Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications
Abstract
:1. Introduction
2. Dielectric Characterization of Human Tissues
- Description of the standard calibration procedure and related confounders (e.g., environmental variables, VNA drift, and cable movement);
- The validation procedure with a known reference liquid;
- The uncertainty evaluation, usually based on the guidelines of the National Institute of Standard and Technology (NIST) [41];
- A summary of the comparative studies with both in vivo and ex vivo measurements;
- The best practice measurement steps, with special considerations about the sensing depth, the tissue heterogeneity, and the effect of temperature on tissue DPs.
- Measurement setup (e.g., effects of cable movements on VNA settings, and probe use);
- Calibration;
- Sample characteristics;
- Measurement practices (e.g., repeating the measure several times is highly recommended);
- Data analysis, which comprises data fitting with mathematical equations and uncertainty calculation (according to GUM document by the Joint Committee for Guides in Metrology [51]);
- Data reporting methods, which satisfy EU directives, such as the FAIR guiding principles [52], and promote open-access data collections.
3. Breast Cancer Detection
3.1. Traditional Breast Imaging Techniques
- Mammography employs potentially harmful X-rays, so frequent repeats of the procedure are not recommended, while breast compression-induced pain in some cases discourages women from attending screening programs (involving subjects aged > 40). Among the unresolved issues, there are the low sensitivity (<70%) attested in high-density breasts (usually younger aged women) with respect to 90% achieved in fat-dominated tissues, and high-rate of false-positive recalls. Digital breast tomosynthesis (DBT) partially solves the difficulties related to tissue overlap in dense breasts by collecting 3-D multiple projections, but this implies additional radiation exposure. Nevertheless, mammography provides high spatial resolution, and the performance decreases only when the lesion size is smaller than 20 mm [57].
- Breast sonography, or ultrasound (US), exploits the acoustic impedance of soft tissues, and it is sensitive to differences in fat, fibrous, and glandular components of the breast. It offers a non-ionizing, low-cost tool for the investigation of symptomatic cases and examination of dense breasts complementary to mammography, as well as real-time image-based guidance during needle biopsy. Nevertheless, its role as a screening method is debated due to its dependence on operator skill, long-time requirements, and higher rates of false positives [58]. While recent advancements in US technology have improved the achievable resolution and allow to automate the procedure, its adoption in clinical practice remains to be fully established.
- MRI creates detailed images of soft tissues based on the relaxation properties of the hydrogen atoms in the presence of a strong magnetic field and usually requires a contrast agent. It is applied for the high-resolution assessment of diagnosed breast lesions, for example, before surgery or to evaluate treatment response [55]. The outcomes of recent tests in screening populations foster the choice of this technique for high-risk patients, having higher sensitivity in finding neoplasms. However, its use is constrained by high costs, long acquisition times, limited portability and availability, together with the remaining doubts related to the high number of false detections [56].
3.2. Current Achievements and Drawbacks in Microwave Breast Imaging
3.3. Microwave Breast Imaging Devices
4. Brain Stroke Detection
4.1. Brain Stroke Diagnosis and Treatment
4.2. Microwave Brain Stroke Imaging Devices
5. Non-Invasive Glucose Monitoring
5.1. Diabetes and Blood Glucose Level Sensing: State of the Art
- Region A: measurements within 20% of the reference sensor, corresponding to clinically valid treatment;
- Region B: values outside of 20% but not leading to inappropriate treatment;
- Region C: measurements that result in unnecessary treatment;
- Region D: measurements indicating dangerous failures to detect and treat;
- Region E: “erroneous treatment” zone, where measurements cause confusion between the treatment of hypoglycemia and hyperglycemia.

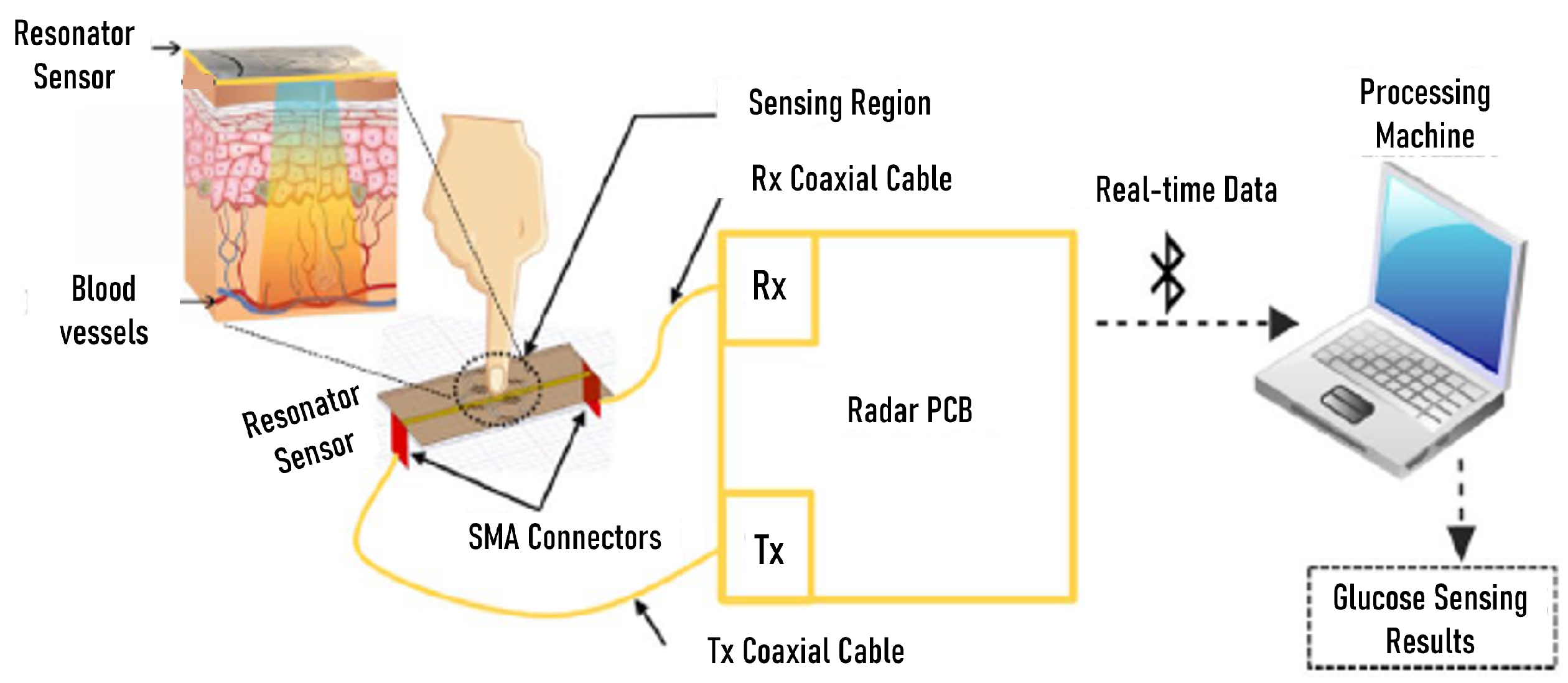
5.2. Microwave Sensors for Glucose Monitoring Tested on Humans
6. Other Medical Applications
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BGL | Blood Glucose Level |
| CEG | Clarke Error Grid |
| CGM | Continuous Glucose Monitoring |
| CM | Coupling Medium |
| CMS | Contact-based Meander-line Sensor |
| CT | Computed Tomography |
| DAS | Delay-and-Sum |
| DBIM | Distorted Born Iterative Method |
| DoI | Domain of Interest |
| DPs | Dielectric Properties |
| EM | Electromagnetic |
| FDTD | Finite Difference Time Domain |
| GT | Ground Truth |
| ICH | Intracerebral Hemorrhages |
| IS | Ischemic Strokes |
| LSTM | Long Short-Term Memory |
| MARD | Mean Absolute Relative Difference |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| MW | Microwave |
| NCT | Neoadjuvant Chemotherapy |
| PCB | Printed Circuit Board |
| RMSE | Root Mean Square Error |
| SRR | Split-Ring Resonators |
| SSPP | Spoof Surface Plasmon Polariton |
| TDS | Time Domain Spectroscopy |
| TSVD | Truncated Singular Value Decomposition |
| TwIST | Two-step Iterative Shrinkage Thresholding |
| US | Ultrasound |
| UWB | Ultra-wideband |
| VNA | Vector Network Analyzer |
| WGR | Whispering Gallery Resonator |
| WHO | World Health Organization |
References
- Larsen, L.E.; Jacobi, J.H. Medical Applications of Microwave Imaging; IEEE Press: New York, NY, USA, 1986; Volume 32. [Google Scholar]
- Bolomey, J.C. Crossed Viewpoints on Microwave-Based Imaging for Medical Diagnosis: From Genesis to Earliest Clinical Outcomes. In The World of Applied Electromagnetics: In Appreciation of Magdy Fahmy Iskander; Lakhtakia, A., Furse, C.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 369–414. [Google Scholar] [CrossRef]
- Mehrotra, P.; Chatterjee, B.; Sen, S. EM-Wave Biosensors: A Review of RF, Microwave, mm-Wave and Optical Sensing. Sensors 2019, 19, 1013. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.; Augello, E.; Battistini, G.; Benassi, F.; Masotti, D.; Paolini, G. Microwave Devices for Wearable Sensors and IoT. Sensors 2023, 23, 4356. [Google Scholar] [CrossRef] [PubMed]
