Wireless and Battery-Free Sensor for Interstitial Fluid Pressure Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensor Fabrication
2.3. Instrumentation and Characterization
3. Results and Discussion
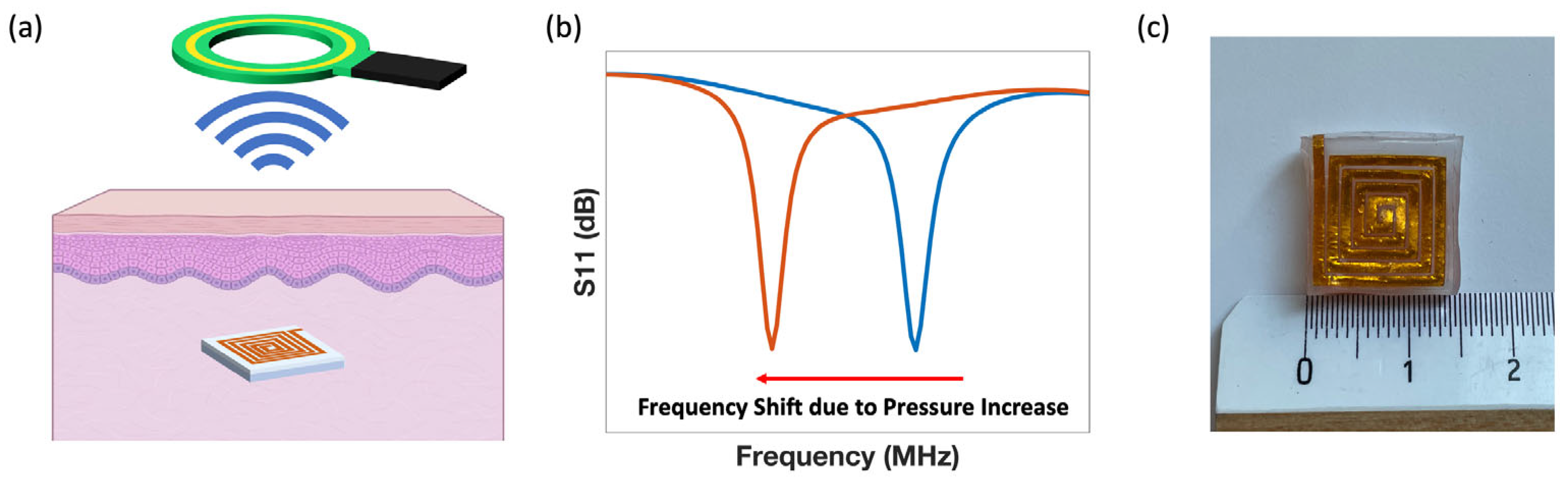
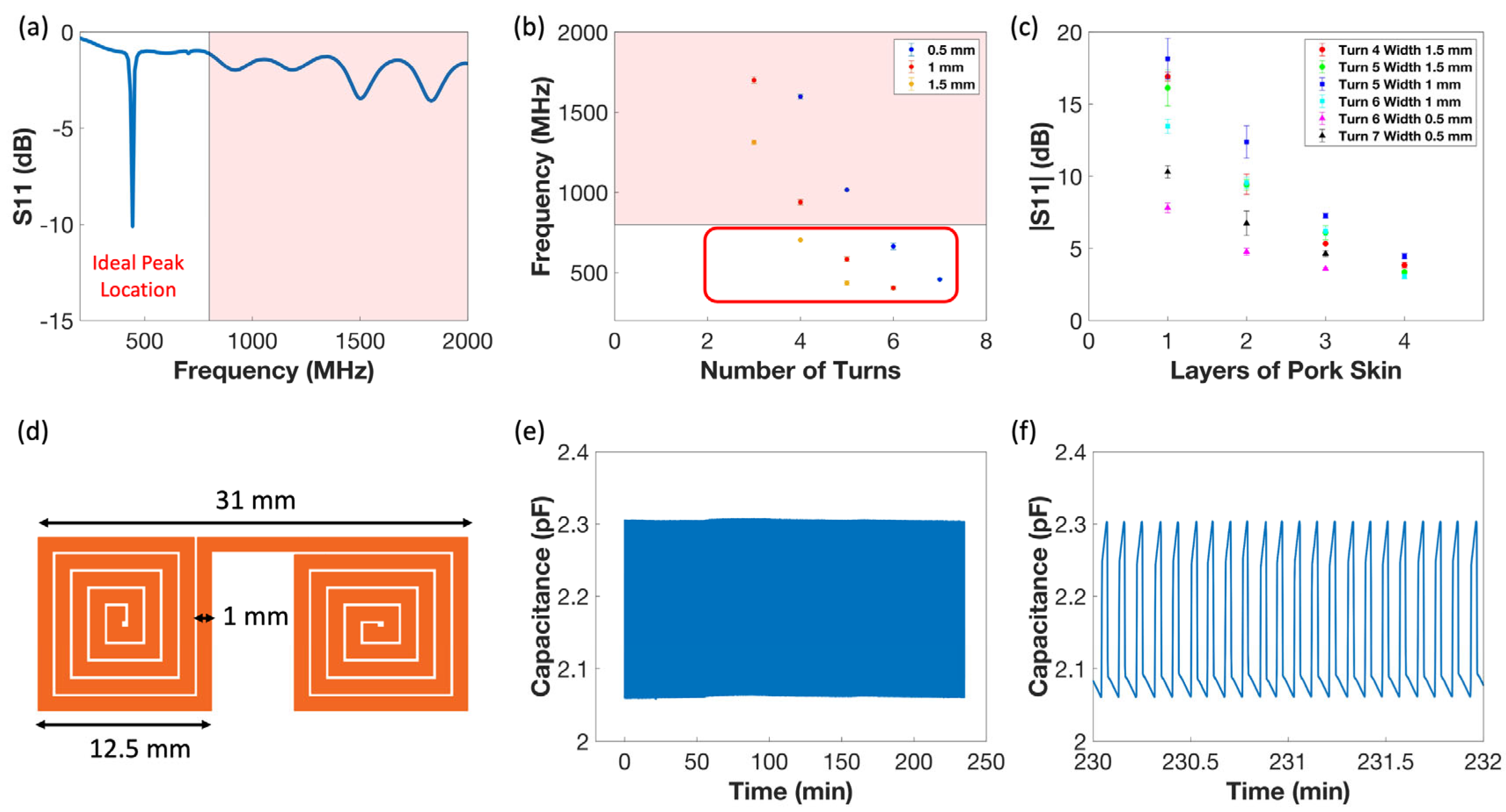
3.1. Sensor Form Factor Determination
3.2. Sensor Sensitivity Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothman, A.M.K.; Zafar, H.; Sandy, R.; Wright, C.; Mitra, S.; Ebah, L.; Ilyas, D.; Hanumapura, P.; Sebastien, S.; Khalifa, A.; et al. A Subcutaneous Multiparameter Sensor With Integrated Interstitial Fluid Pressure Measurement for Remote Heart Failure Monitoring. JACC Basic. Transl. Sci. 2023, 8, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Bruce, C.J.; Pressman, G.S.; Leinveber, P.; Romero-Corral, A.; Korinek, J.; Konecny, T.; Villarraga, H.R.; Kara, T.; Caples, S.M.; et al. Dynamic Changes of Left Ventricular Performance and Left Atrial Volume Induced by the Mueller Maneuver in Healthy Young Adults and Implications for Obstructive Sleep Apnea, Atrial Fibrillation and Heart Failure. Am. J. Cardiol. 2008, 102, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.S.; Horwitz, L.I.; Barreto-Filho, J.A.; Kim, N.; Bernheim, S.M.; Suter, L.G.; et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013, 309, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Rubini, A.; Vilaplana-Prieto, C.; Vázquez-Jarén, E.; Hernández-González, M.; Félix-Redondo, F.J.; Fernández-Bergés, D. Analysis and prediction of readmissions for heart failure in the first year after discharge with INCA score. Sci. Rep. 2023, 13, 22477. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.B.; Riley, S.R.; Prater, L.; Schnell, P.M.; Schuster, A.L.R.; Smith, S.A.; Foreman, B.; Xu, W.Y.; Gustin, J.; Li, Y.; et al. Advance Care Planning (ACP) in Medicare Beneficiaries with Heart Failure. J. Gen. Intern. Med. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for heart failure: Predict or prevent? Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Kalogeropoulos, A.; Georgiopoulou, V.; Belue, R.; Rodondi, N.; Garcia, M.; Bauer, D.C.; Satterfield, S.; Smith, A.L.; Vaccarino, V.; et al. Incident heart failure prediction in the elderly: The health ABC heart failure score. Circ. Heart Fail. 2008, 1, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, S.W.; Clark, K.A.A.; Xin, X.; Parzynski, C.S.; Riello, R.J., III; Sarocco, P.; Ahmad, T.; Desai, N.R. Thirty-Day and 90-Day Episode of Care Spending Following Heart Failure Hospitalization among Medicare Beneficiaries. Circ. Cardiovasc. Qual. Outcomes 2022, 15, E008069. [Google Scholar] [CrossRef]
- Okada, A.; Tsuchihashi-Makaya, M.; Kang, J.; Aoki, Y.; Fukawa, M.; Matsuoka, S. Symptom Perception, Evaluation, Response to Symptom, and Delayed Care Seeking in Patients with Acute Heart Failure: An Observational Study. J. Cardiovasc. Nurs. 2019, 34, 36–43. [Google Scholar] [CrossRef]
