Realistic 3D Phantoms for Validation of Microwave Sensing in Health Monitoring Applications
Abstract
:1. Introduction
1.1. Microwave Sensing and Its Advantages
1.2. State-of-the-Art Tissue-Mimicking Phantom Development for Micalrowaves
1.2.1. Brain Phantoms
1.2.2. Breast Phantoms
1.3. Objectives and Novelty of This Study
2. Materials and Methods
2.1. Materials Used in Phantom Development
2.2. Procedure to Prepare Phantoms of Different Tissues
2.2.1. Brain, Skin, Tumor and Glandular Phantoms
2.2.2. Muscle and Intestinal Phantoms
2.2.3. Fat Phantoms
2.2.4. Measurement of Dielectric Properties of Phantoms
2.2.5. Verification of Phantoms with EM-Simulations
2.3. Phantom Molds for Realistic 3D Emulation Platforms
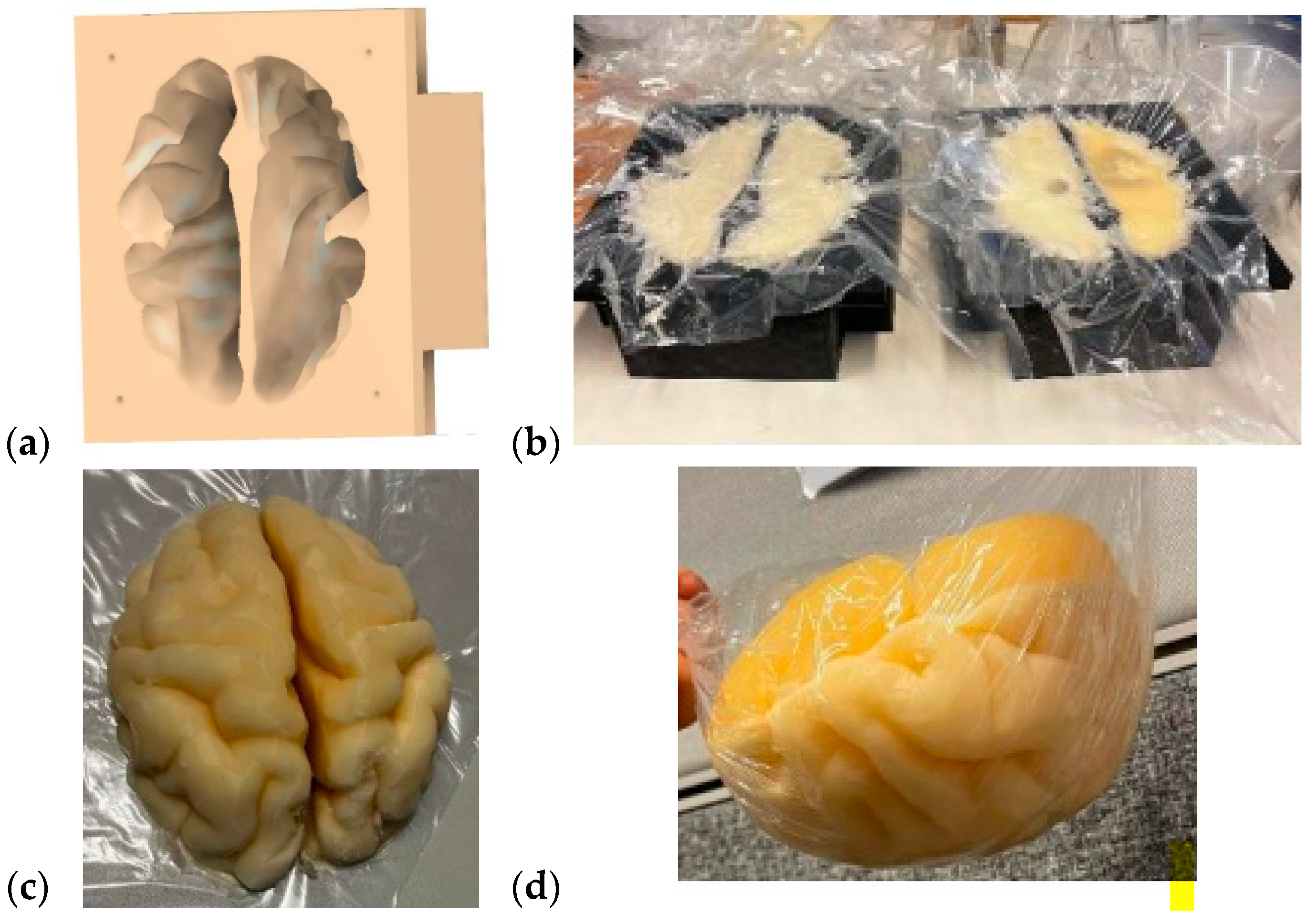
2.3.1. Brain Mold
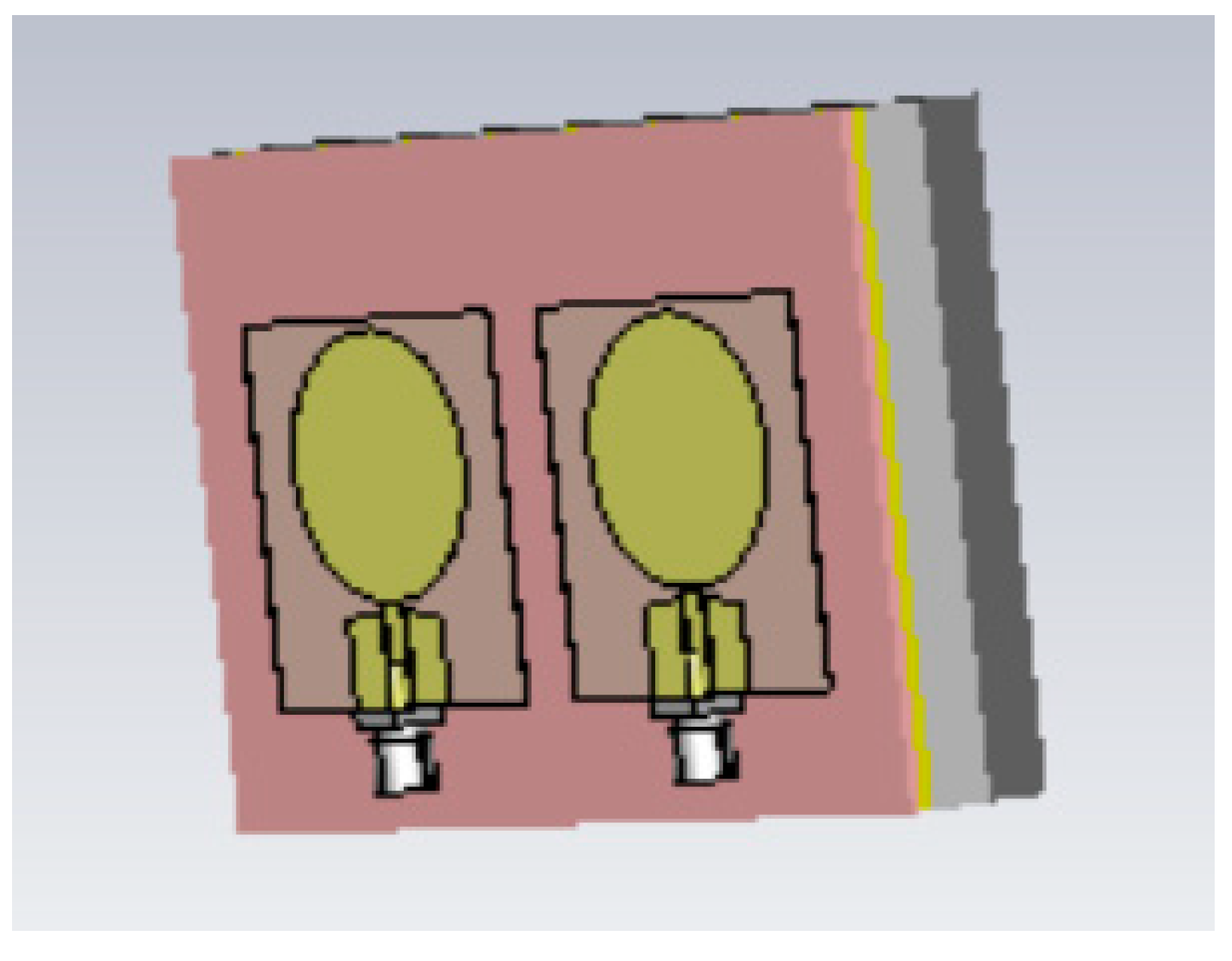
2.3.2. Breast Phantoms for Breast-Tumor-Detection Studies
2.3.3. Abdominal Molds
3. Results
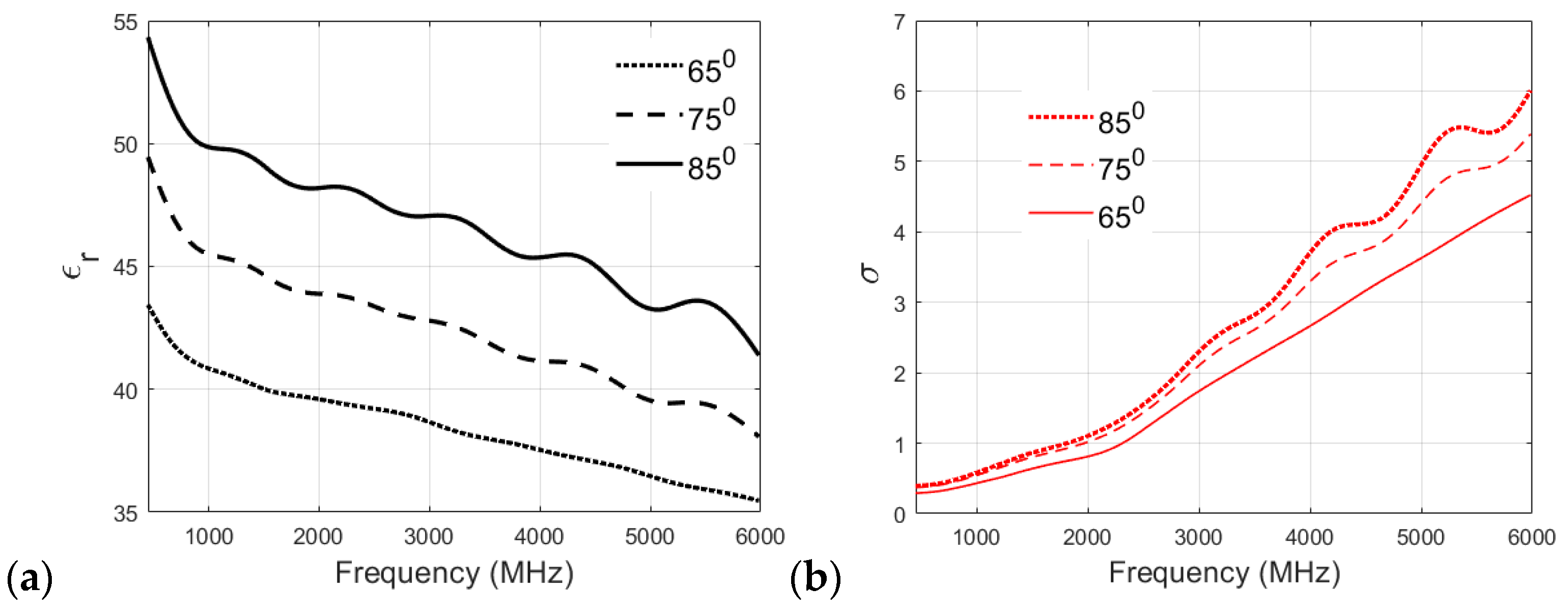
3.1. Effect of Cooking Temperature on the Dielectric Properties of Phantom Recipies
3.2. Evaluation of Dielectric Properties of Phantoms
3.2.1. Measurement Analysis and Summary for Skin and Tumor Phantoms
3.2.2. Glandular Phantom Longevity
3.2.3. Muscle Longevity
3.2.4. Fat Phantom
3.3. Verification of the Final Phantom Recipes with EM Simulations
3.3.1. Verification of the Skin Phantom
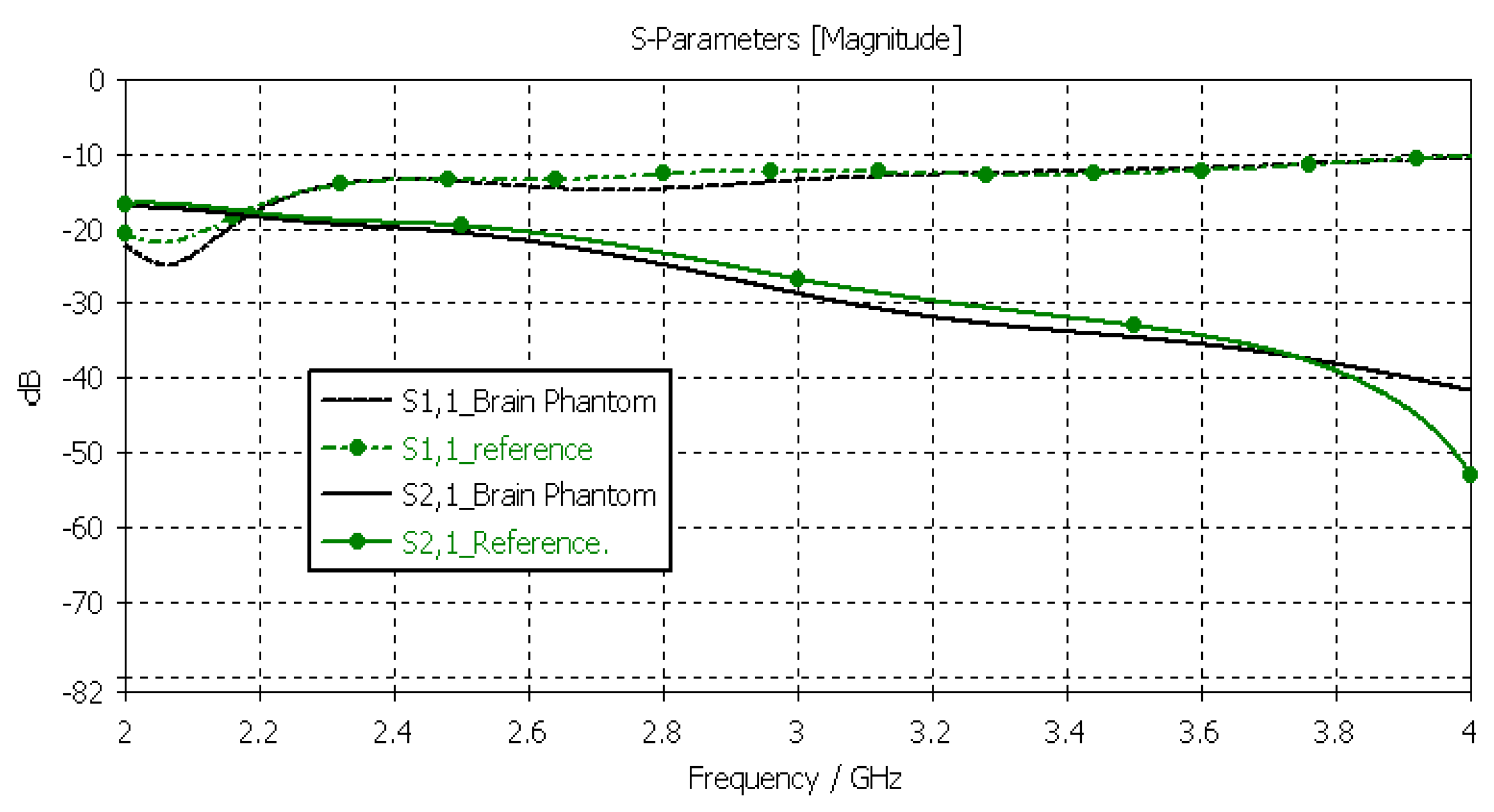
3.3.2. Verification of the Brain Phantom
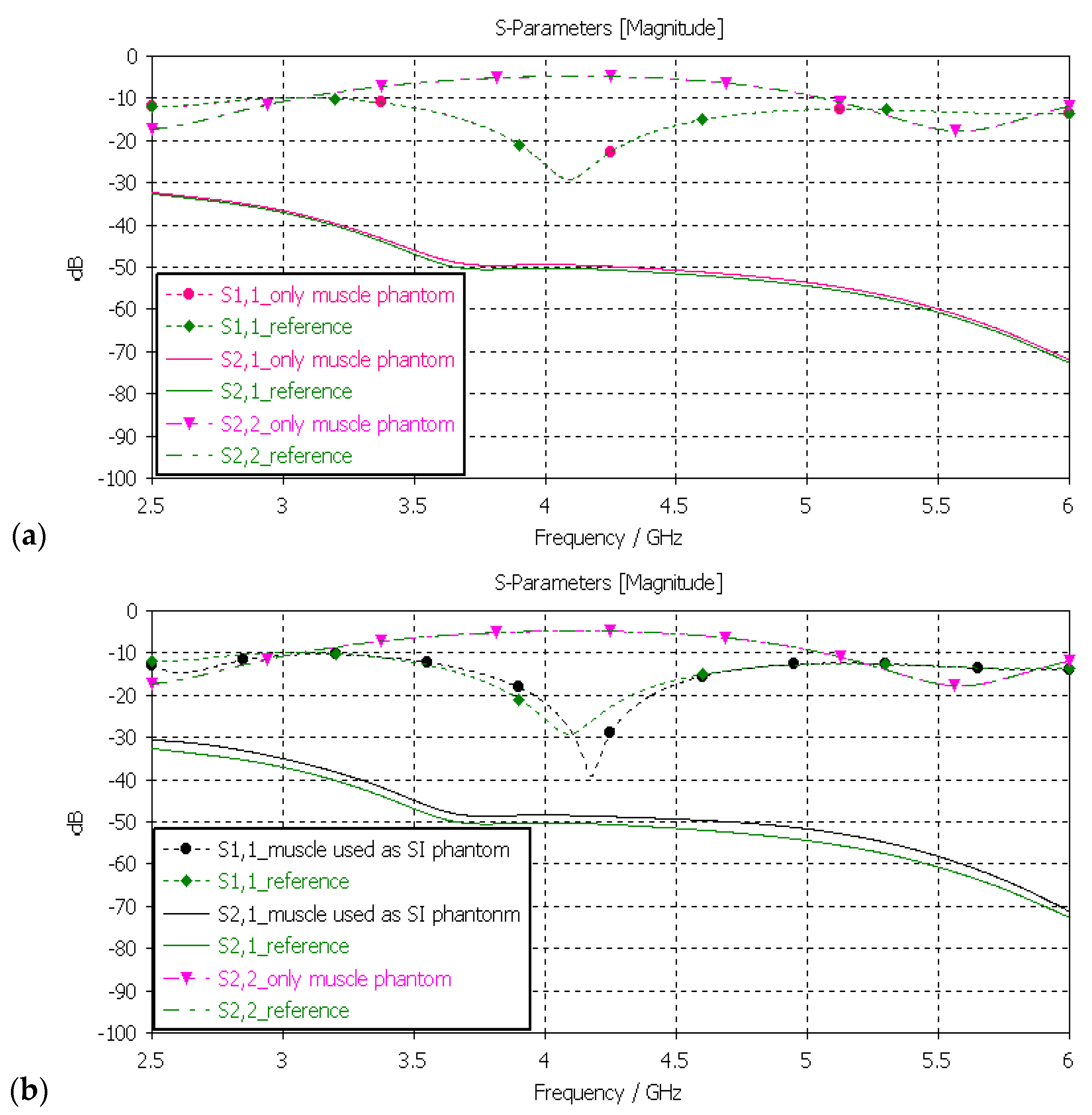
3.3.3. Verification of Muscle and Intestinal Phantoms
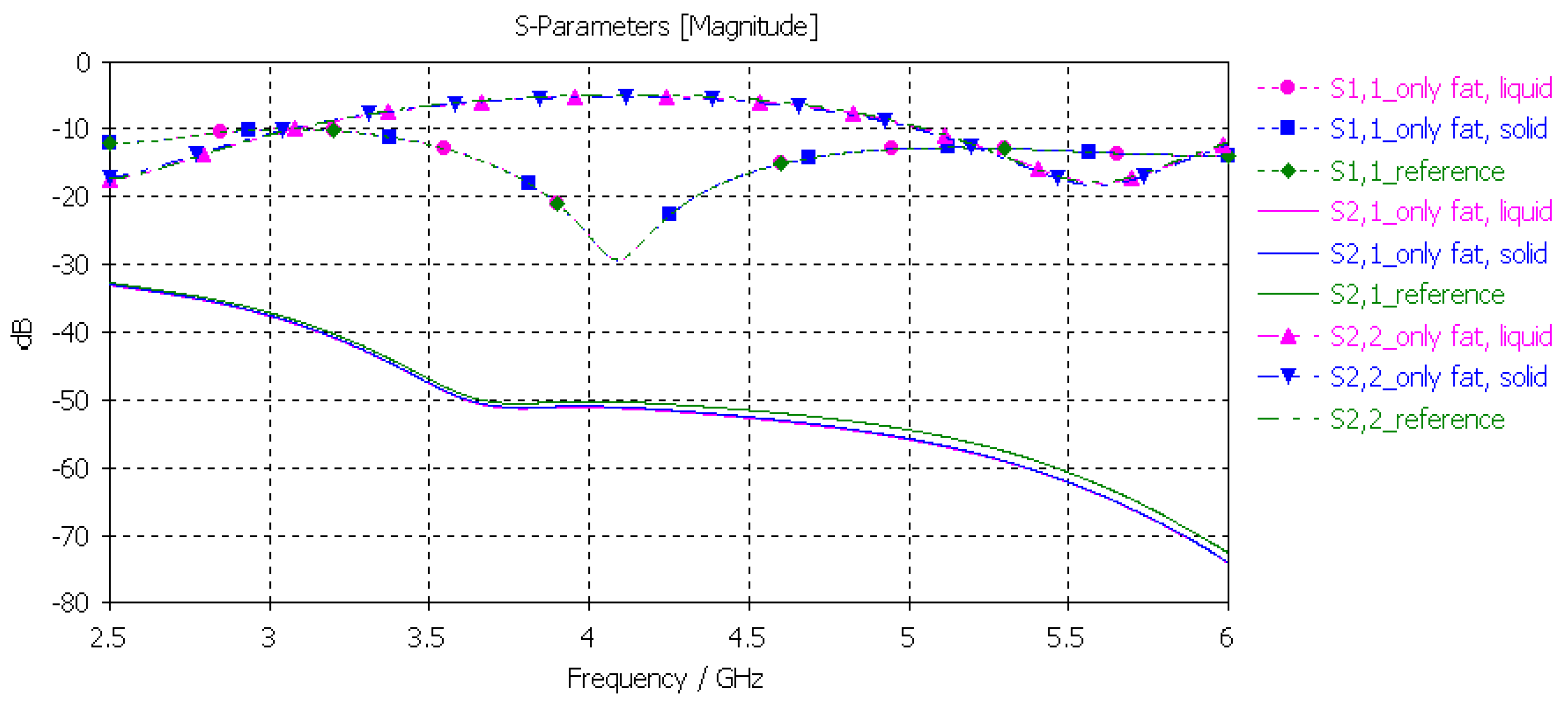
3.3.4. Verification of the Fat Phantom
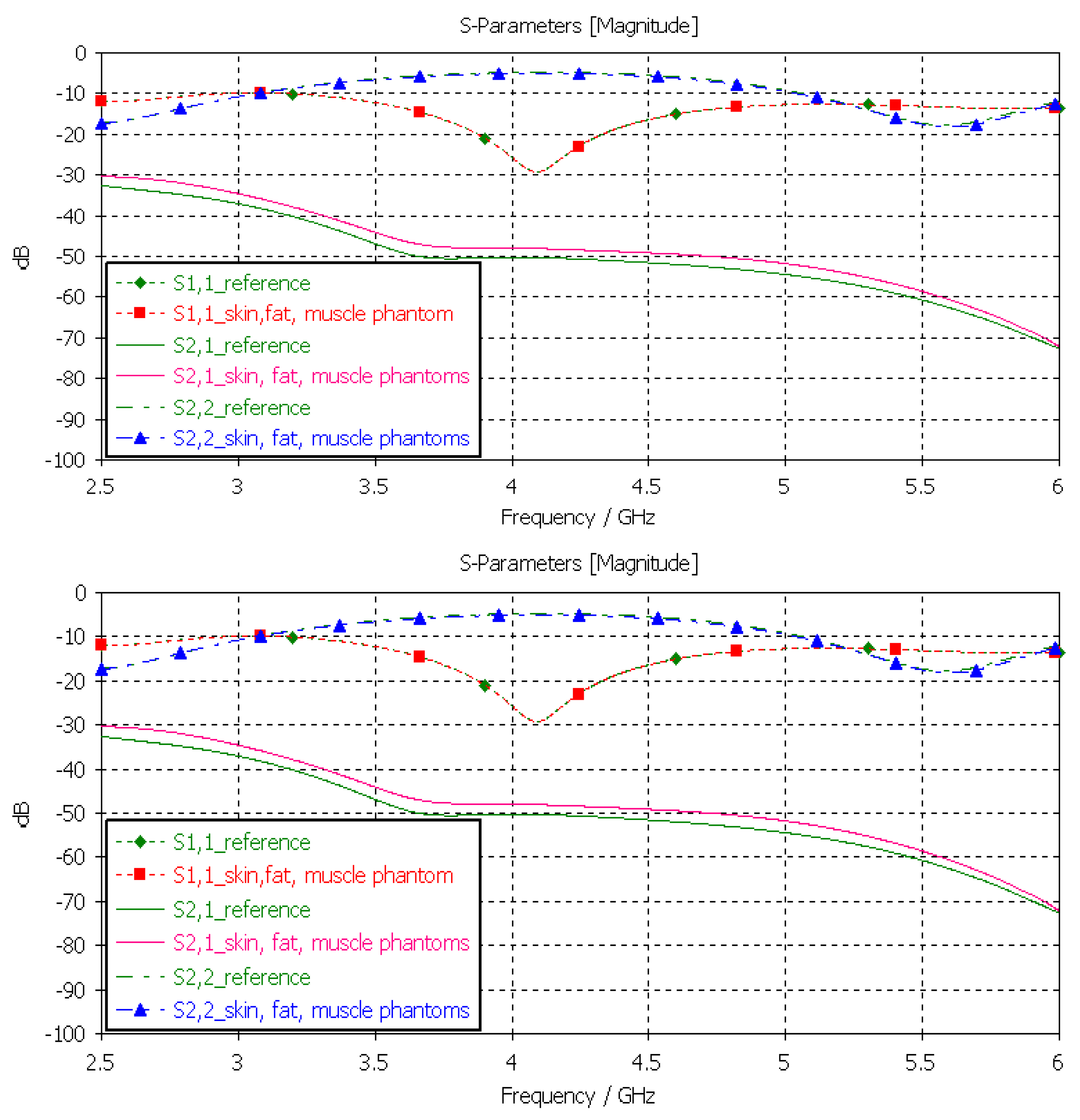
3.3.5. Verification of the Full Abdominal Phantom Layer Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neira, L.M.; Mays, R.O.; Hagness, S.C. Human Breast Phantoms: Test Beds for the Development of Microwave Diagnostic and Therapeutic Technologies. IEEE Pulse 2017, 8, 66–70. [Google Scholar] [CrossRef]
- Costanzo, S.; Cioffi, V.; Qureshi, A.M.; Borgia, A. Gel-Like Human Mimicking Phantoms: Realization Procedure, Dielectric Characterization and Experimental Validations on Microwave Wearable Body Sensors. Biosensors 2021, 11, 111. [Google Scholar] [CrossRef]
- Garrett, J.; Fear, E. Stable and Flexible Materials to Mimic the Dielectric Properties of Human Soft Tissues. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 599–602. [Google Scholar] [CrossRef]
- Castello-Palacios, S.; Garcia-Pardo, C.; Fornes-Leal, A.; Cardona, N.; Valles-Lluch, A. Tailor-Made Tissue Phantoms Based on Acetonitrile Solutions for Microwave Applications up to 18 GHz. IEEE Trans. Microw. Theory Tech. 2016, 64, 3987–3994. [Google Scholar] [CrossRef]
