Potential of a New, Flexible Electrode sEMG System in Detecting Electromyographic Activation in Low Back Muscles during Clinical Tests: A Pilot Study on Wearables for Pain Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
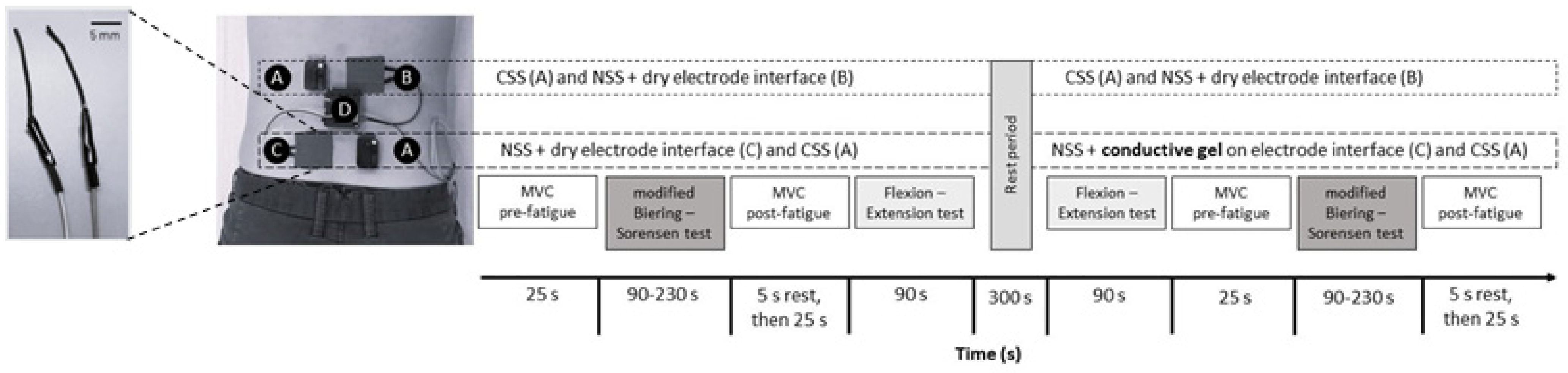
2.2. General Protocol
2.3. Materials
2.3.1. The New sEMG System (NSS)
2.3.2. The Commercial Laboratory sEMG System (CSS)
2.3.3. Dynamometer
2.4. Clinical and Force Tests Descriptions
2.4.1. Force Test: Maximal Voluntary Contraction
2.4.2. Modified Biering–Sorensen Test
2.4.3. Flexion–Extension Test
2.5. sEMG
2.5.1. Set-Up
2.5.2. Signal Processing
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Recordings
3.3. NSS Sensitivity
3.3.1. Fatigue Assessment (Change in MDF)
3.3.2. Muscle Activation Pattern Assessment (RMS Ratios)
3.4. NSS Validity
3.4.1. Validity of Fatigue Assessment (Change in MDF)
3.4.2. Validity of the Muscle Activation Pattern (RMS Ratios)
3.5. Validity over Time
3.6. Impact of NSS Skin–Electrode Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, R.D.; Cumming, R.G.; Bleasel, J.; York, J.; Das, A.; McAuley, J.H. Prognosis in patients with recent onset low back pain in Australian primary care: Inception cohort study. BMJ 2008, 337, a171. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.C.; Chang, W.-J.; Buscemi, V.; Liston, M.; Skippen, P.; Cashin, A.G.; McAuley, J.H.; Schabrun, S.M. Low Somatosensory Cortex Excitability in the Acute Stage of Low Back Pain Causes Chronic Pain. J. Pain 2021, 23, 289–304. [Google Scholar] [CrossRef]
- Korakakis, V.; O’Sullivan, K.; Kotsifaki, A.; Sotiralis, Y.; Giakas, G. Lumbo-pelvic proprioception in sitting is impaired in subgroups of low back pain–But the clinical utility of the differences is unclear. A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250673. [Google Scholar] [CrossRef]
- Laird, R.A.; Gilbert, J.; Kent, P.; Keating, J.L. Comparing lumbo-pelvic kinematics in people with and without back pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2014, 15, 229. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Selen, L.P.J.; Cholewicki, J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J. Electromyogr. Kinesiol. 2003, 13, 333–351. [Google Scholar] [CrossRef]
- Ghamkhar, L.; Kahlaee, A.H. Pain and Pain-Related Disability Associated With Proprioceptive Impairment in Chronic Low Back Pain Patients: A Systematic Review. J. Manip. Physiol. Ther. 2019, 42, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Knox, M.F.; Chipschase, L.; Schabrun, S.; Romero, R.; Marschal, P. Anticipatory and compensatory postural adjustments in people with low back pain: A systematic review and meta-analysis. Spine J. 2018, 18, 1934–1949. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Galea, M.P.; Hodges, P.W. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 2008, 131, 2161–2171. [Google Scholar] [CrossRef]
- Hodges, P.W.; Falla, D. Interaction between Pain and Sensorimotor Control. In Grieve’s Modern Musculoskeletal Physiotherapy; Elsevier: London, UK, 2015; ISBN 978-0-7020-5152-4. [Google Scholar]