- Lin, C.Y.; Dracup, K.; Pelter, M.M.; Biddle, M.J.; Moser, D.K. Association of psychological distress with reasons for delay in seeking medical care in rural patients with worsening heart failure symptoms. J. Rural. Health 2022, 38, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hammash, M.; Miller, J.L.; Schrader, M.; Mudd-Martin, G.; Biddle, M.J.; Moser, D.K. Delay in seeking medical care for worsening heart failure symptoms: Predictors and association with cardiac events. Eur. J. Cardiovasc. Nurs. 2021, 20, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wexler, R.; Elton, T.; Pleister, A.; Feldman, D. Cardiomyopathy: An Overview. Am. Fam. Physician 2009, 79, 778–784. Available online: https://www.aafp.org/pubs/afp/issues/2009/0501/p778.html (accessed on 1 June 2023). [PubMed]
- De Couto, G.; Ouzounian, M.; Liu, P.P. Early detection of myocardial dysfunction and heart failure. Nat. Rev. Cardiol. 2010, 7, 334–344. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Patel, M.R. The Role of Cardiovascular MRI in Heart Failure and the Cardiomyopathies. Magn. Reson. Imaging Clin. N. Am. 2007, 25, 71–95. [Google Scholar] [CrossRef]
- Gehlbach, B.K.; Geppert, E. The Pulmonary Manifestations of Left Heart Failure. Chest 2004, 125, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Simon, B.; Alter, H.J.; Cheung, P. Ability of physicians to diagnose congestive heart failure based on chest X-ray. J. Emerg. Med. 2011, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, K.; Kelly, J. The electrocardiogram in heart failure. Age Ageing 2000, 29, 203–206. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Tong, W. Measuring impedance in congestive heart failure: Current options and clinical applications. Am. Heart J. 2009, 157, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Wang, L.; Chau, E.; Chan, R.H.-W.; Kong, S.-L.; Tang, M.-O.; Christensen, J.; Stadler, R.W.; Lau, C.-P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef]
- Afari, M.E.; Syed, W.; Tsao, L. Implantable devices for heart failure monitoring and therapy. Heart Fail. Rev. 2018, 23, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, R.; Pedram, M.; Ghasemzadeh, H. SmartSock: A wearable platform for context-aware assessment of ankle edema. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Orlando, FL, USA, 16–20 August 2016; pp. 6302–6306. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Cheng, H.; Shin, W.-J.; Fan, J.A.; Liu, Z.; Lu, C.-J.; Kong, G.-W.; Chen, K.; Patnaik, D.; et al. Materials and designs for wireless epidermal sensors of hydration and strain. Adv. Funct. Mater. 2014, 24, 3846–3854. [Google Scholar] [CrossRef]
