Comparison of the Bactericidal Effect of Ultrasonic and Heat Combined with Ultrasonic Treatments on Egg Liquids and Additional Analysis of Their Effect by NIR Spectral Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasonic Treatment
2.2. NIR Measurement
2.3. E. coli Inoculation
2.4. Data Analysis
3. Results
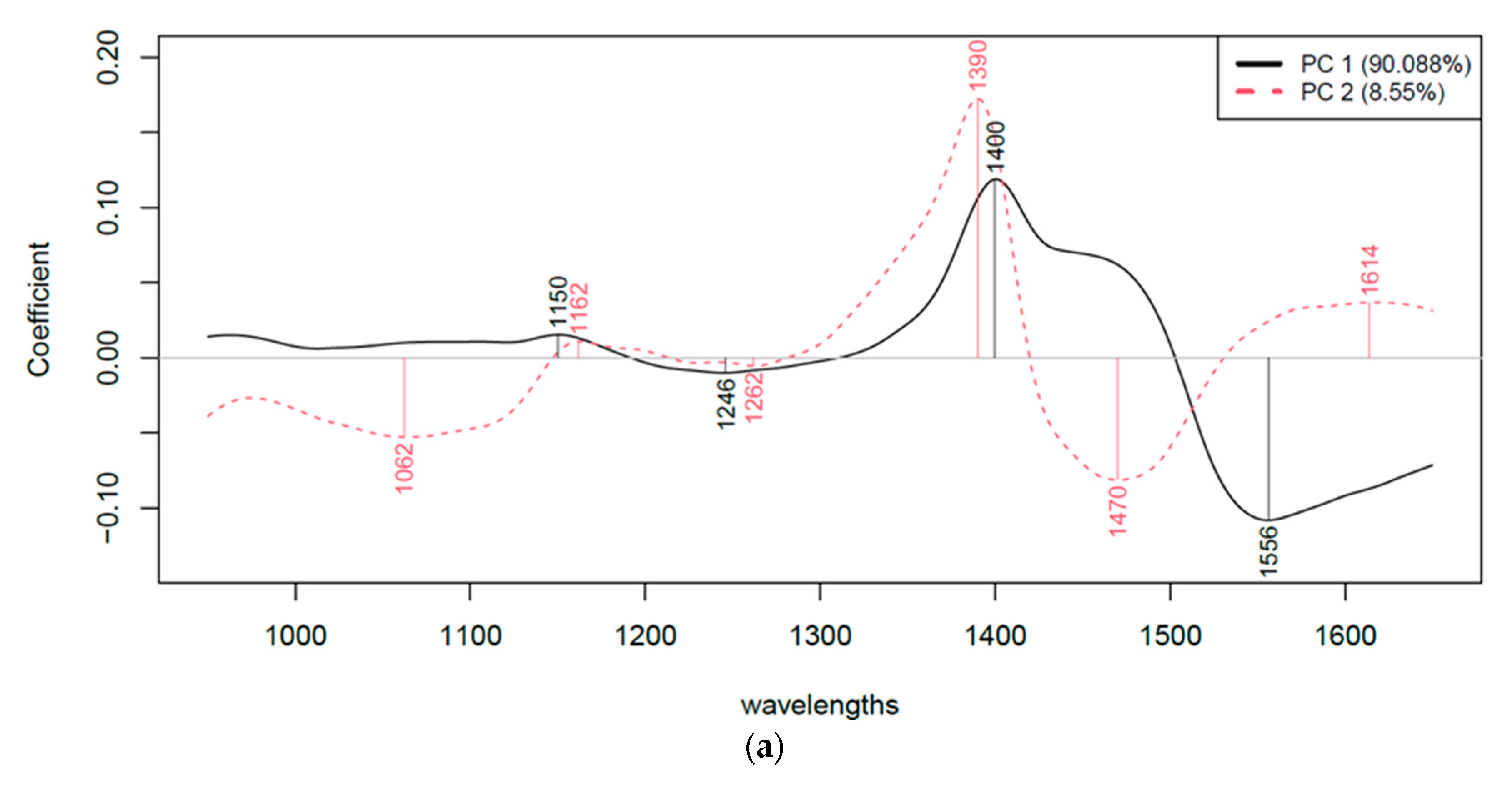
NIR Measurement
- -
- Water bands: Changes in the 1300–1600 nm range could indicate changes in water structure due to treatments. This range will be further analyzed by aquaphotomics.
- -
- Protein bands: Shifts in N-H (around 1500–1570 nm) or C-H (1100–1250 nm) peaks may reflect protein denaturation or changes in secondary structure.