- Pollacco, D.A.; Conti, M.C.; Farrugia, L.; Wismayer, P.S.; Farina, L.; Sammut, C.V. Dielectric properties of muscle and adipose tissue-mimicking solutions for microwave medical imaging applications. Phys. Med. Biol. 2019, 64, 095009. [Google Scholar] [CrossRef]
- Di Meo, S.; Pasotti, L.; Iliopoulos, I.; Pasian, M.; Ettorre, M.; Zhadobov, M.; Matrone, G. Tissue-mimicking materials for breast phantoms up to 50 GHz. Phys. Med. Biol. 2019, 64, 055006. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Madsen, E.L.; Frank, G.R.; Hagness, S.C. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys. Med. Biol. 2005, 50, 4245–4258. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Fakhoury, J.; Oprisor, R.; Coates, M.; Popović, M. Improved tissue phantoms for experimental validation of microwave breast cancer detection. In Proceedings of the Fourth European Conference on Antennas and Propagation, Barcelona, Spain, 12–16 April 2010; pp. 1–5. [Google Scholar]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M. Dielectric Properties Characterization From 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Di Meo, S.; Iliopoulos, I.; Pasian, M.; Ettorre, M.; Pasotti, L.; Zhadobov, M.; Matrone, G. Tissue mimicking materials for breast phantoms using waste oil hardeners. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; pp. 1–4. [Google Scholar]
- Khaleghi, A.; Hasanvand, A.; Balasingham, I. Radio Frequency Backscatter Communication for High Data Rate Deep Implants. IEEE Trans. Microw. Theory Tech. 2018, 67, 1093–1106. [Google Scholar] [CrossRef]
- Anzai, D.; Katsu, K.; Chavez-Santiago, R.; Wang, Q.; Plettemeier, D.; Wang, J.; Balasingham, I. Experimental Evaluation of Implant UWB-IR Transmission with Living Animal for Body Area Networks. IEEE Trans. Microw. Theory Tech. 2013, 62, 183–192. [Google Scholar] [CrossRef]
- Bose, P.; Khaleghi, A.; Mahmood, S.; Albatat, M.; Bergsland, J.; Balasingham, I. Evaluation of Data Telemetry for Future Leadless Cardiac Pacemaker. IEEE Access 2019, 7, 157933–157945. [Google Scholar] [CrossRef]
- Särestöniemi, M.; Pomalaza-Raez, C.; Kissi, C.; Iinatti, J. On the UWB in-body propagation measurements using pork meat. In Proceedings of the International Conference of BodyNets2020, Tallinn, Estonia, 21 October 2020. [Google Scholar]