- Bing, S.; Chawang, K.; Chiao, J.C. A Tuned Microwave Resonant Sensor for Skin Cancerous Tumor Diagnosis. IEEE J. Electromagn. Microwaves Med. Biol. 2023, 7, 320–327. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Wang, L. Microwave Imaging and Sensing Techniques for Breast Cancer Detection. Micromachines 2023, 14, 1462. [Google Scholar] [CrossRef] [PubMed]
- Savazzi, M.; Karadima, O.; Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Vipiana, F.; Kosmas, P.; Fernandes, C.A.; Felício, J.M.; Conceição, R.C. Experimental Assessment of Axillary Lymph Node Microwave Tomography Using Anthropomorphic Phantoms. IEEE J. Electromagn. Microwaves Med. Biol. 2023, 7, 160–167. [Google Scholar] [CrossRef]
- Guo, L.; Alqadami, A.S.M.; Abbosh, A. Stroke Diagnosis Using Microwave Techniques: Review of Systems and Algorithms. IEEE J. Electromagn. RF Microwaves Med. Biol. 2023, 7, 122–135. [Google Scholar] [CrossRef]
- Sultan, K.; Abbosh, A. Advancing Wearable Electromagnetic Knee Imaging: A Comprehensive Review of Systems, Frameworks, Key Challenges, and Future Directions. IEEE J. Electromagn. RF Microwaves Med. Biol. 2023, 7, 468–490. [Google Scholar] [CrossRef]
- Ahdi Rezaeieh, S.; Darvazehban, A.; Janani, A.S.; Abbosh, A.M. Electromagnetic Torso Scanning: A Review of Devices, Algorithms, and Systems. Biosensors 2021, 11, 135. [Google Scholar] [CrossRef]
- Porter, E.; O’Loughlin, D. Pathway to Demonstrating Clinical Efficacy of Microwave Breast Imaging: Qualitative and Quantitative Performance Assessment. IEEE J. Electromagn. RF Microwaves Med. Biol. 2022, 6, 439–448. [Google Scholar] [CrossRef]
- Porter, E.; La Gioia, A.; Salahuddin, S.; Decker, S.; Shahzad, A.; Elahi, A.; O’Halloran, M.; Beyan, O. Minimum information for dielectric measurements of biological tissues (MINDER): A framework for repeatable and reusable data. Int. J. RF Microw. Comput.-Aided Eng. 2017, 28, e21201. [Google Scholar] [CrossRef]
- Farrugia, L.; Porter, E.; Conceição, R.C.; Meo, S.D.; Godinho, D.M.; Bonello, J.; Ragulskis, M.; Ocket, I.; Farina, L.; Cavagnaro, M.; et al. The Complex Permittivity of Biological Tissues: A Practical Measurement Guideline. IEEE Access 2024, 12, 10296–10314. [Google Scholar] [CrossRef]
- Nikolova, N.K. Introduction to Microwave Imaging; EuMA High Frequency Technologies Series; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Benny, R.; Anjit, T.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. B 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Gong, Z.; Chen, Y.; Ding, Y.; Zhang, H. Perspective: Microwave Medical Imaging Using Space-Time-Frequency A Priori Knowledge for Health Monitoring. IEEE J. Electromagn. RF Microwaves Med. Biol. 2024, 8, 2–14. [Google Scholar] [CrossRef]
- Zaatar, O.; Zakaria, A.; Qaddoumi, N. A Novel Switch for Microwave Imaging Systems. IEEE Access 2024, 12, 26978–26990. [Google Scholar] [CrossRef]
- Shahmirzadi, N.V.; Nguyen, J.; Kazemivala, R.; Nikolova, N.K.; Chen, C.H. Electronically Scanned Active Sensor Array for the Imaging of Compressed Breast. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Althubitat Alamro, W.; Seet, B.C. Review of practical antennas for microwave and millimetre-wave medical imaging. In Electromagnetic Waves and Antennas for Biomedical Applications; Healthcare Technologies, Institution of Engineering and Technology: London, UK, 2021; pp. 185–207. [Google Scholar] [CrossRef]
- Bucci, O.M.; Crocco, L.; Scapaticci, R.; Bellizzi, G. On the Design of Phased Arrays for Medical Applications. Proc. IEEE 2016, 104, 633–648. [Google Scholar] [CrossRef]
- Devaney, A. Time reversal imaging of obscured targets from multistatic data. IEEE Trans. Antennas Propag. 2005, 53, 1600–1610. [Google Scholar] [CrossRef]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. SAFE—Microwave Imaging Device for Breast Cancer Early Screening and Diagnostics. In Electromagnetic Imaging for a Novel Generation of Medical Devices: Fundamental Issues, Methodological Challenges and Practical Implementation; Vipiana, F., Crocco, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 273–292. [Google Scholar] [CrossRef]
- Bertero, M.; Boccacci, P. Introduction to Inverse Problems in Imaging; Institute of Physics: Bristol, UK, 1998. [Google Scholar]
- Ghavami, N.; Tiberi, G.; Edwards, D.J.; Monorchio, A. UWB Microwave Imaging of Objects with Canonical Shape. IEEE Trans. Antennas Propag. 2012, 60, 231–239. [Google Scholar] [CrossRef]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. MARIA M4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef]
- Bourqui, J.; Sill, J.; Fear, E. A prototype system for measuring microwave frequency reflections from the breast. Int. J. Biomed. Imaging 2012, 2012, 851234. [Google Scholar] [CrossRef]
- Kibria, S.; Samsuzzaman, M.; Islam, M.T.; Mahmud, M.Z.; Misran, N.; Islam, M.T. Breast Phantom Imaging Using Iteratively Corrected Coherence Factor Delay and Sum. IEEE Access 2019, 7, 40822–40832. [Google Scholar] [CrossRef]
- Smith, K.; Bourqui, J.; Garrett, D.; Zarnke, S.; Owjimehr, M.; Deutscher, D.; Fung, T.; Fear, E. Microwave Imaging of the Breast: Consistency of Measurements Over Time. IEEE J. Electromagn. Microwaves Med. Biol. 2022, 6, 61–67. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fang, Q.; Rubaek, T.; Demidenko, E.; Paulsen, K.D. Log transformation benefits parameter estimation in microwave tomographic imaging. Med. Phys. 2007, 34, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Karadima, O.; Lu, P.; Sotiriou, I.; Kosmas, P. Assessment and Validation of 2-D and 3-D DBIM-TwIST Algorithm for Brain Stroke Detection and Differentiation. In Electromagnetic Imaging for a Novel Generation of Medical Devices: Fundamental Issues, Methodological Challenges and Practical Implementation; Vipiana, F., Crocco, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 131–177. [Google Scholar] [CrossRef]
- Henriksson, T.; Planas, R.; El-Salloum, C.; Gatto, P.; Khavari, A.; Romero-Hinrichsen, F.; Pearce, S. Brain Stroke Detection Using Electromagnetic Tomography: A New Portable Scanner. In Proceedings of the 2023 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (USNC-URSI), Portland, OR, USA, 23–28 July 2023; pp. 315–316. [Google Scholar] [CrossRef]