- Ehrenbrusthoff, K.; Ryan, C.G.; Grüneberg, C.; Martin, D.J. A systematic review and meta-analysis of the reliability and validity of sensorimotor measurement instruments in people with chronic low back pain. Musculoskelet. Sci. Pract. 2018, 35, 73–83. [Google Scholar] [CrossRef]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.P.; Menezes Costa, L.C.; Ostelo, R.W.J.G.; Macedo, L.G. Motor control exercise for chronic non-specific low-back pain. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; p. CD012004. [Google Scholar] [CrossRef]
- Patricio, P.; Roy, J.-S.; Macedo, L.; Roy, M.; Léonard, G.; Hodges, P.; Massé-Alarie, H. Repetitive transcranial magnetic stimulation alone and in combination with motor control exercise for the treatment of individuals with chronic non-specific low back pain (ExTraStim trial): Study protocol for a randomised controlled trial. BMJ Open 2021, 11, e045504. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, C.; Vanderthommen, M.; Duysens, C.; Crielaard, J.-M. Spinal muscle evaluation using the Sorensen test: A critical appraisal of the literature. Joint Bone Spine 2006, 73, 43–50. [Google Scholar] [CrossRef]
- Hirsch, G.; Beach, G.; Cooke, C.; Menard, M.; Shelag, L. Relationship Between Performance on Lumbar Dynamometry and Waddell Score in a Population with Low-Back Pain. Spine 1991, 16, 1039–1043. [Google Scholar] [CrossRef]
- Demoulin, C.; Boyer, M.; Duchateau, J.; Grosdent, S.; Jidovtseff, B.; Crielaard, J.-M.; Vanderthommen, M. Is the Sørensen test valid to assess muscle fatigue of the trunk extensor muscles? J. Back Musculoskelet. Rehabil. 2016, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sorensen Physical Measurements as Risk Indicators for Low-Back Trouble Over a One-Year Period. Spine 1984, 9, 106–119. [CrossRef] [PubMed]
- Perret, C.; Poiraudeau, S.; Fermanian, J.; Colau, M.M.L.; Benhamou, M.A.M.; Revel, M. Validity, reliability, and responsiveness of the fingertip-to-floor test. Arch. Phys. Med. Rehabil. 2001, 82, 1566–1570. [Google Scholar] [CrossRef]
- Robinson, H.S.; Mengshoel, A.M. Assessments of Lumbar Flexion Range of Motion: Intertester Reliability and Concurrent Validity of 2 Commonly Used Clinical Tests. Spine 2014, 39, E270–E275. [Google Scholar] [CrossRef] [PubMed]
- Oddsson, L.I.E.; De Luca, C.J. Activation imbalances in lumbar spine muscles in the presence of chronic low back pain. J. Appl. Physiol. 2003, 94, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and Biophysics of Surface EMG for Physiotherapists and Kinesiologists: Toward a Common Language With Rehabilitation Engineers. Front. Neurol. 2020, 11, 576729. [Google Scholar] [CrossRef]
- Arvanitidis, M.; Bikinis, N.; Petrakis, S.; Gkioka, A.; Tsimpolis, D.; Falla, D.; Martinez-Valdes, E. Spatial distribution of lumbar erector spinae muscle activity in individuals with and without chronic low back pain during a dynamic isokinetic fatiguing task. Clin. Biomech. 2021, 81, 105214. [Google Scholar] [CrossRef]
- Oddsson, L.I.E.; Giphart, J.E.; Buijs, R.J.C.; Roy, S.H.; Taylor, H.P.; De Luca, C.J. Development of new protocols and analysis procedures for the assessment of LBP by surface EMG techniques. J. Rehabil. Res. Dev. 1997, 34, 415–426. [Google Scholar] [PubMed]