- Särestöniemi, M.; Myllymäki, S.; Reponen, J.; Myllylä, T. Remote diagnostics and monitoring using microwave technique—Improving healthcare in rural areas and in exceptional situations. Finn. J. eHealth eWelfare 2023, 15, 6–22. [Google Scholar] [CrossRef]
- Chandra, R.; Zhou, H.; Balasingham, I.; Narayanan, R.M. On the Opportunities and Challenges in Microwave Medical Sensing and Imaging. IEEE Trans. Biomed. Eng. 2015, 62, 1667–1682. [Google Scholar] [CrossRef]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of Microwaves Techniques for Breast Cancer Detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Manjula, J. Non-invasive microwave head imaging to detect tumors and to estimate their size and location. Phys. Med. 2022, 13, 100047. [Google Scholar] [CrossRef]
- Costanzo, A.; Augello, E.; Battistini, G.; Benassi, F.; Masotti, D.; Paolini, G. Microwave Devices for Wearable Sensors and IoT. Sensors 2023, 23, 4356. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Duarte, D.O.; Origlia, C.; Vasquez, J.A.T.; Scapaticci, R.; Crocco, L.; Vipiana, F. Experimental Assessment of Real-Time Brain Stroke Monitoring via a Microwave Imaging Scanner. IEEE Open J. Antennas Propag. 2022, 3, 824–835. [Google Scholar] [CrossRef]
- Hossain, A.; Islam, M.T.; Hoque, A.; Rahim, S.K.A.; Alshammari, A.S.; Chowdhury, M.E.; Soliman, M.S. Sensor-based microwave brain imaging system (SMBIS): An experimental six-layered tissue based human head phantom model for brain tumor diagnosis using electromagnetic signals. Eng. Sci. Technol. Int. J. 2023, 45, 101491. [Google Scholar] [CrossRef]
- Islam, M.S.; Hoque, A.; Islam, T.; Amin, N.; Chowdhury, M.E.H. A Portable Electromagnetic Head Imaging System Using Metamaterial Loaded Compact Directional 3D Antenna. IEEE Access 2021, 9, 50893–50906. [Google Scholar] [CrossRef]
- Karadima, O.; Rahman, M.; Sotiriou, I.; Ghavami, N.; Lu, P.; Ahsan, S.; Kosmas, P. Experimental validation of microwave tomography with the DBIM-TwIST algorithm for brain stroke detection and classification. Sensors 2020, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.J.; Abbosh, M. Realistic head phantom to test microwave systems for brain imaging. Microw. Opt. Technol. Lett. 2014, 56, 979–982. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Abbosh, A.M.; Mustafa, S.; Ireland, D. Microwave system for head imaging. IEEE Trans. Instrum. Meas. 2013, 63, 117–123. [Google Scholar] [CrossRef]
- Najafi, M.; Teimouri, J.; Shirazi, A.; Geraily, G.; Esfahani, M.; Shafaei, M. Technical Note: Construction of heterogeneous head phantom for quality control in stereotactic radiosurgery. Med. Phys. 2017, 44, 5070–5074. [Google Scholar] [CrossRef]