- Schwan, H.; Foster, K. RF-field interactions with biological systems: Electrical properties and biophysical mechanisms. Proc. IEEE 1980, 68, 104–113. [Google Scholar] [CrossRef]
- Schepps, J.L.; Foster, K.R. The UHF and microwave dielectric properties of normal and tumour tissues: Variation in dielectric properties with tissue water content. Phys. Med. Biol. 1980, 25, 1149. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.M.; Land, D.V. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193. [Google Scholar] [CrossRef]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994, 21 4, 547–550. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.; Lindstrom, M.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric Properties Characterization From 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Sasaki, K.; Porter, E.; Rashed, E.A.; Farrugia, L.; Schmid, G. Measurement and image-based estimation of dielectric properties of biological tissues —past, present, and future—. Phys. Med. Biol. 2022, 67, 14TR01. [Google Scholar] [CrossRef]
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef]
- The NIST Reference on Constants, Units, and Uncertainty. 2017. Available online: https://physics.nist.gov/cuu/Uncertainty/basic.html (accessed on 15 February 2024).
- La Gioia, A.; O’Halloran, M.; Porter, E. Modelling the Sensing Radius of a Coaxial Probe for Dielectric Characterisation of Biological Tissues. IEEE Access 2018, 6, 46516–46526. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Andreuccetti, D.; Fossi, R.; Petrucci, C. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10 Hz–100 GHz. IFAC-CNR, Florence (Italy). 1997. Available online: https://niremf.ifac.cnr.it/tissprop/htmlclie/htmlclie.php (accessed on 15 February 2024).
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 4.1. 22 February 2022. Available online: https://itis.swiss/virtual-population/tissue-properties/downloads/database-v4-1/ (accessed on 15 February 2024).
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Converse, M.C.; Booske, J.H.; Hagness, S.C. Ultrawideband temperature-dependent dielectric properties of animal liver tissue in the microwave frequency range. Phys. Med. Biol. 2006, 51, 1941. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Khan, S.; Jones, M.; Dwyer, R.M.; O’Halloran, M. Investigation of the effect of dehydration on tissue dielectric properties in ex vivo measurements. Biomed. Phys. Eng. Express 2017, 3, 045001. [Google Scholar] [CrossRef]
- Maenhout, G.; Santorelli, A.; Porter, E.; Ocket, I.; Markovic, T.; Nauwelaers, B. Effect of Dehydration on Dielectric Measurements of Biological Tissue as Function of Time. IEEE J. Electromagn. RF Microwaves Med. Biol. 2020, 4, 200–207. [Google Scholar] [CrossRef]
- Working Group 1 of the Joint Committee for Guides in Metrology (JCGM/WG1). Evaluation of Measurement Data-Guide to the Expression of Uncertainty in Measurement. Document JCGM 100:2008, GUM 1995 with Minor Corrections. 2008. Available online: https://www.bipm.org/documents/20126/2071204/JCGM_100_2008_E.pdf (accessed on 15 February 2024).
- H2020 Programme. Guidelines on FAIR Data Management in Horizon 2020. 2020. Available online: https://ec.europa.eu/research/participants/data/ref/h2020/grants_manual/hi/oa_pilot/h2020-hi-oa-data-mgt_e (accessed on 19 February 2024).
- MyWAVE COST Action: Open-Access Repository of Dielectric and Thermal Properties of Biological Tissues. Available online: https://www.um.edu.mt/projects/mywave/data-repository (accessed on 19 February 2024).
- World Health Organization. Fact Scheets–Breast Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 15 February 2024).
- Moloney, B.M.; O’Loughlin, D.; Abd Elwahab, S.; Kerin, M.J. Breast Cancer Detection—A Synopsis of Conventional Modalities and the Potential Role of Microwave Imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef]
- Nicosia, L.; Gnocchi, G.; Gorini, I.; Venturini, M.; Fontana, F.; Pesapane, F.; Abiuso, I.; Bozzini, A.C.; Pizzamiglio, M.; Latronico, A.; et al. History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century. Healthcare 2023, 11, 1596. [Google Scholar] [CrossRef]
- Wang, J.; Gottschal, P.; Ding, L.; Veldhuizen, D.; Lu, W.; Houssami, N.; Greuter, M.; de Bock, G. Mammographic sensitivity as a function of tumor size: A novel estimation based on population-based screening data. Breast 2021, 55, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Geisel, J.; Raghu, M.; Hooley, R. The Role of Ultrasound in Breast Cancer Screening: The Case for and Against Ultrasound. Semin. Ultrasound CT MRI 2018, 39, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of Microwaves Techniques for Breast Cancer Detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef]
- AlSawaftah, N.; El-Abed, S.; Dhou, S.; Zakaria, A. Microwave Imaging for Early Breast Cancer Detection: Current State, Challenges, and Future Directions. J. Imaging 2022, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef]
- Reimer, T.; Pistorius, S. Review and Analysis of Tumour Detection and Image Quality Analysis in Experimental Breast Microwave Sensing. Sensors 2023, 23, 5123. [Google Scholar] [CrossRef]
- Cheng, Y.; Fu, M. Dielectric properties for non-invasive detection of normal, benign, and malignant breast tissues using microwave theories: Microwave properties of breast tissues. Thorac. Cancer 2018, 9, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Canicattì, E.; Sánchez-Bayuela, D.A.; Romero Castellano, C.; Aguilar Angulo, P.M.; Giovanetti González, R.; Cruz Hernández, L.M.; Ruiz Martín, J.; Tiberi, G.; Monorchio, A. Dielectric Characterization of Breast Biopsied Tissues as Pre-Pathological Aid in Early Cancer Detection: A Blinded Feasibility Study. Diagnostics 2023, 13, 3015. [Google Scholar] [CrossRef]
- Bellizzi, G.; Bellizzi, G.; Bucci, O.; Crocco, L.; Helbig, M.; Ley, S.; Sachs, J. Optimization of the Working Conditions for Magnetic Nanoparticle-Enhanced Microwave Diagnostics of Breast Cancer. IEEE Trans. Biomed. Eng. 2018, 65, 1607–1616. [Google Scholar] [CrossRef]
- Mahmud, M.Z.; Islam, M.T.; Misran, N.; Almutairi, A.F.; Cho, M. Ultra-Wideband (UWB) Antenna Sensor Based Microwave Breast Imaging: A Review. Sensors 2018, 18, 2951. [Google Scholar] [CrossRef]
- MammoWave. Available online: https://www.ubt-tech.com/mammowave/ (accessed on 2 May 2024).