- Yu, F.; Bilberg, A.; Xiao, L.; Yderstraede, K.B. Foot edema simulation and monitoring using dielectric electro-active polymer sensors. Sens. Actuators A Phys. 2015, 225, 33–40. [Google Scholar] [CrossRef]
- Kim, S.; Iravantchi, Y.; Gajos, K.Z. SwellFit: Developing A Wearable Sensor for Monitoring Peripheral Edema. Available online: http://hdl.handle.net/10125/59823 (accessed on 22 May 2023). [CrossRef]
- Gehin, C.; Grenier, E.; Chaigneau, C.; Reinaudo, J.; Claude, A.; Massot, B.; Montalibet, A.; McAdams, E. Ambulatory sensor for the monitoring of the edema circumference in lower limbs. Sens. Actuators A Phys. 2018, 272, 83–91. [Google Scholar] [CrossRef]
- Fallahzadeh, R.; Ma, Y.; Ghasemzadeh, H. Context-Aware System Design for Remote Health Monitoring: An Application to Continuous Edema Assessment. IEEE Trans. Mob. Comput. 2017, 16, 2159–2173. [Google Scholar] [CrossRef]
- Yamada, E.F.; Villaverde, A.G.J.B.; Munin, E.; Zangaro, R.A.; Pacheco, M.T.T. Effect of low-power laser therapy on edema dynamics: Sensing by using the electrical capacitance method. Laser Interact. Tissue Cells XV 2004, 5319, 355–362. [Google Scholar] [CrossRef]
- Ponikowski, P.; Spoletini, I.; Coats, A.J.S.; Piepoli, M.F.; Rosano, G.M.C. Heart rate and blood pressure monitoring in heart failure. Eur. Heart J. Suppl. 2019, 21, M13–M16. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.C.; Cho, H.J. Blood pressure and heart failure. Clin. Hypertens. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Tegtmeyer, K.; Brady, G.; Lai, S.; Hodo, R.; Braner, D. Placement of an Arterial Line. N. Engl. J. Med. 2006, 354, e13. [Google Scholar] [CrossRef]
- Adamson, P.B.; Magalski, A.; Braunschweig, F.; Böhm, M.; Reynolds, D.; Steinhaus, D.; Luby, A.; Linde, C.; Ryden, L.; Cremers, B.; et al. Ongoing right ventricular hemodynamics in heart failure: Clinical value of measurements derived from an implantable monitoring system. J. Am. Coll. Cardiol. 2003, 41, 565–571. [Google Scholar] [CrossRef]
- Ritzema, J.; Melton, I.C.; Richards, A.M.; Crozier, I.G.; Frampton, C.; Doughty, R.N.; Whiting, J.; Kar, S.; Eigler, N.; Krum, H.; et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: Initial experience with a new permanent implantable device. Circulation 2007, 116, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Benza, R.L. Ambulatory Hemodynamic Monitoring in the Management of Pulmonary Arterial Hypertension. Adv. Pulm. Hypertens. 2014, 13, 81–85. [Google Scholar] [CrossRef]
- Volterrani, M.; Spoletini, I.; Angermann, C.; Rosano, G.; Coats, A.J. Implantable devices for heart failure monitoring: The CardioMEMSTM system. Eur. Heart J. Suppl. 2019, 21, M50–M53. [Google Scholar] [CrossRef] [PubMed]
