors 2023, 11, 108. [Google Scholar] [CrossRef]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part, I. Inorganic cations. Pure Appl. Chem. 2002, 74, 923–994. [Google Scholar] [CrossRef]
- De Souza, F.C.; Vegas, C.G.; da Silva, D.A.I.; Ribeiro, M.S.; Cabral, M.F.; de Melo, M.A.; Mattos, R.M.T.; Faria, R.B.; D’Elia, E. Amperometric and potentiometric determination of iodide using carbon electrodes modified with salophen complex. J. Electroanal. Chem. 2016, 783, 49–55. [Google Scholar] [CrossRef]
- Seah, G.E.; Tan, A.Y.; Neo, Z.H.; Lim, J.Y.; Goh, S.S. Halogen bonding ionophore for potentiometric iodide sensing. Anal. Chem. 2021, 93, 15543–15549. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Shehab, O.R. Comparative Study of Carbon Paste, Screen Printed, and PVC Potentiometric Sensors Based on Copper-sulphamethazine Schiff Base Complex for Determination of Iodide—Experimental and Theoretical Approaches. Electroanalysis 2015, 28, 800–807. [Google Scholar] [CrossRef]
- Machado, A.; Mesquita, R.B.R.; Oliveira, S.; Bordalo, A.A. Development of a robust, fast screening method for the potentiometric determination of iodide in urine and salt samples. Talanta 2017, 167, 688–694. [Google Scholar] [CrossRef]
- Hossieni, O.; Maashi, S.A.; Chamkouri, N.; Sabati, Z.; Nourbakhsh, P.; Sajedinejad, M.; Gholamiyan, A. A Selective Membrane Electrode for Iodide Ion based on New Ionophore and its Application to Pharmaceutical Samples. Indian J. Forensic Med. Toxicol. 2020, 14, 1394. [Google Scholar] [CrossRef]
- Aslaner, S.İ.; Demirel Özel, A. The use of nanocomposite approach in the construction of carbon paste electrode and its application for the potentiometric determination of iodide. Monatshefte Chem.-Chem. Mon. 2022, 153, 881–893. [Google Scholar] [CrossRef]


| Ionophore (g) | Plasticizer (g) | NaTPB (g) | PVC | THF (mL) | ||
|---|---|---|---|---|---|---|
| 0.0045 | DOP | o-NPOE | DOS | 0.0009 | 0.1485 | 2 |
| 0.303 | ||||||
| 0.288 | ||||||
| 0.333 | ||||||
| Sensor | Anion | Selectivity Coefficients (10−3 M) |
|---|---|---|
| DOP | 0.00 | |
| −3.12 | ||
| −2.84 | ||
| −3.26 | ||
| −3.07 | ||
| −2.78 |
| Samples | Sample Amount Labeled (mg) | Potentiometric Detection a (mg) |
|---|---|---|
| Iod Forte 600 (Pharmapharm, Targu-Mures, Romania) | 0.600 | 0.597 ± 0.2 |
| KI + Silymarin (Arena Group SA, Bucuresti, Romania) | 1 | 0.95 ± 0.1 |
| Synthetic sample | 100 | 98 ± 0.9 |
| Ionophore/Electrode | Detection Limit (mol/L) | Detection Domain (mol/L) | Ref. |
|---|---|---|---|
| N,N′-disalicylidene-1,2-phenylendiamine dianion | 1.0 × 10−5 | 10−5–1 | [64] |
| 5,5′-(((5-((1-benzyl-5-iodo-1H-1,2,3-triazol-4-yl)methoxy)-1,3-phenylene)bis(oxy))bis(methylene))bis(1-benzyl-4-iodo-1H-1,2,3-triazole) | 1.25 × 10−6 | 10−9–10−1 | [65] |
| N-(4,6-Dimethyl-pyrimidin-2-yl)-4-[(2-hydroxybenzylidene)amino] benzene sulfonamide copper(II) dihydrated complex | 3.2 × 10−6 | - | [66] |
| Combined iodide commercial electrode (Hanna, RI, USA) | 1.39 × 10−6 | 2.5 × 10−6–1.0 × 10−3 | [67] |
| Imidazolidine-2tion | 7 × 10−7 | 1 × 10−6–1 × 10−1 | [68] |
| Dichloro[1,1′-bis(diphenylphosphino)ferrocene]palladium(II) complex | 2.9 × 10−8 | 1.0 × 10−6–1.0 × 10−1 | [69] |
| Mn(III)Cl-COOH-TPOPP namely 5-(4-carboxy-phenyl)-10,15,20-tris-(4-phenoxy-phenyl)-porphyrinmanganese(III) chloride | 8 × 10−6 | 1.0 × 10−5–1.0 × 10−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, D.; Epuran, C.; Fringu, I.; Fratilescu, I.; Lascu, A.; Macsim, A.-M.; Chiriac, V.; Gherban, M.; Vlascici, D.; Fagadar-Cosma, E. Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound. Sensors 2024, 24, 5517. https://doi.org/10.3390/s24175517
Anghel D, Epuran C, Fringu I, Fratilescu I, Lascu A, Macsim A-M, Chiriac V, Gherban M, Vlascici D, Fagadar-Cosma E. Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound. Sensors. 2024; 24(17):5517. https://doi.org/10.3390/s24175517
Chicago/Turabian StyleAnghel, Diana, Camelia Epuran, Ionela Fringu, Ion Fratilescu, Anca Lascu, Ana-Maria Macsim, Vlad Chiriac, Mihaela Gherban, Dana Vlascici, and Eugenia Fagadar-Cosma. 2024. "Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound" Sensors 24, no. 17: 5517. https://doi.org/10.3390/s24175517
APA StyleAnghel, D., Epuran, C., Fringu, I., Fratilescu, I., Lascu, A., Macsim, A.-M., Chiriac, V., Gherban, M., Vlascici, D., & Fagadar-Cosma, E. (2024). Double Type Detection of Triiodide and Iodide Ions Using a Manganese(III) Porphyrin as Sensitive Compound. Sensors, 24(17), 5517. https://doi.org/10.3390/s24175517