- Villafañe, J.H.; Gobbo, M.; Peranzoni, M.; Naik, G.; Imperio, G.; Cleland, J.A.; Negrini, S. Validity and everyday clinical applicability of lumbar muscle fatigue assessment methods in patients with chronic non-specific low back pain: A systematic review. Disabil. Rehabil. 2016, 38, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.; Arsenault, A.B.; Gravel, D.; Larivière, C.; de Oliveira, E. Back muscle strength and fatigue in healthy and chronic low back pain subjects: A comparative study of 3 assessment protocols. Arch. Phys. Med. Rehabil. 2005, 86, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Hinrichs, R.N. The Biomechanical and Clinical Significance of the Lumbar Erector Spinae Flexion-Relaxation Phenomenon: A Review of Literature. J. Manip. Physiol. Ther. 2005, 28, 623–631. [Google Scholar] [CrossRef]
- Neblett, R.; Brede, E.; Mayer, T.G.; Gatchel, R.J. What is the Best Surface EMG Measure of Lumbar Flexion-Relaxation for Distinguishing Chronic Low Back Pain Patients From Pain-free Controls? Clin. J. Pain 2013, 29, 334–340. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zuriaga, D.; López-Pascual, J.; Garrido-Jaén, D.; García-Mas, M.A. A Comparison of Lumbopelvic Motion Patterns and Erector Spinae Behavior Between Asymptomatic Subjects and Patients With Recurrent Low Back Pain During Pain-Free Periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Forestier, N.; Nougier, V. The effects of muscular fatigue on the coordination of a multijoint movement in human. Neurosci. Lett. 1998, 252, 187–190. [Google Scholar] [CrossRef]
- Routhier, F.; Duclos, N.C.; Lacroix, É.; Lettre, J.; Turcotte, E.; Hamel, N.; Michaud, F.; Duclos, C.; Archambault, P.S.; Bouyer, L.J. Clinicians’ perspectives on inertial measurement units in clinical practice. PLoS ONE 2020, 15, e0241922. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Simpson, L.; Maharaj, M.M.; Mobbs, R.J. The role of wearables in spinal posture analysis: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Roudjane, M.; Tam, S.; Mascret, Q.; Fall, C.L.; Bielmann, M.; de Faria, R.A.D.; Bouyer, L.J.; Gosselin, B.; Messaddeq, Y. Detection of Neuromuscular Activity Using New Non-Invasive and Flexible Multimaterial Fiber Dry-Electrodes. IEEE Sens. J. 2019, 19, 11624–11633. [Google Scholar] [CrossRef]
- Gauthier, N.; Roudjane, M.; Frasie, A.; Loukili, M.; Saad, A.B.; Pagé, I.; Messaddeq, Y.; Bouyer, L.J.; Gosselin, B. Multimodal Electrophysiological Signal Measurement using a New Flexible and Conductive Polymer Fiber-electrode. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4373–4376. [Google Scholar]
- Côté-Picard, C.; Tittley, J.; Mailloux, C.; Perreault, K.; Mercier, C.; Dionne, C.E.; Roy, J.-S.; Massé-Alarie, H. Effect of thermal therapy and exercises on acute low back pain: A protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2020, 21, 814. [Google Scholar] [CrossRef]
- Tsao, H.; Druitt, T.R.; Schollum, T.M.; Hodges, P.W. Motor Training of the Lumbar Paraspinal Muscles Induces Immediate Changes in Motor Coordination in Patients With Recurrent Low Back Pain. J. Pain 2010, 11, 1120–1128. [Google Scholar] [CrossRef]