- Joachimowicz, N.; Duchêne, B.; Conessa, C.; Meyer, O. Anthropomorphic breast and head phantoms for microwave imaging. Diagnostics 2018, 8, 85. [Google Scholar] [CrossRef]
- Pokorny, T.; Vrba, D.; Tesarik, J.; Rodrigues, D.B.; Vrba, J. Anatomically and dielectrically realistic 2.5 D 5-layer reconfigurable head phantom for testing microwave stroke detection and classification. Int. J. Antennas Propag. 2019, 2019, 5459391. [Google Scholar] [CrossRef]
- Mobashsher, A.T.; Abbosh, A.M. Three-dimensional human head phantom with realistic electrical properties and anatomy. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 1401–1404. [Google Scholar] [CrossRef]
- Mustafa, S.; Mohammed, B.; Abbosh, A. Novel preprocessing techniques for accurate microwave imaging of human brain. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 460–463. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Jofre, L.; Hawley, M.S.; Broquetas, A.; de Los Reyes, E.; Ferrando, M.; Elias-Fuste, A.R. Medical imaging with a microwave tomographic scanner. IEEE Trans. Biomed. Eng. 1990, 37, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, K.T.; Gouzouasis, I.A.; Karanasiou, I.S.; Stratakos, G.; Uzunoglu, N.K. Passive focused monitoring and non-invasive irradiation of head tissue phantoms at microwave frequencies. In Proceedings of the 2008 8th IEEE International Conference on Bioinformatics and BioEngineering (BIBE), Athens, Greece, 8–10 October 2008; pp. 1–6. [Google Scholar]
- Looi, C.K.; Chen, Z.N. Design of a human-head-equivalent phantom for ISM 2.4-GHz applications. Microw. Opt. Technol. Lett. 2005, 47, 163–166. [Google Scholar] [CrossRef]
- Mohammed, B.; Abbosh, A.; Henin, B.; Sharpe, P. Head phantom for testing microwave systems for head imaging. In Proceedings of the 2012 Cairo International Biomedical Engineering Conference (CIBEC), Giza, Egypt, 20–22 December 2012; pp. 191–193. [Google Scholar]
- Islam, M.S.; Almutairi, A.F. A portable non-invasive microwave based head imaging system using compact metamaterial loaded 3D unidirectional antenna for stroke detection. Sci. Rep. 2022, 12, 8895. [Google Scholar] [CrossRef] [PubMed]
- Otterskog, M.; Petrovic, N.; Risman, P.O. A multi-layered head phantom for microwave investigations of brain hemorrhages. In Proceedings of the 2016 IEEE Conference on Antenna Measurements & Applications (CAMA), Syracuse, NY, USA, 23–27 October 2016; pp. 1–3. [Google Scholar]