- Wang, L.; Peng, H.; Ma, J. Microwave Breast Imaging Techniques and Measurement Systems. In Breast Imaging; Malik, A.M., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 4. [Google Scholar] [CrossRef]
- Katrin, S.; Jeremie, B.; Zefang, W.; Brendon, B.; Mark, L.E.A. Microwave imaging for monitoring breast cancer treatment: A pilot study. Med. Phys. 2023, 50, 7118–7129. [Google Scholar] [CrossRef]
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer – Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2022, 29, S211–S222. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Cayoren, M.; Akduman, I.; Yilmaz, T.; Onemli, E.; Bugdayci, O.; Aribal, M.E. SAFE: A Novel Microwave Imaging System Design for Breast Cancer Screening and Early Detection—Clinical Evaluation. Diagnostics 2021, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Akduman, I.; Cayoren, M.; Bugdayci, O.; Aribal, M.E. Microwave Breast Lesion Classification—Results from Clinical Investigation of the SAFE Microwave Breast Cancer System. Acad. Radiol. 2023, 30, S1–S8. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar] [CrossRef]
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; diFlorio Alexander, R.M.; Paulsen, K.D. Fast 3-D Tomographic Microwave Imaging for Breast Cancer Detection. IEEE Trans. Med. Imaging 2012, 31, 1584–1592. [Google Scholar] [CrossRef]
- Meaney, P.M.; Kaufman, P.A.; Muffly, L.S.; Click, M.; Poplack, S.P.; Wells, W.A.; Schwartz, G.N.; di Florio-Alexander, R.M.; Tosteson, T.D.; Li, Z.; et al. Microwave imaging for neoadjuvant chemotherapy monitoring: Initial clinical experience. Breast Cancer Res. 2013, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sani, L.; Vispa, A.; Loretoni, R.; Duranti, M.; Ghavami, N.; Alvarez Sánchez-Bayuela, D.; Caschera, S.; Paoli, M.; Bigotti, A.; Badia, M.; et al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PLoS ONE 2021, 16, e0250005. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Loretoni, R.; Duranti, M.; Ghavami, M.; Dudley, S.; Tiberi, G. Radiation-Free Microwave Technology for Breast Lesion Detection Using Supervised Machine Learning Model. Tomography 2023, 9, 105–129. [Google Scholar] [CrossRef]
- Álvarez Sánchez-Bayuela, D.; Ghavami, N.; Romero Castellano, C.; Bigotti, A.; Badia, M.; Papini, L.; Raspa, G.; Palomba, G.; Ghavami, M.; Loretoni, R.; et al. A Multicentric, Single Arm, Prospective, Stratified Clinical Investigation to Confirm MammoWave’s Ability in Breast Lesions Detection. Diagnostics 2023, 13, 2100. [Google Scholar] [CrossRef]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave Breast Imaging with a Monostatic Radar-Based System: A Study of Application to Patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sidebottom, R.; Webb, D.; Bishop, B.; Kabir, M.; Allen, S. Results for the London investigation into dielectric scanning of lesions study of the MARIA M6 breast imaging system. Br. J. Radiol. 2024, 97, 549–552. [Google Scholar] [CrossRef]
- Fasoula, A.; Arvanitis, P.; Duchesne, L. Repeatability assessement of the Wavelia # 2 Microwave Breast Imaging scan: Experimental performance analysis prior to clinical investigation. In Microwave Technologies—Recent Advances and New Trends and Applications; Şerif Savcı, H., Ed.; IntechOpen: Rijeka, Croatia, 2023; Chapter 3. [Google Scholar] [CrossRef]
- MITOS. Available online: https://www.mitosmedikal.com (accessed on 2 May 2024).
- Kurrant, D.; Bourqui, J.; Fear, E. Surface Estimation for Microwave Imaging. Sensors 2017, 17, 1658. [Google Scholar] [CrossRef]
- Wave View. Available online: https://www.waveviewimaging.com (accessed on 2 May 2024).
- Micrima. Available online: https://www.micrima.com (accessed on 29 April 2024).
- Song, H.; Sasada, S.; Kadoya, T.; Arihiro, K.; Okada, M.; Xiao, X.; Ishikawa, T.; O’Loughlin, D.; Takada, J.I.; Kikkawa, T. Cross-Correlation of Confocal Images for Excised Breast Tissues of Total Mastectomy. IEEE Trans. Biomed. Eng. 2024, 71, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Bahrami, H.; Santorelli, A.; Gosselin, B.; Rusch, L.A.; Popović, M. A Wearable Microwave Antenna Array for Time-Domain Breast Tumor Screening. IEEE Trans. Med. Imaging 2016, 35, 1501–1509. [Google Scholar] [CrossRef]
- World Stroke Organization. Available online: https://www.world-stroke.org/about-wso (accessed on 10 March 2024).