- Reghunathan, A.; Chick, J.F.B.; Gemmete, J.J.; Hage, A.; Mahn, J.; Khaja, M.S.; Srinivasa, R.N. Endovascular retrieval of a CardioMEMS heart failure system. Radiol. Case Rep. 2018, 13, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Atwood, J.E. Peripheral edema. Am. J. Med. 2002, 113, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Burch, E.; George; DePasquale, N.P. Congestive Heart Failure-Acute Pulmonary Edema. JAMA 1969, 208, 1895–1897. [Google Scholar] [CrossRef]
- Kumarasinghe, G.; Carroll, G. A guide to peripheral oedema. Med. Today 2015, 16, 26–34. [Google Scholar]
- Guyton, A.C.; Coleman, T.G. Regulation of interstitial fluid volume and pressure. Ann. N. Y Acad. Sci. 1968, 150, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Granger, H.J.; Taylor, A.E. Interstitial Fluid Pressure. Physiol. Rev. 1971, 51, 527–563. [Google Scholar] [CrossRef]
- Guyton, A.C. A Concept of Negative Interstitial Pressure Based on Pressures in Implanted Perforated Capsules. Circ. Res. 1963, 12, 399–414. [Google Scholar] [CrossRef]
- Ozerdem, U.; Hargens, A.R. A simple method for measuring interstitial fluid pressure in cancer tissues. Microvasc. Res. 2005, 70, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Hargens, A.R.; Miller, S.L. Negative Pressure in the Interstitial Fluid of Animals. New Ser. 1968, 161, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ozerdem, U. Measuring interstitial fluid pressure with fiberoptic pressure transducers. Microvasc. Res. 2009, 77, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, H.O.; Reed, R.K.; Aukland, K. Interstitial Fluid Pressure in Rats Measured With a Modified Wick Technique. Mlcrovascljlar Res. 1977, 14, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wiederhielm, C.A.; Woodbury, J.W.; Kirk, S.; Rushmer, R.F. Pulsatile pressures in the microcirculation of frog’s mesentery. AM J. Physiol. 1964, 207, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W.; Darouiche, R.O. Catheter-Associated Infections Pathogenesis Affects Prevention. Arch. Intern. Med. 2004, 164, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Sax, H.; Pittet, D. Catheter-related infections. Microbes Infect. 2004, 6, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Available online: https://www.science.org (accessed on 12 June 2023).
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Arshad, F.; Eissa, S.; Safavieh, M.; Alattas, S.G.; Ahmed, M.U.; Zourob, M. Recent developments towards portable point-of-care diagnostic devices for pathogen detection. Sens. Diagn. 2022, 1, 87–105. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab. A Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Kenry; Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pegan, J.D.; Zhang, J.; Chu, M.; Nguyen, T.; Park, S.-J.; Paul, A.; Kim, J.; Bachman, M.; Khine, M. Skin-mountable stretch sensor for wearable health monitoring. Nanoscale 2016, 8, 17295–17303. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Nguyen, T.; Pandey, V.; Zhou, Y.; Pham, H.N.; Bar-Yoseph, R.; Radom-Aizik, S.; Jain, R.; Cooper, D.M.; Khine, M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit. Med. 2019, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Byun, J.; Seong, N.; Ha, J.; Kim, H.; Kim, S.; Kim, T.; Im, H.; Kim, D.; Hong, Y. Silver nanowire-embedded PDMS with a multiscale structure for a highly sensitive and robust flexible pressure sensor. Nanoscale 2015, 7, 6208–6215. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Jang, H.; Kim, S.Y.; Jeong, H.; Han, S.; Jang, Y.; Kim, D.H.; Lee, H.S. Flexible piezocapacitive sensors based on wrinkled microstructures: Toward low-cost fabrication of pressure sensors over large areas. RSC Adv. 2017, 7, 39420–39426. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, J.; Chu, M.; Khine, M. Flexible Piezoresistive Pressure Sensor Using Wrinkled Carbon Nanotube Thin Films for Human Physiological Signals. Adv. Mater. Technol. 2018, 3, 1700158. [Google Scholar] [CrossRef]
- Kim, J.; Chou, E.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv. Healthc. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Lee, G.Y.; Jang, J.; Yun, S.M.; Kim, E.; Park, J.U. Liquid Metal-Based Soft Electronics for Wearable Healthcare. Adv. Healthc. Mater. 2021, 10, 2002280. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Lee, S.; Seo, J.; Lee, J.; Son, S.; Cho, H.J.; Algadi, H.; Al-Sayari, S.; Kim, D.E.; et al. Ag nanowire reinforced highly stretchable conductive fibers for wearable electronics. Adv. Funct. Mater. 2015, 25, 3114–3121. [Google Scholar] [CrossRef]
- Tang, W.; Fu, C.; Xia, L.; Lyu, P.; Li, L.; Fu, Z.; Pan, H.; Zhang, C.; Xu, W. A flexible and sensitive strain sensor with three-dimensional reticular structure using biomass Juncus effusus for monitoring human motions. Chem. Eng. J. 2022, 438, 135600. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Fu, Z.; Zhu, Q.; Zhou, Z.; Gong, J.; Zhu, N.; Wang, X.; Wei, X.; Xia, L.; et al. Color construction of multi-colored carbon fibers using glucose. Nat. Commun. 2024, 15, 1979. [Google Scholar] [CrossRef] [PubMed]
- Rwei, P.; Qian, C.; Abiri, A.; Zhou, Y.; Chou, E.F.; Tang, W.C.; Khine, M. Soft Iontronic Capacitive Sensor for Beat-to-Beat Blood Pressure Measurements. Adv. Mater. Interfaces 2022, 9, 2200294. [Google Scholar] [CrossRef]
- Abiri, A.; Chou, E.F.; Qian, C.; Rinehart, J.; Khine, M. Intra-beat biomarker for accurate continuous non-invasive blood pressure monitoring. Sci. Rep. 2022, 12, 16772. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Masu, K.; Machida, K.; Yamane, D.; Judy, J.W. Microelectromechanical systems (MEMS):fabrication, design and applications. Smart Mater. Struct. 2001, 10, 1115. [Google Scholar] [CrossRef]
