Glycemic Control Assessed by Intermittently Scanned Glucose Monitoring in Type 1 Diabetes during the COVID-19 Pandemic in Austria
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Main Analysis
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Sample-ID | Pre-Lockdown (Same Season) | Pre-Lockdown | Lockdown | Phases (n) | Baselines |
|---|---|---|---|---|---|
| 1001 | yes | no | no | 1 | yes |
| 1003 | yes | yes | yes | 3 | yes |
| 1004 | yes | yes | yes | 3 | yes |
| 1006 | yes | yes | yes | 3 | yes |
| 1009 | yes | yes | yes | 3 | yes |
| 1014 | no | yes | yes | 2 | yes |
| 1015 | no | yes | yes | 2 | yes |
| 1019 | yes | yes | yes | 3 | yes |
| 1020 | yes | yes | yes | 3 | yes |
| 1028 | yes | yes | yes | 3 | yes |
| 1032 | yes | yes | yes | 3 | yes |
| 1033 | yes | yes | yes | 3 | yes |
| 1034 | no | yes | yes | 2 | yes |
| 1035 | yes | yes | yes | 3 | yes |
| 1036 | yes | yes | yes | 3 | yes |
| 1037 | no | yes | yes | 2 | yes |
| 1038 | yes | yes | yes | 3 | yes |
| 1040 | yes | yes | yes | 3 | yes |
| 1041 | yes | yes | yes | 3 | yes |
| 1044 | no | yes | yes | 2 | yes |
| 1047 | no | yes | yes | 2 | yes |
| 1048 | yes | yes | yes | 3 | yes |
| 1050 | yes | yes | yes | 3 | yes |
| 1051 | yes | yes | yes | 3 | yes |
| 1054 | no | yes | yes | 2 | yes |
| 1055 | yes | yes | yes | 3 | yes |
| 1056 | yes | yes | yes | 3 | yes |
| 1057 | yes | yes | yes | 3 | yes |
| 1059 | yes | yes | yes | 3 | yes |
| 1060 | yes | yes | yes | 3 | yes |
| 1061 | yes | yes | yes | 3 | yes |
| 1065 | yes | yes | yes | 3 | yes |
| 1070 | yes | yes | yes | 3 | yes |
| 1072 | yes | yes | yes | 3 | yes |
| 1073 | yes | yes | yes | 3 | yes |
| 1075 | yes | yes | yes | 3 | yes |
| 1076 | yes | yes | yes | 3 | yes |
| 1079 | no | yes | yes | 2 | yes |
| 1081 | yes | yes | yes | 3 | yes |
| 1085 | yes | yes | yes | 3 | yes |
| 1086 | yes | yes | yes | 3 | yes |
| 1087 | yes | yes | yes | 3 | yes |
| 1088 | no | yes | yes | 2 | yes |
| 1090 | yes | yes | yes | 3 | yes |
| 1093 | yes | yes | yes | 3 | yes |
| 1094 | yes | yes | yes | 3 | yes |
| 1095 | yes | yes | yes | 3 | yes |
| 1096 | yes | yes | yes | 3 | yes |
| 1097 | yes | yes | yes | 3 | yes |
| 1098 | yes | yes | yes | 3 | yes |
| 1100 | yes | yes | yes | 3 | yes |
| 1101 | yes | yes | yes | 3 | yes |
| 1103 | no | yes | yes | 2 | yes |
| 1104 | yes | yes | yes | 3 | yes |
| 1106 | yes | yes | yes | 3 | yes |
| 1107 | yes | yes | yes | 3 | yes |
| 1111 | yes | yes | yes | 3 | yes |
| 1113 | yes | yes | no | 2 | yes |
| 1114 | yes | yes | yes | 3 | yes |
| 1122 | yes | yes | yes | 3 | yes |
| 1123 | yes | yes | yes | 3 | yes |
| 1125 | yes | yes | yes | 3 | yes |
| 1127 | no | yes | yes | 2 | yes |
| 1130 | yes | yes | yes | 3 | yes |
| 1131 | no | yes | yes | 2 | yes |
| 1132 | no | yes | yes | 2 | yes |
| 1134 | yes | yes | yes | 3 | yes |
| 1135 | no | yes | yes | 2 | yes |
| 1137 | yes | yes | yes | 3 | yes |
| 1140 | yes | yes | yes | 3 | yes |
| 1143 | yes | yes | yes | 3 | yes |
| 1144 | yes | yes | yes | 3 | yes |
| 1145 | no | yes | yes | 2 | yes |
| 1146 | yes | yes | yes | 3 | yes |
| 1150 | yes | yes | yes | 3 | yes |
| 1153 | yes | yes | yes | 3 | yes |
| 1154 | yes | yes | yes | 3 | yes |
| 1158 | yes | yes | yes | 3 | yes |
| 1160 | yes | yes | yes | 3 | yes |
| 1163 | yes | yes | yes | 3 | yes |