- Montaño, A.; Hanley, D.F.; Hemphill, J.C. Chapter 13—Hemorrhagic stroke. In Handbook of Clinical Neurology; Hetts, S.W., Cooke, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 176, pp. 229–248. [Google Scholar] [CrossRef]
- Anderson, J.A. Acute ischemic stroke: The golden hour. Nurs. Crit. Care 2016, 11, 28–36. [Google Scholar] [CrossRef]
- Mathur, S.; Walter, S.; Grunwald, I.Q.; Helwig, S.A.; Lesmeister, M.; Fassbender, K. Improving Prehospital Stroke Services in Rural and Underserved Settings with Mobile Stroke Units. Front. Neurol. 2019, 10, 159. [Google Scholar] [CrossRef]
- Shahrestani, S.; Wishart, D.; Han, S.M.J.; Strickland, B.A.; Bakhsheshian, J.; Mack, W.J.; Toga, A.W.; Sanossian, N.; Tai, Y.C.; Zada, G. A systematic review of next-generation point-of-care stroke diagnostic technologies. Neurosurg. Focus 2021, 51, E11. [Google Scholar] [CrossRef]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A feasibility study on microwave imaging for brain stroke monitoring. Prog. Electromagn. Res. B 2012, 40, 305–324. [Google Scholar] [CrossRef]
- Hossain, A.; Islam, M.; Gan, K.B.; Kashem, S.; Soliman, M.; Misran, N.; Chowdhury, M. Microwave brain imaging system to detect brain tumor using metamaterial loaded stacked antenna array. Sci. Rep. 2022, 12, 16478. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Duarte, D.O.; Origlia, C.; Tobon Vasquez, J.A.; Scapaticci, R.; Crocco, L.; Vipiana, F. Experimental Assessment of Real-Time Brain Stroke Monitoring via a Microwave Imaging Scanner. IEEE Open J. Antennas Propag. 2022, 3, 824–835. [Google Scholar] [CrossRef]
- Origlia, C.; Gugliermino, M.; Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Vipiana, F. Anthropomorphic Multi-tissue Head Phantom for Microwave Imaging Devices Testing. In Proceedings of the 2023 17th European Conference on Antennas and Propagation (EuCAP), Florence, Italy, 26–31 March 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Hossain, A.; Tariqul Islam, M.; Hoque, A.; Kamal Abdul Rahim, S.; Alshammari, A.S.; Chowdhury, M.E.; Soliman, M.S. Sensor-based microwave brain imaging system (SMBIS): An experimental six-layered tissue based human head phantom model for brain tumor diagnosis using electromagnetic signals. Eng. Sci. Technol. Int. J. 2023, 45, 101491. [Google Scholar] [CrossRef]
- McDermott, B.; Porter, E.; Santorelli, A.; Divilly, B.; Morris, L.; Jones, M.; Mc Ginley, B.; O’Halloran, M. Anatomically and dielectrically realistic microwave head phantom with circulation and reconfigurable lesions. Prog. Electromagn. Res. B 2017, 78, 47–60. [Google Scholar] [CrossRef]
- Salimitorkamani, M.; Mehranpour, M.; Cansiz, G.; Joof, S.; Akinci, M.N.; Akduman, I.; Odabasi, H. A low-cost and multifunctional long-life anthropomorphic head phantom for microwave brain imaging systems. Microw. Opt. Technol. Lett. 2024, 66, e34095. [Google Scholar] [CrossRef]
- Mohammed, B.; Bialkowski, K.; Hill, S.; Stancombe, A.; Alqadami, A.; Heitzmann, M.T.; Abbosh, A. Stable and Lifelong Head Phantoms Using Polymer Composition Mimicking Materials to Test Electromagnetic Medical Imaging Systems. IEEE J. Electromagn. Microwaves Med. Biol. 2021, 5, 322–328. [Google Scholar] [CrossRef]
- Shahidul Islam, M.; Islam, M.T.; Hoque, A.; Islam, M.T.; Amin, N.; Chowdhury, M.E.H. A Portable Electromagnetic Head Imaging System Using Metamaterial Loaded Compact Directional 3D Antenna. IEEE Access 2021, 9, 50893–50906. [Google Scholar] [CrossRef]
- Bisio, I.; Estatico, C.; Fedeli, A.; Lavagetto, F.; Pastorino, M.; Randazzo, A.; Sciarrone, A. Variable-Exponent Lebesgue-Space Inversion for Brain Stroke Microwave Imaging. IEEE Trans. Microw. Theory Tech. 2020, 68, 1882–1895. [Google Scholar] [CrossRef]
- Cuccaro, A.; Dell’Aversano, A.; Basile, B.; Maisto, M.A.; Solimene, R. Subcranial Encephalic Temnograph-Shaped Helmet For Brain Stroke Monitoring. Sensors 2024, 24, 2887. [Google Scholar] [CrossRef]
- Razzicchia, E.; Ghavami, N.; Karadima, O.; Kosmas, P. Metasurface Technology for Medical Imaging. In Electromagnetic Imaging for a Novel Generation of Medical Devices: Fundamental Issues, Methodological Challenges and Practical Implementation; Vipiana, F., Crocco, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 69–99. [Google Scholar] [CrossRef]
- Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Rodriguez-Duarte, D.O.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; et al. A Prototype Microwave System for 3D Brain Stroke Imaging. Sensors 2020, 20, 2607. [Google Scholar] [CrossRef]
- Rodriguez-Duarte, D.O.; Gugliermino, M.; Origlia, C.; Tobon Vasquez, J.A.; Scapaticci, R.; Crocco, L.; Vipiana, F. Advancements in the Experimental Validation of a Wearable Microwave Imaging System for Brain Stroke Monitoring. In Proceedings of the 2024 18th European Conference on Antennas and Propagation (EuCAP), Glasgow, UK, 17–22 March 2024; pp. 1–3. [Google Scholar] [CrossRef]
- Gugliermino, M.; Rodriguez-Duarte, D.O.; Origlia, C.; Tobon Vasquez, J.A.; Bolomey, J.C.; Scapaticci, R.; Crocco, L.; Vipiana, F. Assessment of a Brain Stroke Microwave Scanner Based on Off-the-Shelf Solid-State Switching. In Proceedings of the 2024 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (AP-S/URSI), Florence, Italy, 14–19 July 2024; pp. 1–2. [Google Scholar]
- Medfield Diagnostics AB. Available online: https://www.medfielddiagnostics.com (accessed on 25 March 2024).
- EMTensor. Available online: https://www.emtensor.com/applications/post-stroke-monitoring (accessed on 25 March 2024).
- EMVision. Available online: https://emvision.com.au (accessed on 25 March 2024).
- Poltschak, S.; Freilinger, M.; Feger, R.; Stelzer, A.; Hamidipour, A.; Henriksson, T.; Hopfer, M.; Planas, R.; Semenov, S. A multiport vector network analyzer with high-precision and realtime capabilities for brain imaging and stroke detection. Int. J. Microw. Wirel. Technol. 2018, 10, 605–612. [Google Scholar] [CrossRef]
- Henriksson, T.; Sahebdivan, S.; Planas, R.; Brunner, C.; Kellermair, L.; Guger, M.; Struhal, W.; Fuchs, P.; Reichl, J.; Stelzer, A.; et al. Human Brain Imaging by Electromagnetic Tomography: A mobile brain scanner for clinical settings. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Hopfer, M.; Planas, R.; Hamidipour, A.; Henriksson, T.; Semenov, S. Electromagnetic Tomography for Detection, Differentiation, and Monitoring of Brain Stroke: A Virtual Data and Human Head Phantom Study. IEEE Antennas Propag. Mag. 2017, 59, 86–97. [Google Scholar] [CrossRef]
- Abbosh, A.; Bialkowski, K.; Guo, L.; Al-Saffar, A.; Zamani, A.; Trakic, A.; Brankovic, A.; Bialkowski, A.; Zhu, G.; Cook, D.; et al. Clinical electromagnetic brain scanner. Sci. Rep. 2024, 14, 5760. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Fhager, A.; Trefna, H.; Yu, Y.; Mckelvey, T.; Pegenius, G.; Karlsson, J.E.; Elam, M. Microwave-Based Stroke Diagnosis Making Global Prehospital Thrombolytic Treatment Possible. IEEE Trans. Bio-Med. Eng. 2014, 61, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Fhager, A.; Candefjord, S.; Elam, M.; Persson, M. Microwave Diagnostics Ahead: Saving Time and the Lives of Trauma and Stroke Patients. IEEE Microw. Mag. 2018, 19, 78–90. Available online: https://api.semanticscholar.org/CorpusID:4612847 (accessed on 15 February 2024). [CrossRef]
- Ljungqvist, J.; Candefjord, S.; Persson, M.; Jönsson, L.; Skoglund, T.; Elam, M. Clinical Evaluation of a Microwave-Based Device for Detection of Traumatic Intracranial Hemorrhage. J. Neurotrauma 2017, 34, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsis, D.; Manioti, E.; Touris, G.; Kyriakakis, E.; Tsamopoulos, N.; Gamvroudi, M. Detecting Stroke at the Emergency Department by a Point of Care Device: A Multicenter Feasibility Study. Med. Devices Evid. Res. 2024, 17, 107–112. [Google Scholar] [CrossRef] [PubMed]
- NSW Health Report. Available online: https://www.ambulance.nsw.gov.au/news/news-items/nsw-ambulance-first-in-the-world-to-trial-new-stroke-care-technology (accessed on 25 March 2024).