- Kumar, S.S.; Pant, B.D. Design principles and considerations for the ‘ideal’ silicon piezoresistive pressure sensor: A focused review. Microsyst. Technol. 2014, 20, 1213–1247. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, Y.; Kim, G.; Blaauw, D. A Pressure Sensing System with ±0.75 mmHg (3σ) Inaccuracy for Battery-Powered Low Power IoT applications. In Proceedings of the 2020 IEEE Symposium on VLSI Circuits, Honolulu, HI, USA, 16–19 June 2020; pp. 1–2. [Google Scholar] [CrossRef]
- Mejia-Aranda, A.R.; Basurto-Pensado, M.A.; Antunez-Ceron, E.E.; Castro-Gómez, L.L.; Urquiza-Beltran, G.; Rodriguez, J.A.; García, J.C.; Sánchez-Mondragón, J.J.; Ruiz-Pérez, V.I. Fiber Optic Pressure Sensor of 0-0.36 psi by Multimode Interference Technique. J. Appl. Res. Technol. 2013, 11, 695–701. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J.B. Flexible Piezoelectric-Induced Pressure Sensors for Static Measurements Based on Nanowires/Graphene Heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.X.; Yang, Y.J. A Wireless Cardiovascular Pressure Sensor Based on an Iontronic Film with High Sensitivity. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 135–138. [Google Scholar] [CrossRef]
- Tai, Y.; Yang, Z. Toward Flexible Wireless Pressure-Sensing Device via Ionic Hydrogel Microsphere for Continuously Mapping Human-Skin Signals. Adv. Mater. Interfaces 2017, 4, 1700496. [Google Scholar] [CrossRef]
- Song, S.H.; Brown, M.; Maleki, T.; Ziaie, B. A wireless interstitial pressure sensor with a Guyton chamber. In Proceedings of the 2013 Transducers and Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Transducers and Eurosensors, Barcelona, Spain, 16–20 June 2013; pp. 2161–2164. [Google Scholar] [CrossRef]
- Song, P.; Ma, Z.; Ma, J.; Yang, L.; Wei, J.; Zhao, Y.; Zhang, M.; Yang, F.; Wang, X. Recent progress of miniature MEMS pressure sensors. Micromachines 2020, 11, 56. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Shi, Y.; Li, L.; Wang, W.; He, L.; Liu, R. Recent developments for flexible pressure sensors: A review. Micromachines 2018, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fang, H.; Xu, B.; Yang, L.; Niu, H.; Wang, H.; Chen, D.; Liu, Y.; Wang, Z.; Wang, Y.; et al. Flexible wireless passive lc pressure sensor with design methodology and cost-effective preparation. Micromachines 2021, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Huang, R.; Yao, T.; Zhang, Y.; Miao, Y.; Liu, C.; Liu, J.; Chen, X. Textile-Based Wireless Pressure Sensor Array for Human-Interactive Sensing. Adv. Funct. Mater. 2019, 29, 1808786. [Google Scholar] [CrossRef]
- Palmroth, A.; Salpavaara, T.; Lekkala, J.; Kellomäki, M. Fabrication and Characterization of a Wireless Bioresorbable Pressure Sensor. Adv. Mater. Technol. 2019, 4, 1900428. [Google Scholar] [CrossRef]