| 1164 | yes | no | yes | 2 | yes |
| 1092 | yes |
References
- Kluemper, J.R.; Smith, A.; Wobeter, B. Diabetes: The role of continuous glucose monitoring. Drugs Context 2022, 11, 2021-9-13. [Google Scholar] [CrossRef] [PubMed]
- Clodi, M.; Abrahamian, H.; Brath, H.; Schernthaner, G.; Brix, J.; Ludvik, B.; Drexel, H.; Saely, C.H.; Fasching, P.; Rega-Kaun, G.; et al. Antihyperglycemic treatment guidelines for diabetes mellitus type 2 (Update 2019). Wien. Klin. Wochenschr. 2019, 131, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lechleitner, M.; Kaser, S.; Hoppichler, F.; Roden, M.; Weitgasser, R.; Ludvik, B.; Fasching, P.; Winhofer-Stöckl, Y.; Kautzky-Willer, A.; Schernthaner, G.; et al. Diagnosis and insulin therapy of type 1 diabetes mellitus (Update 2019). Wien. Klin. Wochenschr. 2019, 131, 77–84. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.; Welsh, J.B.; Hirsch, I.B.; Garg, S.K. Real-Time Continuous Glucose Monitoring during the Coronavirus Disease 2019 Pandemic and Its Impact on Time in Range. Diabetes Technol. Ther. 2021, 23, S-1. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.A.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.; Leclerc, P.; Tremblay, C.; Tannenbaum, T.-N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010, 33, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Orioli, L.; Hermans, M.P.; Thissen, J.-P.; Maiter, D.; Vandeleene, B.; Yombi, J.-C. COVID-19 in diabetic patients: Related risks and specifics of management. Ann. D‘endocrinologie 2020, 81, 101–109. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- OEDG-Leitlinien-2023-Kurzversion.pdf. Available online: https://www.oedg.at/pdf/OEDG-Leitlinien-2023-Kurzversion.pdf (accessed on 10 July 2024).
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.; Kroon, L. Ultra-Rapid-Acting Insulins: How Fast Is Really Needed? Clin. Diabetes 2021, 39, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Bode, B.; Cho, J.I.; Liu, R.; Tobian, J.; Hardy, T.; Chigutsa, F.; Phillip, M.; Horowitz, B.; Ignaut, D. Improved postprandial glucose control with ultra rapid lispro versus lispro with continuous subcutaneous insulin infusion in type 1 diabetes: PRONTO-Pump-2. Diabetes Obes. Metab. 2021, 23, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef] [PubMed]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Kaafarani, H.M.A.; El Moheb, M.; Hwabejire, J.O.; Naar, L.; Christensen, M.A.; Breen, K.; Gaitanidis, A.; Alser, O.; Mashbari, H.; Bankhead-Kendall, B.; et al. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Ann. Surg. 2020, 272, e61–e62. [Google Scholar] [CrossRef]
- Schiaffini, R.; Barbetti, F.; Rapini, N.; Inzaghi, E.; Deodati, A.; Patera, I.P.; Matteoli, M.C.; Ciampalini, P.; Carducci, C.; Lorubbio, A.; et al. School and pre-school children with type 1 diabetes during COVID-19 quarantine: The synergic effect of parental care and technology. Diabetes Res. Clin. Pract. 2020, 166, 108302. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cheng, Z.; Luo, L.; Zhu, Y.; Lin, W.; Ming, Z.; Chen, W.; Hu, Y. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb. Res. 2021, 201, 23–29. [Google Scholar] [CrossRef] [PubMed]
- FreeStyle Libre|FreeStyle Abbott. Available online: https://www.freestyle.abbott/at-de/home.html (accessed on 24 April 2024).