- Semenov, S.; Hopfer, M.; Planas, R.; Hamidipour, A.; Henriksson, T. Electromagnetic tomography for brain imaging: 3D reconstruction of stroke in a human head phantom. In Proceedings of the 2016 IEEE Conference on Antenna Measurements and Applications (CAMA), Syracuse, NY, USA, 23–27 October 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Fedeli, A.; Schenone, V.; Randazzo, A.; Pastorino, M.; Henriksson, T.; Semenov, S. Nonlinear S-Parameters Inversion for Stroke Imaging. IEEE Trans. Microw. Theory Tech. 2021, 69, 1760–1771. [Google Scholar] [CrossRef]
- Zhu, G.; Bialkowski, A.; Crozier, S.; Guo, L.; Nguyen, P.T.; Stancombe, A.E.; Abbosh, A. Brain Injury Localization and Size Estimation Using Electromagnetic Symmetric Crossing Lines Method. IEEE Trans. Instrum. Meas. 2023, 72, 1–11. [Google Scholar] [CrossRef]
- Alqadami, A.S.M.; Zamani, A.; Trakic, A.; Abbosh, A. Flexible Electromagnetic Cap for Three-Dimensional Electromagnetic Head Imaging. IEEE Trans. Biomed. Eng. 2021, 68, 2880–2891. [Google Scholar] [CrossRef]
- WHO Fact Sheets—Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 8 May 2024).
- Shang, T.; Zhang, J.Y.; Thomas, A.; Arnold, M.A.; Vetter, B.N.; Heinemann, L.; Klonoff, D.C. Products for Monitoring Glucose Levels in the Human Body with Noninvasive Optical, Noninvasive Fluid Sampling, or Minimally Invasive Technologies. J. Diabetes Sci. Technol. 2022, 16, 168–214. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Andrea, F. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating Clinical Accuracy of Systems for Self-Monitoring of Blood Glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lübke, M. Commercial and Scientific Solutions for Blood Glucose Monitoring—A Review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Rezaei, P.; Fakhr, M. Dual-Frequency Microwave Resonant Sensor to Detect Noninvasive Glucose-Level Changes Through the Fingertip. IEEE Trans. Instrum. Meas. 2021, 70, 1–8. [Google Scholar] [CrossRef]
- Kazemi, N.; Abdolrazzaghi, M.; Light, P.E.; Musilek, P. In-human testing of a non-invasive continuous low-energy microwave glucose sensor with advanced machine learning capabilities. Biosens. Bioelectron. 2023, 241, 115668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, X.; Pang, Y.; Su, W.; Kikkawa, T. Noninvasive, Intelligent Blood Glucose Monitoring on Fingertip Using Dual-Band Fusion and LSTM-R Network. IEEE Sens. J. 2024, 24, 3465–3476. [Google Scholar] [CrossRef]
- Deshmukh, V.; Chorage, S. Non-invasive determination of blood glucose level using narrowband microwave sensor. J. Ambient. Intell. Humaniz. Comput. 2021, 1–16. [Google Scholar] [CrossRef]
- Kandwal, A.; Nie, Z.; Igbe, T.; Li, J.; Liu, Y.; Liu, L.W.; Hao, Y. Surface Plasmonic Feature Microwave Sensor with Highly Confined Fields for Aqueous-Glucose and Blood-Glucose Measurements. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Kandwal, A.; Liu, L.W.Y.; Li, J.; Liu, Y.; Tang, H.; Ju, Z.; Igbe, T.; Jasrotia, R.; Nie, Z. Designing Highly Sensitive Microwave Antenna Sensor with Novel Model for Noninvasive Glucose Measurements. Prog. Electromagn. Res. 2023, 176, 129–141. [Google Scholar] [CrossRef]
- Kandwal, A.; Liu, L.W.; Deen, M.J.; Jasrotia, R.; Kanaujia, B.K.; Nie, Z. Electromagnetic Wave Sensors for Noninvasive Blood Glucose Monitoring: Review and Recent Developments. IEEE Trans. Instrum. Meas. 2023, 72, 1–15. [Google Scholar] [CrossRef]
- Gelosi, I.E.; Avalos Ribas, R.; Uriz, A.J.; Castiñeira Moreira, J.; Fuentes, N. Design and Implementation of a Non-Invasive RF Device to Measure Glucose in Blood. IEEE Lat. Am. Trans. 2022, 20, 813–822. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R. Low-cost Portable Microwave Sensor for Non-Invasive Monitoring of Blood Glucose Level: Novel Design Utilizing a Four-Cell CSRR Hexagonal Configuration. Nature 2020, 10, 15200. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, S.J.J.; Bennet, M.A.; Shanker, N.R. Antiallergic Abdominal Belt for Human Glucose Level Measurement Using Microwave Active Sensor Antenna. IEEE Sens. J. 2023, 23, 16227–16237. [Google Scholar] [CrossRef]
- Liu, L.W.Y.; Kandwal, A.; Kogut, A.; Eremenko, Z.E.; Kogut, E.; Islam, M.T.; Dolia, R.; Nosatiuk, S.; Nguyen, S.T. In-Vivo and Ex-Vivo Measurements of Blood Glucose Using Whispering Gallery Modes. Sensors 2020, 20, 830. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.E.; Hojjati-Firoozabadi, A.; Gigoyan, S.; Safavi-Naeini, S.; Shaker, G. Non-Reciprocal Whispering-Gallery-Mode Resonator for Sensitive Blood Glucose Monitoring. IEEE Trans. Instrum. Meas. 2022, 71, 1–12. [Google Scholar] [CrossRef]
- Afontechnology. Available online: https://afontechnology.com (accessed on 8 May 2024).
- Know Labs. Available online: https://www.knowlabs.co (accessed on 8 May 2024).