- Zhai, Y.; Lee, J.; Hoang, Q.; Sievenpipper, D.; Garudadri, H.; Ng, T.N. A printed wireless fluidic pressure sensor. Flex. Print. Electron. 2018, 3, 035006. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Deng, Y.; Yang, Q.; Zhao, J.; Seo, M.-H.; Bai, W.; MacEwan, M.R.; Huang, Y.; Ray, W.Z.; et al. Bioresorbable Wireless Sensors as Temporary Implants for In Vivo Measurements of Pressure. Adv. Funct. Mater. 2020, 30, 2003754. [Google Scholar] [CrossRef]
- Kou, H.; Zhang, L.; Tan, Q.; Liu, G.; Dong, H.; Zhang, W.; Xiong, J. Wireless wide-range pressure sensor based on graphene/PDMS sponge for tactile monitoring. Sci. Rep. 2019, 9, 3916. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Iqbal, T.; Vazquez, P.; Farid, N.; Thampi, S.; Wijns, W.; Shahzad, A. Thin-film flexible wireless pressure sensor for continuous pressure monitoring in medical applications. Sensors 2020, 20, 6653. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef]
- Li, W.; Liu, A.; Wang, Y.; Qu, K.; Wen, H.; Zhao, J.; Shi, Y.; Wang, H.; Ye, M.; Guo, W. Implantable and Degradable Wireless Passive Protein-Based Tactile Sensor for Intracranial Dynamic Pressure Detection. Electronics 2023, 12, 2466. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Liu, J.; Qian, J.; Ren, Q.; An, G.; Xiong, J. An LC wireless passive pressure sensor based on single-crystal MgO MEMS processing technique for high temperature applications. Sensors 2021, 21, 6602. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, W.; Li, A.L.; Zhan, Z.; Wang, L.Y.; Sun, D.H. Design and manufacturing of a passive pressure sensor based on LC resonance. Micromachines 2016, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Lucarotti, C.; Oddo, C.M.; Vitiello, N.; Carrozza, M.C. Synthetic and bio-artificial tactile sensing: A review. Sensors 2013, 13, 1435–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, R.; Tao, J.; Peng, Y.; Pan, C. Recent progress in flexible pressure sensor arrays: From design to applications. J. Mater. Chem. C 2018, 6, 11878–11892. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Di, C.A.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horiz. 2015, 2, 140–156. [Google Scholar] [CrossRef]
- Wei, J.C.; Cartmill, I.D.; Kendall, M.A.; Crichton, M.L. In vivo, in situ and ex vivo comparison of porcine skin for microprojection array penetration depth, delivery efficiency and elastic modulus assessment. J. Mech. Behav. Biomed. Mater. 2022, 130, 105187. [Google Scholar] [CrossRef] [PubMed]
- Ranamukhaarachchi, S.A.; Lehnert, S.; Ranamukhaarachchi, S.L.; Sprenger, L.; Schneider, T.; Mansoor, I.; Rai, K.; Häfeli, U.O.; Stoeber, B. A micromechanical comparison of human and porcine skin before and after preservation by freezing for medical device development. Sci. Rep. 2016, 6, 32074. [Google Scholar] [CrossRef]
- Vardaxis, N.J.; Brans, T.A.; Boon, M.E.; Kreis, R.W.; Marres, L.M. Confocal laser scanning microscopy of porcine skin: Implications for human wound healing studies. J. Anat. 1997, 190, 601–611. [Google Scholar] [CrossRef]