- Islam, M.S.; Almutairi, A.F. Experimental tissue mimicking human head phantom for estimation of stroke using IC-CF-DMAS algorithm in microwave based imaging system. Sci. Rep. 2021, 11, 22015. [Google Scholar] [CrossRef] [PubMed]
- Velander, J.; Redzwan, S.; Perez, M.D.; Asan, N.B.; Nowinski, D.; Lewen, A.; Enblad, P.; Augustine, R. A four-layer phantom for testing in-vitro microwave-based sensing approach in intra-cranial pressure monitoring. In Proceedings of the 2018 IEEE International Microwave Biomedical Conference (IMBioC), Philadelphia, PA, USA, 14–15 June 2018; pp. 49–51. [Google Scholar]
- Joachimowicz, N.; Vasquez, J.T.; Turvani, G.; Dassano, G.; Casu, M.R.; Vipiana, F.; Duchene, B.; Scapaticci, R.; Crocco, L. Head phantoms for a microwave imaging system dedicated to cerebrovascular disease monitoring. In Proceedings of the 2018 IEEE Conference on Antenna Measurements & Applications (CAMA), Västerås, Sweden, 3–6 September 2018; pp. 1–3. [Google Scholar]
- Joachimowicz, N.; Conessa, C.; Henriksson, T.; Duchêne, B. Breast phantoms for microwave imaging. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 1333–1336. [Google Scholar] [CrossRef]
- Mashal, A.; Gao, F.; Hagness, S.C. Heterogeneous anthropomorphic phantoms with realistic die-lectric properties for microwave breast imaging experiments. Microw. Opt. Technol. Lett. 2011, 53, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Samsuzzaman; Kibria, S.; Islam, M.T. Experimental breast phantoms for estimation of breast tumor using microwave imaging systems. IEEE Access 2018, 6, 78587–78597. [Google Scholar] [CrossRef]
- Vigneras, V.; Bonnaudin, F. Biological tissues equivalent liquids in the frequency range 900–3000 MHz. In Proceedings of the XXVIIIth URSI General Assembly, New Delhi, India, 23–29 October 2005; pp. 1–4. [Google Scholar]
- Gunnarsson, T.; Joachimowicz, N.; Joisel, A.; Conessa, C.; Diet, A.; Bolomey, J.C. Quantitative microwave breast phantom imaging using a planar 2.45 GHz system. In Proceedings of the XXIXth URSI General Assembly, Chicago, IL, USA, 7–16 August 2008. [Google Scholar]
- Henriksson, T.; Joachimowicz, N.; Conessa, C.; Bolomey, J.-C. Quantitative microwave imaging for breast cancer detection using a planar 2.45 GHz system. IEEE Trans. Instrum. Meas. 2010, 59, 2691–2699. [Google Scholar] [CrossRef]
- Henriksson, T. Contribution to Quantitative Microwave Imaging Techniques for Biomedical Applications. Ph.D. Thesis, Mälardalen University, Västerås, Sweden, 2009. [Google Scholar]
- Romeo, S.; Di Donato, L.; Bucci, O.M.; Catapano, I.; Crocco, L.; Scarfì, M.R.; Massa, R. Dielectric characterization study of liquid-based materials for mimicking breast tissues. Microw. Opt. Technol. Lett. 2011, 53, 1276–1280. [Google Scholar] [CrossRef]