- Cataldo, A.; Cino, L.; Distante, C.; Maietta, G.; Masciullo, A.; Mazzeo, P.L.; Schiavoni, R. Integrating microwave reflectometry and deep learning imaging for in-vivo skin cancer diagnostics. Measurement 2024, 235, 114911. [Google Scholar] [CrossRef]
- Alamro, W.; Seet, B.C.; Wang, L.; Parthiban, P. Experimental Detection of Early-Stage Lung and Skin Tumors Based on Super Wideband Imaging. IEEE J. Electromagn. Microwaves Med. Biol. 2024, 8, 182–189. [Google Scholar] [CrossRef]
- Guardiola, M.; Dghoughi, W.; Sont, R.; Garrido, A.; Marcoval, S.; Neira, L.M.; Belda, I.; Fernández-Esparrach, G. MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models. Sensors 2022, 22, 4902. [Google Scholar] [CrossRef]
- Rezaeieh, S.A.; Abbosh, A. Review of systems for the detection and monitoring of accumulated fluids in the human torso. In Proceedings of the 2015 International Symposium on Antennas and Propagation (ISAP), Hobart, Australia, 9–12 November 2015; pp. 1–4. Available online: https://ieeexplore.ieee.org/document/7447329 (accessed on 15 February 2024).
- Wang, M.; Scapaticci, R.; Cavagnaro, M.; Crocco, L. Towards a Microwave Imaging System for Continuous Monitoring of Liver Tumor Ablation: Design and In Silico Validation of an Experimental Setup. Diagnostics 2021, 11, 866. [Google Scholar] [CrossRef]
- Prokhorova, A.; Ley, S.; Helbig, M. Quantitative Interpretation of UWB Radar Images for Non-Invasive Tissue Temperature Estimation during Hyperthermia. Diagnostics 2021, 11, 818. [Google Scholar] [CrossRef]
- González-López, G.; Jofre Roca, L.; Amorós García de Valdecasas, S.; Rodríguez-Leor, O.; Gálvez-Montón, C.; Bayés-Genís, A.; O’Callaghan, J. Resonance-Based Microwave Technique for Body Implant Sensing. Sensors 2019, 19, 4828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18, 153473541987634. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, R.; ter Haar, G.; Nightingale, K.; Marks, L.; Natarajan, S. Methods of monitoring thermal ablation of soft tissue tumors—A comprehensive review. Med. Phys. 2022, 49, 769–791. [Google Scholar] [CrossRef]
- Ley, S.; Schilling, S.; Fiser, O.; Vrba, J.; Sachs, J.; Helbig, M. Ultra-Wideband Temperature Dependent Dielectric Spectroscopy of Porcine Tissue and Blood in the Microwave Frequency Range. Sensors 2019, 19, 1707. [Google Scholar] [CrossRef]
- Scapaticci, R.; Bellizzi, G.G.; Cavagnaro, M.; Lopresto, V.; Crocco, L. Exploiting Microwave Imaging Methods for Real-Time Monitoring of Thermal Ablation. Int. J. Antennas Propag. 2017, 2017, 5231065. [Google Scholar] [CrossRef]
- Scapaticci, R.; Lopresto, V.; Pinto, R.; Cavagnaro, M.; Crocco, L. Monitoring Thermal Ablation via Microwave Tomography: An Ex Vivo Experimental Assessment. Diagnostics 2018, 8, 81. [Google Scholar] [CrossRef]
- Wang, M.; Crocco, L.; Costanzo, S.; Scapaticci, R.; Cavagnaro, M. A Compact Slot-Loaded Antipodal Vivaldi Antenna for a Microwave Imaging System to Monitor Liver Microwave Thermal Ablation. IEEE Open J. Antennas Propag. 2022, 3, 700–708. [Google Scholar] [CrossRef]
- Zeinali, N.; Sebek, J.; Fallahi, H.; Pfannenstiel, A.; Prakash, P. Transmission Coefficient-Based Monitoring of Microwave Ablation: Development and Experimental Evaluation in Ex Vivo Tissue. IEEE Trans. Biomed. Eng. 2024, 71, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.H.; Moosazadeh, M.; Guo, L.; Abbosh, A.M. Compact Dual-Polarized Cavity-Backed Antenna with Wideband Performance for Deep Torso Imaging. IEEE Trans. Antennas Propag. 2024, 72, 2217–2227. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Li, M.; Yang, F.; Xu, S.; Cao, Y.; Yu, Z.; Abubakar, A. Study on Microwave Thorax Imaging for Human Respiration Monitoring. IEEE J. Electromagn. RF Microwaves Med. Biol. 2024, 8, 190–197. [Google Scholar] [CrossRef]
- Dagheyan, A.G.; Molaei, A.; Obermeier, R.; Martinez, A.K.; Lorenzo, J.M. Near-Field Radar Microwave Imaging as an Add-on Modality to Mammography. In Breast Imaging; Malik, A.M., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 2. [Google Scholar] [CrossRef]
- Khoshdel, V.; Ashraf, A.; LoVetri, J. Enhancement of Multimodal Microwave-Ultrasound Breast Imaging Using a Deep-Learning Technique. Sensors 2019, 19, 4050. [Google Scholar] [CrossRef]