- -
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruxton, C.H.S.; Derbyshire, E.; Gibson, S. The nutritional properties and health benefits of eggs. Nutr. Food Sci. 2010, 40, 263–279. [Google Scholar] [CrossRef]
- Jin, H.; Li, P.; Jin, Y.; Sheng, L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem. 2021, 352, 129457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Li, Z.; Jin, H.; Shu, D.; Jin, Y.; Jin, G.; Sheng, L. Research advances on the effects of thermal and non-thermal processing techniques on the physicochemical properties and microbiological control of liquid eggs. Food Control 2024, 155, 110106. [Google Scholar] [CrossRef]
- Ayari, E.; Németh, C.; Hidas, K.I.; Tóth, A.; Láng, D.; Friedrich, L. Heat treatment of liquid egg yolk. Prog. Agric. Eng. Sci. 2020, 16, 121–127. [Google Scholar] [CrossRef]
- Nemeth, C.S.; Friedrich, L.; Pásztor-Huszár, K.; Pipoly, E.; Suhajda, Á.; Balla, C.S. Thermal destruction of Listeria monocytogenes in liquid egg products with heat treatment at lower temperature and longer than pasteurization. African J. Food Sci. 2011, 5, 161–167. [Google Scholar]
- Li, P.; Jin, Y.; Sheng, L. Impact of microwave assisted phosphorylation on the physicochemistry and rehydration behaviour of egg white powder. Food Hydrocoll. 2020, 100, 105380. [Google Scholar] [CrossRef]
- Farid, N.; Waheed, A.; Motwani, S. Synthetic and natural antimicrobials as a control against food borne pathogens: A review. Heliyon 2023, 9, e17021. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli (accessed on 14 May 2024).
- EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2073-20200308 (accessed on 14 May 2024).
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gamlath, C.J.; Pathak, R.; Martin, G.J.O.; Ashokkumar, M. Ultrasound—The Physical and Chemical Effects Integral to Food Processing. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–358. [Google Scholar]
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207. [Google Scholar] [CrossRef]
- de São José, J.F.B.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Cameron, M.; McMaster, L.D.; Britz, T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008, 15, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, X.; Meng, L.; Li, J. Stresses make microbe undergo programmed cell death: Mechanisms and opportunities. Food Res. Int. 2022, 157, 111273. [Google Scholar] [CrossRef]
- Hashemi Moosavi, M.; Mousavi Khaneghah, A.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Huseyn, E.; Sant’Ana, A.S. A review of recent advances in the decontamination of mycotoxin and inactivation of fungi by ultrasound. Ultrason. Sonochem. 2021, 79, 105755. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing—Food quality assurance and food safety. Trends Food Sci. Technol. 2012, 26, 88–98. [Google Scholar] [CrossRef]
- Meena, L.; Malini, B.; Byresh, T.; Sunil, C.; Rawson, A.; Venkatachalapathy, N. Ultrasound as a pre-treatment in millet-based probiotic beverage: It’s effect on fermentation kinetics and beverage quality. Food Chem. Adv. 2024, 4, 100631. [Google Scholar] [CrossRef]
- Moura Brito, L.; Araújo Teixeira da Costa, G.; Campos Carvalhaes Reis, P.; de Toledo Guimarães, J.; Luis de Paiva Anciens Ramos, G.; Gomes da Cruz, A.; Cristina Alves Lacerda, I.; Ortiz Alvarenga, V. Impact of high-intensity ultrasound on fermentation, viability and predictive growth of lactic acid cultures: A study with conventional and probiotic fermented milks. J. Food Eng. 2024, 371, 111990. [Google Scholar] [CrossRef]
- Safwa, S.M.; Ahmed, T.; Talukder, S.; Sarker, A.; Rana, M.R. Applications of non-thermal technologies in food processing Industries—A review. J. Agric. Food Res. 2023, 100917. [Google Scholar] [CrossRef]
- Bariya, A.R.; Rathod, N.B.; Patel, A.S.; Nayak, J.K.B.; Ranveer, R.C.; Hashem, A.; Abd_Allah, E.F.; Ozogul, F.; Jambrak, A.R.; Rocha, J.M. Recent developments in ultrasound approach for preservation of animal origin foods. Ultrason. Sonochem. 2023, 101, 106676. [Google Scholar] [CrossRef] [PubMed]
- Piñon, M.; Alarcon-Rojo, A.; Paniwnyk, L.; Mason, T.; Luna, L.; Renteria, A. Ultrasound for improving the preservation of chicken meat. Food Sci. Technol. 2019, 39, 129–135. [Google Scholar] [CrossRef]