- Bergenstal, R.M.; Beck, R.W.; Close, K.L.; Grunberger, G.; Sacks, D.B.; Kowalski, A.; Brown, A.S.; Heinemann, L.; Aleppo, G.; Ryan, D.B.; et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care 2018, 41, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Tornese, G.; Ceconi, V.; Monasta, L.; Carletti, C.; Faleschini, E.; Barbi, E. Glycemic Control in Type 1 Diabetes Mellitus during COVID-19 Quarantine and the Role of In-Home Physical Activity. Diabetes Technol. Ther. 2020, 22, 462–467. [Google Scholar] [CrossRef]
- Bonora, B.M.; Boscari, F.; Avogaro, A.; Bruttomesso, D.; Fadini, G.P. Glycaemic Control Among People with Type 1 Diabetes during Lockdown for the SARS-CoV-2 Outbreak in Italy. Diabetes Ther. 2020, 11, 1369–1379. [Google Scholar] [CrossRef]
- Ghesquière, L.; Garabedian, C.; Drumez, E.; Lemaître, M.; Cazaubiel, M.; Bengler, C.; Vambergue, A. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes Metab. 2021, 47, 101201. [Google Scholar] [CrossRef]
- Sfinari, A.; Pervanidou, P.; Chouliaras, G.; Zoumakis, E.; Vasilakis, I.A.; Nicolaides, N.C.; Kanaka-Gantenbein, C. Perceived Changes in Emotions, Worries and Everyday Behaviors in Children and Adolescents Aged 5–18 Years with Type 1 Diabetes during the COVID-19 Pandemic. Children 2022, 9, 736. [Google Scholar] [CrossRef]
- Giansanti, D.; Morone, G.; Loreti, A.; Germanotta, M.; Aprile, I. A Narrative Review of the Launch and the Deployment of Telemedicine in Italy during the COVID-19 Pandemic. Healthcare 2022, 10, 415. [Google Scholar] [CrossRef]
- Forlani, S.; Mastrosimone, E.; Paglia, S.; Protti, S.; Ferraris, M.P.; Casale, M.C.; Di Capua, M.; Grossi, M.G.; Esposti, M.; Randazzo, D.; et al. The First Italian Telemedicine Program for Non-Critical COVID-19 Patients: Experience from Lodi (Italy). JCM 2022, 11, 5322. [Google Scholar] [CrossRef] [PubMed]