- Meaney, P.M.; Raynolds, T.; Geimer, S.D.; Ouma, D.; Player, G.M.; Yang, X.; Paulsen, K.D. Motion-Based Microwave Tomographic Measurement Device for 3D Coverage in an MR System. Med. Phys. 2022, 49, 7638–7647. [Google Scholar] [CrossRef]
- Bolomey, J.C.; Pichot, C.; Jofre-Roca, L. Microwaves for medical diagnostic radiology: From proofs-of-concept to real world needs. In Proceedings of the 2023 IEEE Conference on Antenna Measurements and Applications (CAMA), Genoa, Italy, 15–17 November 2023; pp. 107–112. [Google Scholar] [CrossRef]

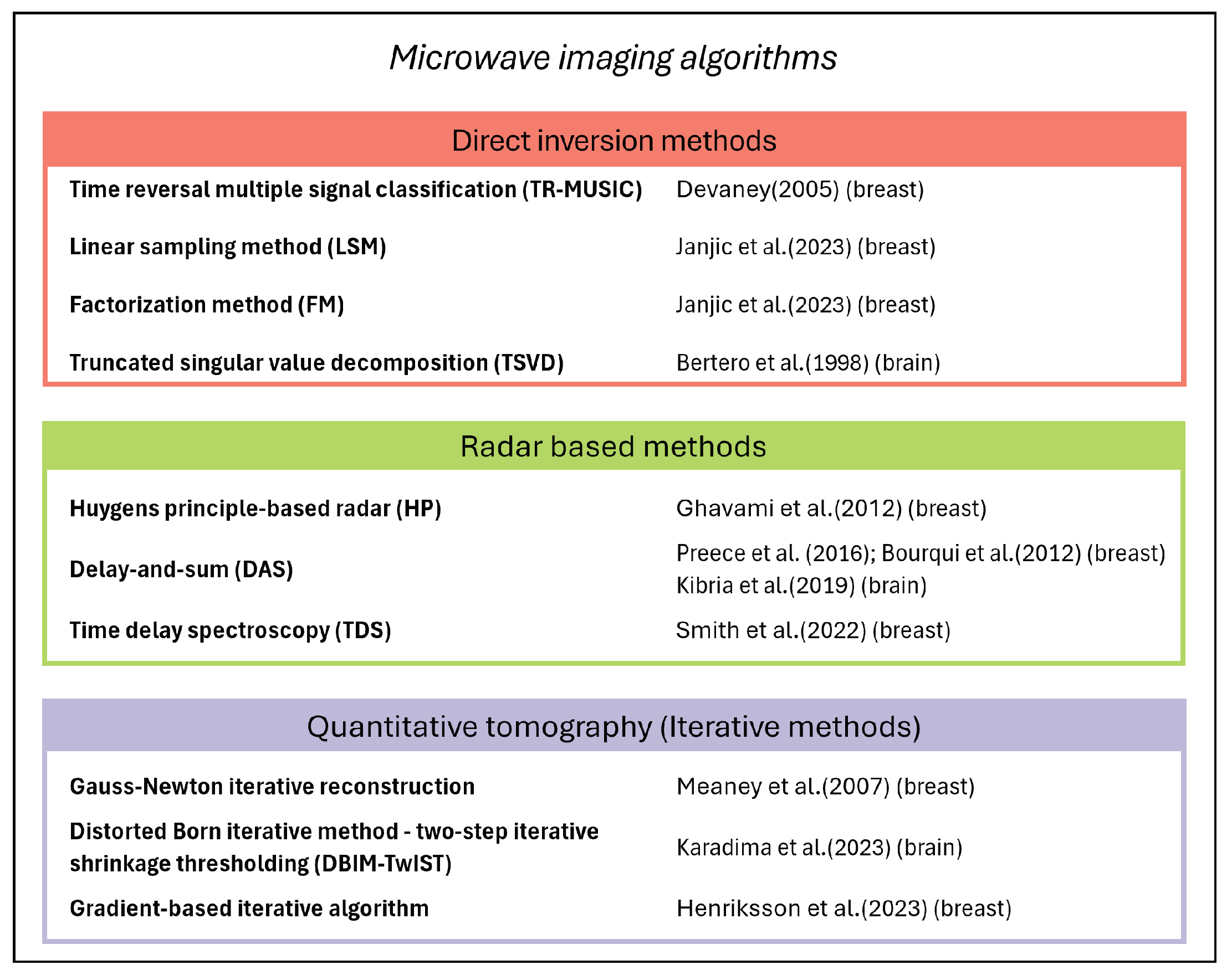
| D-C [73,74,75]  | MammoWave [76,77,78]  | TSAR [27,79]  | MITS [29,69]  | MARIA [26,80,81]  | Wavelia [70,82]  | SAFE [23,71,83]  | |
|---|---|---|---|---|---|---|---|
| Array type | synthetic | synthetic | synthetic | hardware | hardware | synthetic | synthetic |
| Geometry | cylindrical | cylindrical | conformal | planar | hemispherical | cylindrical | cylindrical |
| Antenna | monopole | horn | Vivaldi | Vivaldi | slot | Vivaldi | Vivaldi |
| No. of antennas | 16 | 2 | 1 | 10 | 60 | 21 | 2 |
| Frequency (GHz) | 0.7–1.7 | 1–9 | 2.4–15 | 0.1–10 | 3–10 | 0.8–4 | 1–8 |
| Coupling medium | liquid | no | liquid | no | shell + liquid | creamy liquid | shell |
| Algorithm | tomography | HP | DAS | TDS | DAS | TR-MUSIC | LSM + FM |
| Scan-time (min) | 2 | 7 | 30 | 0.25 | 0.17 | 15 | 7 |
| Largest trial | 400 | 103 | 8 | 15 | 389 | 24 | 115 |
| Sensitivity (%) | - | 74 | - | - | 47 | 87 | 63 |
| Strokefinder MD100 [73,74,75]  | EMTensor [32,112,113,114]  | EMVision [115]  | |
|---|---|---|---|
| Geometry | conformal | hemispherical | ring |
| Antenna | patch | waveguide | waveguide |
| No. of antennas | 8 | 128 | 16 |
| Frequency (GHz) | 0.1–1.95 | 0.92–1.08 | 0.7–1.8 |
| Coupling medium | no | gel | liquid |
| Classification (C)/Imaging (I) | C | I | C/I |
| Algorithm | ML (CLAFIC) | gradient-based iterative algorithm | custom data-driven algorithm |
| Scan-time (s) | 45 | 2.5 | - |
| Largest Trial | 71 | 52 | 50 |
| Accuracy (%) | - | - | 98 (C) |
| Ref. | Sensor Type | Frequency (GHz) | Sensed Variables | Detection Range (mg/dL) | Estimation Method | Trial Population | Sensitivity | MARD |
|---|---|---|---|---|---|---|---|---|
| [131] | two cross- shaped resonator | 5.5, 8.5 | S21 freq. shift | 89–262 | linear interpolation | 11 ND | 3.53, 3.58 MHz/(mg/dL) | 3% (N = 6) |
| [134] | microstrip antenna | 1.3 | S11 freq. shift | 60–400 | linear regression | 75 ND, 50 PD, 125 D | 11.4 MHz/(mg/dL) | 4.20% (N = 125) |
| [135] | SSPP endfire antenna | 8–12 | S11 freq. shift | 75–150 | not applied | 5 ND | 3.3 MHz/(mg/dL) | - |
| [136] | CMS | 4.5–5 | S11 amplitude + freq. shift | 50–280 | not applied | 5 ND | 1.49 MHz/(mg/dL) 0.073 dB/(mg/dL) | - |
| [138] | not available | 1.8–2.2 | S11 freq. shift | 100–250 | 3rd order regression | 43 ND, 1 D | not available | 7.8% (N = 107) |
| [140] | HSIS antenna | 4.2 | S11 amplitude | 149–290 | linear regression | 30 D | 0.056 (before) 0.027 (after) * db/(mg/dL) | 7.34% (N = 410) |
| [133] | two monopole slot antennas | 3.67, 8.35 | S11, S21 amplitude | 20–500 | feature extr. + non-linear regression | 10 ND | 0.0072 dB/(mg/dL) | 5.9% (N = 750) |
| [132] | patch + split ring resonator | 3.6 | S11 amplitude | 0–500 | linear regression | 2 ND, 2 D | 0.02 db/(mg/dL) | 3.62–6.21% (N = 3000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Origlia, C.; Rodriguez-Duarte, D.O.; Tobon Vasquez, J.A.; Bolomey, J.-C.; Vipiana, F. Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications. Sensors 2024, 24, 4515. https://doi.org/10.3390/s24144515
Origlia C, Rodriguez-Duarte DO, Tobon Vasquez JA, Bolomey J-C, Vipiana F. Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications. Sensors. 2024; 24(14):4515. https://doi.org/10.3390/s24144515
Chicago/Turabian StyleOriglia, Cristina, David O. Rodriguez-Duarte, Jorge A. Tobon Vasquez, Jean-Charles Bolomey, and Francesca Vipiana. 2024. "Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications" Sensors 24, no. 14: 4515. https://doi.org/10.3390/s24144515
APA StyleOriglia, C., Rodriguez-Duarte, D. O., Tobon Vasquez, J. A., Bolomey, J.-C., & Vipiana, F. (2024). Review of Microwave Near-Field Sensing and Imaging Devices in Medical Applications. Sensors, 24(14), 4515. https://doi.org/10.3390/s24144515