- Neira, L.M.; Mays, R.O.; Hagness, S.C. Development and application of human breast phantoms in microwave diagnostic and therapeutic technologies. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6018–6021. [Google Scholar]
- Garrett, J.; Fear, E. A New breast phantom with a durable skin layer for microwave breast imaging. IEEE Trans. Antennas Propag. 2015, 63, 1693–1700. [Google Scholar] [CrossRef]
- Särestöniemi, M.; Dessai, R.; Myllymäki, S.; Myllylä, T. A Novel Durable Fat Tissue Phantom for Microwave Based Medical Monitoring Applications. In Proceedings of the EAI BICT 2023—14th EAI International Conference on Bio-Inspired Information and Communications Technologies, Okinawa, Japan, 11–12 April 2023. [Google Scholar]
- Dessai, R. Evaluating a Breast Tumor Monitoring Vest with Flexible UWB Antennas and Realistic Phantoms—A Proof-of -Concept Study. Master’s Thesis, University of Oulu, Oulu, Finland, 2022. [Google Scholar]
- 2022. Available online: https://www.itis.ethz.ch/virtual-population/tissue-properties/databaseM (accessed on 27 May 2023).
- Dassault Simulia CST Suite. 2022. Available online: https://www.3ds.com/ (accessed on 15 April 2023).
- Orfanidis, S.J. Electromagnetic Waves and Antennas. 2002. Revised 2016. Available online: http://www.ece.rutgers.edu/~orfanidi/ewa/ (accessed on 18 March 2023).
- Särestöniemi, M.; Sonkki, M.; Myllymäki, S.; Pomalaza-Raez, C. Wearable Flexible Antenna for UWB On-body and Implant Communications. Telecom 2021, 2, 285–301. [Google Scholar] [CrossRef]
- Available online: https://www.embodi3d.com/files/category/7-brain/ (accessed on 30 March 2023).
- Ghanim, R.; Kaushik, A.; Park, J.; Abramson, A. Communication protocols integrating wearables, ingestibles, and implantables for closed-loop therapies. Device 2023, 1, 100092. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Särestöniemi, M.; Taparugssanagorn, A.; Wisanmongkol, J.; Hämäläinen, M.; Iinatti, J. Comprehensive Analysis of Wireless Capsule Endoscopy Radio Channel Characteristics Using Anatomically Realistic Gastrointestinal Simulation Model. IEEE Access 2023, 11, 35649–35669. [Google Scholar] [CrossRef]
- Kiourti, A.; Konstantina, S.N. A review of in-body biotelemetry devices: Implantables, ingestibles, and injectables. IEEE Trans. Biomed. Eng. 2017, 64, 1422–1430. [Google Scholar] [CrossRef] [PubMed]






| Tissue | Frequency | |||
|---|---|---|---|---|
| 2 GHz | 4 GHz | 6 GHz | 8 GHz | |