- Bouabida, K.; Lebouché, B.; Pomey, M.-P. Telehealth and COVID-19 Pandemic: An Overview of the Telehealth Use, Advantages, Challenges, and Opportunities during COVID-19 Pandemic. Healthcare 2022, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Oliva, S.; Covelli, E.; Del Casale, A.; Da Molin, G.; Liguori, G.; Orsi, G.B.; Napoli, C. Introducing Telemedicine in Italy: Citizens’ Awareness of a New Healthcare Resource. Healthcare 2023, 11, 2157. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.; Garagiola, E.; Bellavia, D.; Rossetto, F.; Baglio, F. Digital technology and COVID-19 pandemic: Feasibility and acceptance of an innovative telemedicine platform. Technovation 2024, 130, 102941. [Google Scholar] [CrossRef]

| Categorical parameters | Number (n) | Percent (%) |
| Gender [male/female] | 22 females | 34 |
| 42 males | 66 | |
| System of use [pen/pump] | 48 pen | 75 |
| 16 pump | 25 | |
| Numeric parameters | Median (Q1–Q3) | Min–Max |
| Age [years] | 33.5 (26.3; 49.5) | 19.7–73.3 |
| Diabetes duration [years] | 13.5 (5.5; 22.0) | 1.45–64.5 |
| Height [m] | 1.8 (1.7; 1.8) | 1.6–1.9 |
| Weight [kg] | 76.0 (68.0; 88.3) | 49.0–134.0 |
| BMI [kg/m2] | 24.6 (22.3; 27.0) | 18.4–37.9 |
| HbA1c [mmol/mol] | 58.5 (51.0; 69.3) | 37.0–100.0 |
| Creatinine [mg/dL] | 0.9 (0.8; 1.0) | 0.6–4.4 |
| isCGM use duration [months] | 68.5 (58.0; 79.3) | 51.0–92.0 |
| isCGM use duration [years] | 5.7 (4.8; 6.6) | 4.3–7.7 |
| Visits during lockdown 2020 [n] | 0.0 (0.0; 1.0) | 0.0–6.0 |
| Visits during pre-lockdown [n] | 1.0 (0.0; 1.0) | 0.0–6.0 |
| Visits during pre-pandemic [n] | 1.0 (0.0; 2.3) | 0.0–11.0 |
| Phase | Individuals (N) | Parameters | Median (Q1–Q3) | Min–Max |
|---|---|---|---|---|
| Pre- pandemic 2019 | 64 | Days (n) | 93.0 (85.0–93.0) | 5.0–93.0 |
| isCGM values (n) | 8311.0 (5804.5–8677.5) | 399.0–9177.0 | ||
| isCGM activity (%) | 95.2 (88.1–97.7) | 44.8–102.8 | ||
| Mean glucose (mg/dL) | 176.5 (155.9–196.1) | 117.6–275.8 | ||
| CV | 0.4 (0.4–0.4) | 0.2–0.5 | ||
| GMI | 7.5 (7.0–8.0) | 6.1–9.9 | ||
| MAGE | 187.3 (170.5–207.9) | 123.9–286.1 | ||
| Pre- lockdown 2020 | 64 | Days (n) | 91.0 (85.5–91.0) | 4.0–91.0 |
| isCGM values (n) | 8009.0 (6875.0–8357.0) | 143.0–10312.0 | ||
| isCGM activity (%) | 94.40 (87.2–95.9) | 37.2–118.0 | ||
| Mean glucose (mg/dL) | 168.4 (154.9–197.0) | 113.9–299.4 | ||
| CV | 0.4 (0.3–0.4) | 0.2–0.5 | ||