- Avon, S.L.; Wood, R.E. Porcine skin as an porcine skin as an porcine skin as an porcine skin as an porcine skin as an in-vivo in-vivo in-vivo in-vivo in-vivo model for model for model for model for. J. Forensic Odontostomatol. 2005, 23, 30–39. [Google Scholar] [PubMed]
- Nopper, R.; Niekrawietz, R.; Reindl, L. Wireless readout of passive LC sensors. IEEE Trans. Instrum. Meas. 2010, 59, 2450–2457. [Google Scholar] [CrossRef]
- Nopper, R.; Has, R.; Reindl, L. A wireless sensor readout system-circuit concept, simulation, and accuracy. IEEE Trans. Instrum. Meas. 2011, 60, 2976–2983. [Google Scholar] [CrossRef]
- Fonseca, M.A.; English, J.M.; Von Arx, M.; Allen, M.G. Wireless micromachined ceramic pressure sensor for high-temperature applications. J. Microelectromechanical Syst. 2002, 11, 337–343. [Google Scholar] [CrossRef]
- Stauffer, F.; Zhang, Q.; Tybrandt, K.; Llerena Zambrano, B.; Hengsteler, J.; Stoll, A.; Trüeb, C.; Hagander, M.; Sujata, J.-M.; Hoffmann, F.; et al. Soft Electronic Strain Sensor with Chipless Wireless Readout: Toward Real-Time Monitoring of Bladder Volume. Adv. Mater. Technol. 2018, 3, 1800031. [Google Scholar] [CrossRef]
- Jain, S.; Pandey, K.; Lahoti, A.; Rao, P. Evaluation of skin and subcutaneous tissue thickness at insulin injection sites in Indian, insulin naïve, type-2 diabetic adult population. Indian. J. Endocrinol. Metab. 2013, 17, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.J.; Wang, L.F.; Dong, L.; Huang, Q.A. Symmetric LC circuit configurations for passive wireless multifunctional sensors. J. Microelectromechanical Syst. 2019, 28, 344–350. [Google Scholar] [CrossRef]
- Deng, W.J.; Wang, L.F.; Dong, L.; Huang, Q.A. LC Wireless Sensitive Pressure Sensors with Microstructured PDMS Dielectric Layers for Wound Monitoring. IEEE Sens. J. 2018, 18, 4886–4892. [Google Scholar] [CrossRef]
- Dautta, M.; Alshetaiwi, M.; Escobar, A.; Torres, F.; Bernardo, N.; Tseng, P. Multi-Functional Hydrogel-Interlayer RF/NFC Resonators as a Versatile Platform for Passive and Wireless Biosensing. Adv. Electron. Mater. 2020, 6, 1901311. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, C.; Ye, F.; Li, J.; Tseng, P.; Khine, M. Wireless and Battery-Free Sensor for Interstitial Fluid Pressure Monitoring. Sensors 2024, 24, 4429. https://doi.org/10.3390/s24144429
Qian C, Ye F, Li J, Tseng P, Khine M. Wireless and Battery-Free Sensor for Interstitial Fluid Pressure Monitoring. Sensors. 2024; 24(14):4429. https://doi.org/10.3390/s24144429
Chicago/Turabian StyleQian, Chengyang, Fan Ye, Junye Li, Peter Tseng, and Michelle Khine. 2024. "Wireless and Battery-Free Sensor for Interstitial Fluid Pressure Monitoring" Sensors 24, no. 14: 4429. https://doi.org/10.3390/s24144429