| Brain (gray matter) | 49.7 | 46.6 | 43.7 | 40.9 |
| Brain (white matter) | 36.7 | 34.5 | 32.4 | 30.4 |
| Brain tumor | 59.0 | 55.7 | 52.2 | 48.6 |
| Fat | 5.33 | 5.12 | 4.84 | 4.46 |
| Glandular tissue | 58.1 | 54.9 | 51.7 | 48.4 |
| Breast tumor | 63.0 | 59.1 | 56.6 | 55.4 |
| Skin | 38.6 | 36.6 | 34.9 | 33.2 |
| Muscle | 53.3 | 50.8 | 48.2 | 45.5 |
| Large intestine | 54.7 | 51.3 | 48.1 | 45.0 |
| Large intestine lumen | 53.3 | 50.8 | 48.2 | 45.5 |
| Small intestine | 55.4 | 51.6 | 48.3 | 45.1 |
| Small intestine lumen | 53.3 | 50.8 | 48.2 | 45.5 |
| Phantom Type | Concentration of Ingredients | |||||||
|---|---|---|---|---|---|---|---|---|
| DI Water (mL) | Gelatine (g) | Sunflower Oil (mL) | DW Liquid 1 (mL) | Xanthan Gum (g) | PG 2 (mL) | Sugar (g) | NaCl (g) | |
| Skin | 10 | 3.01 | 1.68 | 0.83 | - | - | ||
| Tumor | 20.3 | 1.63 | 1.1 | 0.9 | - | |||
| Brain | 9 | 1.5 | 1.1 | 0.5 | - | |||
| Glandular tissue | 25.2 | 5.05 | - | - | - | - | 0.525 | - |
| Muscle/Intestine | 20 | 6.02 | 3.36 | 1.67 | 1.67 | - | - | - |
| Fat | 3 | 2 | - | 0.5 | 1 | 50 | - | - |
| Skin (mm) | Fat (mm) | Skull Bone (mm) | Brain (mm) | Muscle (mm) | Small Intestine (mm) | |
|---|---|---|---|---|---|---|
| Layer model 1 (head) | 1.2 | 1.2 | 7.5 | 7.5 | - | - |
| Layer model 2 (abdomen) | 2.2 | 10 | - | - | 8 | 20 |
| Phantom Type | Sample Trial | Concentration of Ingredients | |||
|---|---|---|---|---|---|
| Water (mL) | Gelatin (g) | Oil (mL) | Dishwasher (mL) | ||
| Tumor | TS1 | 22 | 1.7 | 4.25 | 0.95 |
| TS2 | 8 | 1.7 | 4.25 | 0.95 | |
| TS3 | 12.3 | 1.63 | 1.1 | 0.9 | |
| TS4 | 14.3 | 1.63 | 1.1 | 0.9 | |
| TS5 | 16.3 | 1.63 | 1.1 | 0.9 | |
| TS6 | 18.3 | 1.63 | 1.1 | 0.9 | |
| TS7 | 20.3 | 1.63 | 1.1 | 0.9 | |
| TS8 | 22.3 | 1.63 | 1.1 | 0.9 | |
| Skin | SS1 | 6 | 3.01 | 1.68 | 0.83 |
| SS2 | 8 | 3.01 | 1.68 | 0.83 | |
| SS3 | 10 | 3.01 | 1.68 | 0.83 | |
| Phantom Type | Sample Trial | Relative Permittivity/Conductivity(S/m) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| After 5 h | After 24 h | After 7 days | After 10 days | ||||||
| 2.5 GHz | 6 GHz | 2.5 GHz | 6 GHz | 2.5 GHz | 6 GHz | 2.5 GHz | 6 GHz | ||
| Tumor | TS1 | 43.2/1.38 | 38.6/4.97 | 41.06/1.08 | 36.61/4.26 | 40.8/1.36 | 36.96/4.99 | 38.06/1.56 | 33.3/4.32 |
| TS2 | 28.4/2.87 | 24.6/3.16 | 28.7/2.81 | 24.6/3.63 | 29.3/2.145 | 24.31/3.62 | 25.3/1.98 | 20.6/3.13 | |
| TS3 | 31.7/1.23 | 29.2/3.9 | 31.2/1.12 | 29.7/4.11 | 30.9/1.3 | 28.2/4.16 | 21.2/1.31 | 18.5/4.8 | |
| TS4 | 38.7/1.03 | 35.12/4.23 | 38.92/1.04 | 34.27/4.09 | 38.7/1.122 | 33.21/4.03 | 37.92/1.02 | 26.25/3.98 | |