| GMI | 7.3 (7.0–8.0) | 6.0–10.5 | ||
| MAGE | 177.0 (165.4–205.6) | 121.3–358.0 | ||
| Lockdown 2020 | 64 | Days (n) | 93.0 (90.0–93.0) | 6.0–93.0 |
| isCGM values (n) | 8308.0 (7245.5–8480.0) | 155.0–12934.0 | ||
| isCGM activity (%) | 93.3 (85.6–95.2) | 26.9–144.9 | ||
| Mean glucose (mg/dL) | 163.6 (154.0–193.0) | 112.4–352.0 | ||
| CV | 0.4 (0.3–0.4) | 0.2–0.6 | ||
| GMI | 7.2 (7.0–7.9) | 6.0–11.7 | ||
| MAGE | 177.5 (163.5–202.4) | 119.0–357.8 |
| Difference * | Parameter | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Lockdown 2020 vs. pre-pandemic 2019 | Days (n) | 2.0 (2.0–2.0) | −56.0–66.0 |
| isCGM values (n) | 185.0 (−2.0–577.5) | −5645.0–4181.0 | |
| isCGM activity (%) | 0.0 (−1.7–1.6) | −70.5–27.1 | |
| Mean glucose (mg/dL) | −3.8 (−13.5–2.6) | −61.6–66.9 | |
| CV | −0.0 (−0.0–0.0) | −0.1–0.1 | |
| GMI | −0.1 (−0.3–0.1) | −1.5–1.6 | |
| MAGE | −2.3 (−11.8–3.7) | −75.0–80.8 | |
| Lockdown 2020 vs. pre-lockdown 2020 | Days (n) | 0.0 (0.0–1.5) | −87.0–88.0 |
| isCGM values (n) | −42.0 (−471.0–1247.0) | −8701.0–7699.0 | |
| isCGM activity (%) | −1.0 (−5.9–1.2) | −72.3–42.2 | |
| Mean glucose (mg/dL) | −3.6 (−17.2–6.3) | −42.9–83.0 | |
| CV | −0.0 (−0.1–0.0) | −0.2–0.1 | |
| GMI | −0.1 (−0.4–0.2) | −1.0–2.0 | |
| MAGE | −6.5 (−17.1–8.7) | −43.3–94.9 |
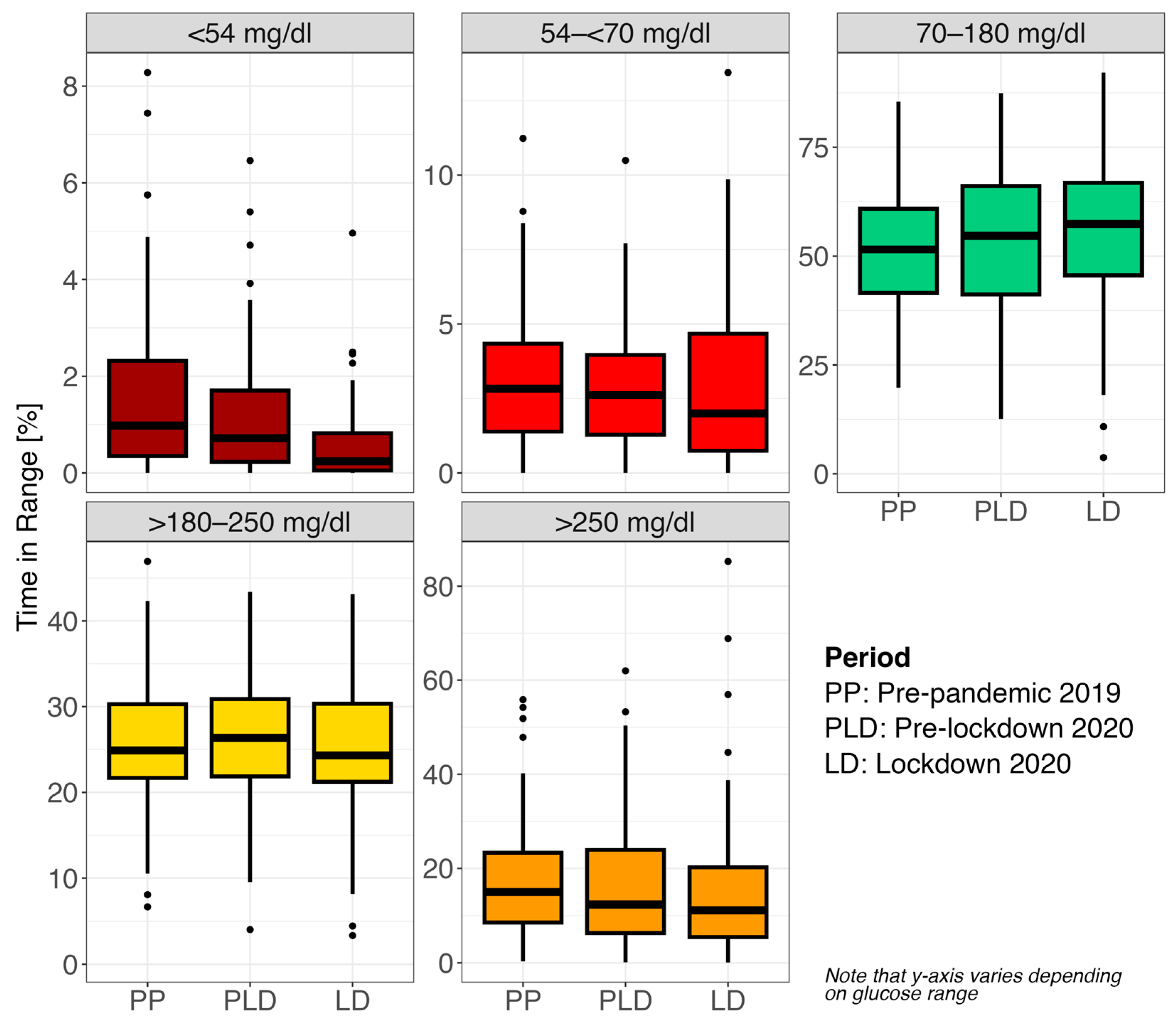
| Phase | Glucose Range | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Pre-pandemic 2019 | <54 mg/dL | 1.0 (0.4–2.3) | 0.0–8.3 |
| 54–<70 mg/dL | 2.8 (1.4–4.3) | 0.0–11.2 | |
| <70 mg/dL | 3.6 (1.8–6.5) | 0.0–14.9 | |
| 70–180 mg/dL | 51.5 (41.5–60.9) | 19.8–85.5 | |
| >180–250 mg/dL | 24.9 (21.7–30.3) | 6.7–47.0 | |
| >180 mg/dL | 44.6 (31.5–55.2) | 7.2–80.0 | |
| >250 mg/dL | 15.0 (8.6–23.3) | 0.3–55.9 | |
| Pre-lockdown 2020 | <54 mg/dL | 0.72 (0.2–1.7) | 0.0–6.5 |
| 54–<70 mg/dL | 2.61 (1.3–4.0) | 0.0–10.5 | |
| <70 mg/dL | 3.6 (1.6–5.6) | 0.0–15.2 | |
| 70–180 mg/dL | 54.7 (41.2–66.1) | 12.6–87.4 | |
| >180–250 mg/dL | 26.4 (21.9–30.9) | 4.0–43.4 | |
| >180 mg/dL | 40.6 (30.0–56.1) | 4.5–87.4 | |
| >250 mg/dL | 12.3 (6.3–24.0) | 0.1–62.0 | |
| Lockdown 2020 | <54 mg/dL | 0.2 (0.1–0.8) | 0.0–5.0 |