- Park, R.J.; Tsao, H.; Cresswell, A.G.; Hodges, P.W. Differential activity of regions of the psoas major and quadratus lumborum during submaximal isometric trunk efforts. J. Orthop. Res. 2012, 30, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Ebert, V.; Kinzl, L.; Dehner, C.; Elbel, M.; Hartwig, E. Surface electromyography of the paravertebral muscles in patients with chronic low back pain. Arch. Phys. Med. Rehabil. 2005, 86, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.H.; De Luca, C.J.; Casavant, D.A. Lumbar Muscle Fatigue and Chrnoic Lower back Pain. Spine 1989, 14, 992–1001. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface ElectroMyoGraphy; Roessingh Research and Development: Enschede, The Netherlands, 1999; Volume 8, pp. 13–54. ISBN 90-75452-15-2. [Google Scholar]
- Dupuis, F.; Sole, G.; Wassinger, C.; Bielmann, M.; Bouyer, L.J.; Roy, J.-S. Fatigue, induced via repetitive upper-limb motor tasks, influences trunk and shoulder kinematics during an upper limb reaching task in a virtual reality environment. PLoS ONE 2021, 16, e0249403. [Google Scholar] [CrossRef]
- Alschuler, K.N.; Neblett, R.; Wiggert, E.; Haig, A.J.; Geisser, M.E. Flexion-relaxation and Clinical Features Associated With Chronic Low Back Pain: A Comparison of Different Methods of Quantifying Flexion-relaxation. Clin. J. Pain 2009, 25, 760–766. [Google Scholar] [CrossRef]
- Li, L.; Caldwell, G.E. Coefficient of cross correlation and the time domain correspondence. J. Electromyogr. Kinesiol. 1999, 9, 385–389. [Google Scholar] [CrossRef]
- Wren, T.A.L.; Patrick Do, K.; Rethlefsen, S.A.; Healy, B. Cross-correlation as a method for comparing dynamic electromyography signals during gait. J. Biomech. 2006, 39, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Niijima, A.; Isezaki, T.; Aoki, R.; Watanabe, T.; Yamada, T. Biceps fatigue estimation with an E-textile headband. In Proceedings of the 2018 ACM International Symposium on Wearable Computers, Singapore, 8–12 October 2018; ACM: New York, NY, USA, 2018; pp. 222–223. [Google Scholar]
- Belbasis, A.; Fuss, F.K. Muscle Performance Investigated With a Novel Smart Compression Garment Based on Pressure Sensor Force Myography and Its Validation Against EMG. Front. Physiol. 2018, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Bootsman, R.; Markopoulos, P.; Qi, Q.; Wang, Q.; Timmermans, A.A. Wearable technology for posture monitoring at the workplace. Int. J. Hum.-Comput. Stud. 2019, 132, 99–111. [Google Scholar] [CrossRef]
- Mokhlespour Esfahani, M.I.; Nussbaum, M.A. A “Smart” Undershirt for Tracking Upper Body Motions: Task Classification and Angle Estimation. IEEE Sens. J. 2018, 18, 7650–7658. [Google Scholar] [CrossRef]
- Zaltieri, M.; Massaroni, C.; Lo Presti, D.; Bravi, M.; Sabbadini, R.; Miccinilli, S.; Sterzi, S.; Formica, D.; Schena, E. A Wearable Device Based on a Fiber Bragg Grating Sensor for Low Back Movements Monitoring. Sensors 2020, 20, 3825. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; Lammers, A.R.; Danial, P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009, 9, 115–120. [Google Scholar] [CrossRef]