| TS5 | 42.27/1.24 | 37.51/4.52 | 43.12/1.32 | 39.21/5.0 | 42.27/1.43 | 38.13/5.1 | 39.18/1.13 | 31.42/4.82 | |
| TS6 | 49/1.45 | 45.2/5.53 | 50.9/1.46 | 45.27/5.6 | 50.34/1.54 | 43.52/5.34 | 40.4/1.32 | 39.7/4.99 | |
| TS7 | 62.8/1.68 | 59.0/6.32 | 62.9/1.69 | 57.2/6.52 | 61.01/1.48 | 56.47/6.13 | 57.3/1.21 | 48.6/5.82 | |
| TS8 | 70.5/1.75 | 67.3/6.84 | 69.0/1.75 | 63.1/7.16 | 69.51/1.48 | 61.26/6.951 | 61.1/1.83 | 56.4/6.27 | |
| Skin | SS1 | 30.07/0.93 | 26.37/3.2 | 35.07/0.08 | 27.14/2.15 | 29.86/1.655 | 25.12/3.478 | 20.26/0.54 | 17.25/2.12 |
| SS2 | 35.1/1.34 | 31.7/3.36 | 38.9/1.07 | 32.8/3.12 | 41.39/1.72 | 31.96/5.38 | 23.5/1.82 | 18.7/2.28 | |
| SS3 | 40.3/1.48 | 36.9/4.78 | 38.2/1.96 | 34.1/3.76 | 41.22/1.54 | 34.11/5.51 | 30.1/0.93 | 26.37/3.2 | |
| Time | Relative Permittivity/Conductivity (S/m) | ||||
|---|---|---|---|---|---|
| Frequency (GHz) | 5 h | 24 h | 7 days | 10 days | |
| 2.5 | 62.03/2.03 | 61.82/2.15 | 62.45/2.15 | 57.1/2.0 | |
| 6 | 50.95/8.23 | 50.78/8.03 | 51.44/8.36 | 50.7/8.34 | |
| 8 | 44.75/12.8 | 45.39/11.58 | 48.11/12.25 | 44.99/12.96 | |
| Time | Relative Permittivity/Conductivity (S/m) | ||||
|---|---|---|---|---|---|
| Frequency (GHz) | 5 h | 24 h | 7 days | 10 days | |
| 2.5 | 54.98/1.75 | 55.1/1.8 | 54.79/1.91 | 51.84/3.67 | |
| 6 | 48.9/5.63 | 48.59/5.399 | 47.99/5.12 | 45.1/7.63 | |
| 8 | 45.7/8.2 | 45.34/8.6 | 45.1/8.59 | 43.3/10.2 | |
| 2 GHz | 6 GHz | 8 GHz | |
| Fat | 6.4/0.75 | 5.0/0.953 | 4.76/1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Särestöniemi, M.; Singh, D.; Dessai, R.; Heredia, C.; Myllymäki, S.; Myllylä, T. Realistic 3D Phantoms for Validation of Microwave Sensing in Health Monitoring Applications. Sensors 2024, 24, 1975. https://doi.org/10.3390/s24061975
Särestöniemi M, Singh D, Dessai R, Heredia C, Myllymäki S, Myllylä T. Realistic 3D Phantoms for Validation of Microwave Sensing in Health Monitoring Applications. Sensors. 2024; 24(6):1975. https://doi.org/10.3390/s24061975
Chicago/Turabian StyleSärestöniemi, Mariella, Daljeet Singh, Rakshita Dessai, Charline Heredia, Sami Myllymäki, and Teemu Myllylä. 2024. "Realistic 3D Phantoms for Validation of Microwave Sensing in Health Monitoring Applications" Sensors 24, no. 6: 1975. https://doi.org/10.3390/s24061975
APA StyleSärestöniemi, M., Singh, D., Dessai, R., Heredia, C., Myllymäki, S., & Myllylä, T. (2024). Realistic 3D Phantoms for Validation of Microwave Sensing in Health Monitoring Applications. Sensors, 24(6), 1975. https://doi.org/10.3390/s24061975