| 54–<70 mg/dL | 2.0 (0.7–4.7) | 0.0–13.4 | |
| <70 mg/dL | 2.3 (0.8–6.1) | 0.0–14.9 | |
| 70–180 mg/dL | 57.4 (45.6–66.8) | 3.7–92.2 | |
| >180–250 mg/dL | 24.3 (21.2–30.3) | 3.4–43.1 | |
| >180 mg/dL | 35.4 (28.9–53.3) | 3.4–96.3 | |
| >250 mg/dL | 11.2 (5.5–20.2) | 0.1–85.3 |
| Difference * | Parameter | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Lockdown 2020 vs. pre-pandemic 2019 | <54 mg/dL | −0.4 (−1.0–−0.1) | −3.7–1.4 |
| 54–<70 mg/dL | −0.1 (−0.6–1.1) | −4.9–4.9 | |
| <70 mg/dL | −0.3 (−1.4–0.7) | −6.3–6.3 | |
| 70–180 mg/dL | 3.9 (−1.6–7.1) | −19.0–23.7 | |
| >180–250 mg/dL | −1.2 (−3.8–1.0) | −16.8–18.1 | |
| >180 mg/dL | −3.0 (−7.3–1.0) | −30.0–20.0 | |
| >250 mg/dL | −0.9 (−4.1–0.7) | −26.4–23.3 | |
| Lockdown 2020 vs. pre-lockdown 2020 | <54 mg/dL | −0.6 (−1.5–−0.1) | −5.0–1.9 |
| 54–<70 mg/dL | −0.2 (−1.1–0.6) | −6.4–6.4 | |
| <70 mg/dL | −1.2 (−2.4–0.1) | −7.4–5.9 | |
| 70–180 mg/dL | 3.2 (−1.7–9.7) | −20.0–27.7 | |
| >180–250 mg/dL | −0.5 (−3.2–1.9) | −16.7–19.3 | |
| >180 mg/dL | −2.0 (−10.0–2.1) | −27.7–21.0 | |
| >250 mg/dL | −1.8 (−5.2–0.9) | −17.0–29.4 |
| Parameter | Phase | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Total isCGM scan frequency | Pre-pandemic 2019 | 899.0 (481.0–1430.5) | 97.0–3961.0 |
| Pre-lockdown 2020 | 817.0 (610.0–1163.0) | 9.0–4193.0 | |
| Lockdown 2020 | 814.0 (551.0–1204.5) | 7.0–3805.0 | |
| Mean daily isCGM scan frequency | Pre-pandemic 2019 | 11.5 (7.8–19.4) | 1.8–42.6 |
| Pre-lockdown 2020 | 9.1 (6.8–13.8) | 1.9–46.1 | |
| Lockdown 2020 | 8.8 (6.3–13.0) | 1.2–41.0 |
| Categorical parameters | Number (n) | Percent (%) |
| Gender [male/female] | 26 females | 32.5 |
| 54 males | 67.5 | |
| System of use [pen/pump] | 60 pen | 75 |
| 20 pump | 25 | |
| Numeric parameters | Median (Q1–Q3) | Min–Max |
| Age [years] | 33.2 (25.5; 49.1) | 19.2–73.3 |
| Diabetes duration [years] | 12.5 (5.5; 20.7) | 1.5–64.5 |
| Height [m] | 1.8 (1.7; 1.8) | 1.5–1.9 |
| Weight [kg] | 76.0 (68.0; 88.3) | 43.0–134.0 |
| BMI [kg/m2] | 24.0 (22.0; 26.9) | 17.4–37.9 |
| HbA1c [mmol/mol] | 57.5 (51.0; 69.3) | 37.0–143.0 |