- Goldsack, J.C.; Coravos, A.; Bakker, J.P.; Bent, B.; Dowling, A.V.; Fitzer-Attas, C.; Godfrey, A.; Godino, J.G.; Gujar, N.; Izmailova, E.; et al. Verification, analytical validation, and clinical validation (V3): The foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). npj Digit. Med. 2020, 3, 55. [Google Scholar] [CrossRef]





| Electrode Location | Fatigue Assessment (Modified Biering–Sorensen Test) | Muscle Activation Pattern Assessment (Flexion–Extension Test) | ||||
|---|---|---|---|---|---|---|
| Early MDF (Hz) | Late MDF (Hz) | Early vs. Late | Full Flexion sEMG RMS (µV) | Extension from Full Flexion RMS (µV) | Full Flexion vs. Extension from Full Flexion | |
| T12-L1 | 84.56 ± 22.44 | 53.11 ± 7.47 | p = 0.018 | 22.21 ± 39.70 | 46.26 ± 63.08 | p = 0.012 |
| L4-L5 | 89.03 ± 20.46 | 59.64 ± 16.05 | p = 0.008 | 8.69 ± 2.66 | 30.51 ± 22.25 | p = 0.012 |
| Electrode Location | EMG System | Fatigue Assessment (Modified Biering–Sorensen Test) | Muscle Activation Pattern Assessment (Flexion–Extension Test) | ||
|---|---|---|---|---|---|
| Drop in Median Frequency (%) | NSS vs. CSS | sEMG RMS Ratio | NSS vs. CSS | ||
| T12-L1 | NSS | −46.89 ± 7.47 (n = 7/12) | p = 0.31 | 0.39 ± 0.15 (n = 8/12) | p = 0.21 |
| CSS | −43.79 ± 11.73 (n = 7/12) | 0.30 ± 0.17 (n = 8/12) | |||
| L4-L5 | NSS | −40.35 ± 16.05 (n = 9/12) | p = 0.52 | 0.28 ± 0.40 (n = 8/12) | p = 0.67 |
| CSS | −37.27 ± 18.71 (n = 9/12) | 0.27 ± 0.12 (n = 8/12) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasie, A.; Massé-Alarie, H.; Bielmann, M.; Gauthier, N.; Roudjane, M.; Pagé, I.; Gosselin, B.; Roy, J.-S.; Messaddeq, Y.; Bouyer, L.J. Potential of a New, Flexible Electrode sEMG System in Detecting Electromyographic Activation in Low Back Muscles during Clinical Tests: A Pilot Study on Wearables for Pain Management. Sensors 2024, 24, 4510. https://doi.org/10.3390/s24144510
Frasie A, Massé-Alarie H, Bielmann M, Gauthier N, Roudjane M, Pagé I, Gosselin B, Roy J-S, Messaddeq Y, Bouyer LJ. Potential of a New, Flexible Electrode sEMG System in Detecting Electromyographic Activation in Low Back Muscles during Clinical Tests: A Pilot Study on Wearables for Pain Management. Sensors. 2024; 24(14):4510. https://doi.org/10.3390/s24144510
Chicago/Turabian StyleFrasie, Antoine, Hugo Massé-Alarie, Mathieu Bielmann, Nicolas Gauthier, Mourad Roudjane, Isabelle Pagé, Benoit Gosselin, Jean-Sébastien Roy, Younes Messaddeq, and Laurent J. Bouyer. 2024. "Potential of a New, Flexible Electrode sEMG System in Detecting Electromyographic Activation in Low Back Muscles during Clinical Tests: A Pilot Study on Wearables for Pain Management" Sensors 24, no. 14: 4510. https://doi.org/10.3390/s24144510
APA StyleFrasie, A., Massé-Alarie, H., Bielmann, M., Gauthier, N., Roudjane, M., Pagé, I., Gosselin, B., Roy, J.-S., Messaddeq, Y., & Bouyer, L. J. (2024). Potential of a New, Flexible Electrode sEMG System in Detecting Electromyographic Activation in Low Back Muscles during Clinical Tests: A Pilot Study on Wearables for Pain Management. Sensors, 24(14), 4510. https://doi.org/10.3390/s24144510