| Creatinine [mg/dL] | 0.9 (0.8; 1.0) | 0.6–4.4 |
| isCGM use duration [months] | 67.0 (55.0; 79.0) | 47.0–92.0 |
| isCGM use duration [years] | 5.6 (4.6; 6.6) | 3.9–7.7 |
| Visits during lockdown 2020 [n] | 0.0 (0.0; 1.0) | 0.0–6.0 |
| Visits during pre-lockdown [n] | 1.0 (0.0; 1.0) | 0.0–7.0 |
| Visits during pre-pandemic) [n] | 1.0 (0.0; 2.0) | 0.0–11.0 |
| Phase | Individuals (N) | Parameters | Median (Q1–Q3) | Min–Max |
|---|---|---|---|---|
| Pre- pandemic 2019 | 64 | Days (n) | 93.0 (85.0–93.0) | 5.0–93.0 |
| isCGM values (n) | 8309.0 (5829.8–8675.3) | 399.0–9177.0 | ||
| isCGM activity (%) | 95.2 (87.9–97.6) | 44.8–102.8 | ||
| Mean glucose (mg/dL) | 177.7 (156.2–195.7) | 117.6–275.8 | ||
| CV | 0.4 (0.4–0.4) | 0.2–0.5 | ||
| GMI | 7.6 (7.1–8.0) | 6.1–9.9 | ||
| MAGE | 187.5 (170.8–207.8) | 123.9–286.1 | ||
| Pre- lockdown 2020 | 78 | Days (n) | 91.0 (82.5–91.0) | 4.0–91.0 |
| isCGM values (n) | 7930.0 (6646.3–8344.5) | 143.0–10312.0 | ||
| isCGM activity (%) | 93.32 (83.7–95.8) | 37.2–118.0 | ||
| Mean glucose (mg/dL) | 166.7 (154.0–195.3) | 107.8–299.4 | ||
| CV | 0.4 (0.3–0.4) | 0.2–0.5 | ||
| GMI | 7.3 (7.0–8.0) | 5.9–10.5 | ||
| MAGE | 175.4 (164.3–203.9) | 121.3–358.0 | ||
| Lockdown 2020 | 79 | Days (n) | 93.0 (90.0–93.0) | 1.0–93.0 |
| isCGM values (n) | 8283.0 (7349.5–8478.0) | 32.0–12934.0 | ||
| isCGM activity (%) | 92.83 (85.6–95.0) | 26.9–144.9 | ||
| Mean glucose (mg/dL) | 163.2 (150.6–193.0) | 110.4–352.0 | ||
| CV | 0.4 (0.3–0.4) | 0.2–0.6 | ||
| GMI | 7.2 (6.9–7.9) | 6.0–11.7 | ||
| MAGE | 175.7 (162.4–197.6) | 119.0–357.8 |
| Phase | Glucose Range | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Pre-pandemic 2019 | <54 mg/dL | 0.9 (0.4–2.3) | 0.0–8.3 |
| 54–<70 mg/dL | 2.8 (1.5–4.3) | 0.0–11.2 | |
| <70 mg/dL | 3.6 (1.9–6.5) | 0.0–14.9 | |
| 70–180 mg/dL | 51.6 (41.7–60.8) | 19.8–85.5 | |
| >180–250 mg/dL | 25.2 (21.7–30.3) | 6.7–47.0 | |
| >180 mg/dL | 44.6 (31.5–55.1) | 7.2–80.0 | |
| >250 mg/dL | 15.3 (8.7–23.2) | 0.3–55.9 | |
| Pre-lockdown 2020 | <54 mg/dL | 0.7 (0.3–1.8) | 0.0–7.7 |
| 54–<70 mg/dL | 2.8 (1.4–4.3) | 0.0–13.9 | |
| <70 mg/dL | 3.7 (1.7–6.1) | 0.0–18.7 | |
| 70–180 mg/dL | 55.7 (41.9–67.5) | 12.6–90.6 | |
| >180–250 mg/dL | 24.7 (21.0–29.7) | 4.0–43.4 | |
| >180 mg/dL | 38.7 (29.3–55.8) | 4.5–87.4 | |
| >250 mg/dL | 11.6 (4.6–24.1) | 0.1–62.0 | |
| Lockdown 2020 | <54 mg/dL | 0.2 (0.1–0.8) | 0.0–5.0 |
| 54–<70 mg/dL | 2.0 (0.8–5.4) | 0.0–13.4 | |
| <70 mg/dL | 2.3 (0.8–6.4) | 0.0–14.9 | |
| 70–180 mg/dL | 58.4 (45.4–68.4) | 3.7–92.2 | |
| >180–250 mg/dL | 24.3 (20.7–28.5) | 3.4–43.1 | |
| >180 mg/dL | 35.3 (27.9–52.9) | 3.4–96.3 | |
| >250 mg/dL | 10.0 (4.9–20.7) | 0.1–85.3 |
| Parameter | Phase | Median (Q1–Q3) | Min–Max |
|---|---|---|---|
| Total isCGM scan frequency | Pre-pandemic 2019 | 900.0 (483.5–1426.8) | 97.0–3961.0 |
| Pre-lockdown 2020 | 810.5 (553.8–1172.0) | 9.0–4193.0 | |
| Lockdown 2020 | 816.0 (567.0–1261.0) | 2.0–3805.0 | |
| Mean daily isCGM scan frequency | Pre-pandemic 2019 | 11.6 (7.8–19.3) | 1.8–42.6 |
| Pre-lockdown 2020 | 9.2 (6.7–14.7) | 1.9–46.1 | |
| Lockdown 2020 | 8.9 (6.4–13.6) | 1.2–40.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secco, K.; Baumann, P.M.; Pöttler, T.; Aberer, F.; Cigler, M.; Elsayed, H.; Harer, C.M.; Weitgasser, R.; Schütz-Fuhrmann, I.; Mader, J.K. Glycemic Control Assessed by Intermittently Scanned Glucose Monitoring in Type 1 Diabetes during the COVID-19 Pandemic in Austria. Sensors 2024, 24, 4514. https://doi.org/10.3390/s24144514
Secco K, Baumann PM, Pöttler T, Aberer F, Cigler M, Elsayed H, Harer CM, Weitgasser R, Schütz-Fuhrmann I, Mader JK. Glycemic Control Assessed by Intermittently Scanned Glucose Monitoring in Type 1 Diabetes during the COVID-19 Pandemic in Austria. Sensors. 2024; 24(14):4514. https://doi.org/10.3390/s24144514
Chicago/Turabian StyleSecco, Katharina, Petra Martina Baumann, Tina Pöttler, Felix Aberer, Monika Cigler, Hesham Elsayed, Clemens Martin Harer, Raimund Weitgasser, Ingrid Schütz-Fuhrmann, and Julia Katharina Mader. 2024. "Glycemic Control Assessed by Intermittently Scanned Glucose Monitoring in Type 1 Diabetes during the COVID-19 Pandemic in Austria" Sensors 24, no. 14: 4514. https://doi.org/10.3390/s24144514