- Luo, M.; Shan, K.; Zhang, M.; Ke, W.; Zhao, D.; Nian, Y.; Wu, J.; Li, C. Application of ultrasound treatment for improving the quality of infant meat puree. Ultrason. Sonochem. 2021, 80, 105831. [Google Scholar] [CrossRef]
- Lan, W.; Lang, A.; Zhou, D.; Xie, J. Combined effects of ultrasound and slightly acidic electrolyzed water on quality of sea bass (Lateolabrax Japonicus) fillets during refrigerated storage. Ultrason. Sonochem. 2021, 81, 105854. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, M.C.; García, M.L.; De La Hoz, L.; Cambero, I.; Ordóñez, J.A. Destruction of Salmonella Senftenberg on the Shells of Intact Eggs by Thermoultrasonication. J. Food Prot. 2005, 68, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.L.A.; do Rosário, D.K.A.; Oliveira, S.B.S.; de Souza, H.L.S.; de Carvalho, R.V.; Carneiro, J.C.S.; Silva, P.I.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. Int. J. Food Microbiol. 2018, 269, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Scudino, H.; Silva, E.K.; Gomes, A.; Guimarães, J.T.; Cunha, R.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Ultrasound stabilization of raw milk: Microbial and enzymatic inactivation, physicochemical properties and kinetic stability. Ultrason. Sonochem. 2020, 67, 105185. [Google Scholar] [CrossRef] [PubMed]
- Techathuvanan, C.; D’Souza, D.H. High Intensity Ultrasound for Salmonella Enteritidis Inactivation in Culture and Liquid Whole Eggs. J. Food Sci. 2018, 83, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Islam, T.; Islam, Z.; Jubayer, F.; Rana, R. Sonication results in variable quality and enhanced sensory attributes of Adajamir (Citrus assamensis) juice: A study on an underutilized fruit. Heliyon 2023, 9, e23074. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit—A Review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Lu, R. Predicting firmness and sugar content of sweet cherries using near–infrared diffuse reflectance spectroscopy. Trans. ASAE 2001, 44, 1265. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.B. Robustness of calibration models based on near infrared spectroscopy for the in-line grading of stonefruit for total soluble solids content. Anal. Chim. Acta 2006, 555, 286–291. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, J.; Sun, L.; Chen, Q.; Zhou, F. Freshness measurement of eggs using near infrared (NIR) spectroscopy and multivariate data analysis. Innov. Food Sci. Emerg. Technol. 2011, 12, 182–186. [Google Scholar] [CrossRef]
- Abu-Khalaf, N.; Hmidat, M. Visible/Near Infrared (VIS/NIR) spectroscopy as an optical sensor for evaluating olive oil quality. Comput. Electron. Agric. 2020, 173, 105445. [Google Scholar] [CrossRef]
- Bimpong, D.; Adofowaa, L.A.; Agyeman, A.; Boakye, A.; Oduro, I.N.; Otoo, E.W.; Zaukuu, J.-L.Z. Authenticating peanut butter and yoghurt in the Kumasi Metropolis of Ghana using near infrared spectroscopy. Prog. Agric. Eng. Sci. 2023, 19, 69–81. [Google Scholar] [CrossRef]
- Diaz-Olivares, J.A.; Adriaens, I.; Stevens, E.; Saeys, W.; Aernouts, B. Online milk composition analysis with an on-farm near-infrared sensor. Comput. Electron. Agric. 2020, 178, 105734. [Google Scholar] [CrossRef]
- Bodor, Z.; Koncz, F.A.; Rashed, M.S.; Kaszab, T.; Gillay, Z.; Benedek, C.; Kovacs, Z. Application of near infrared spectroscopy and classical analytical methods for the evaluation of Hungarian honey. Prog. Agric. Eng. Sci. 2018, 14, 11–23. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Berzaghi, P.; Jansson, L.-M.; Andrighetto, I. The use of near-infrared reflectance spectroscopy (NIRS) in the prediction of chemical composition of freeze-dried egg yolk and discrimination between different n−3 PUFA feeding sources. Anim. Feed Sci. Technol. 2006, 128, 108–121. [Google Scholar] [CrossRef]
- Raso, J.; Mañas, P.; Pagán, R.; Sala, F.J. Influence of different factors on the output power transferred into medium by ultrasound. Ultrason. Sonochem. 1999, 5, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.; Felfoldi, J.; Taczmanne Bruckner, A.; Mohacsi-Farkas, C.; Bodor, Z.; Kertesz, I.; Nemeth, C.; Zsom-Muha, V. Determining Sonication Effect on E. coli in Liquid Egg, Egg Yolk and Albumen and Inspecting Structural Property Changes by Near-Infrared Spectra. Sensors 2021, 21, 398. [Google Scholar] [CrossRef]
- Tsenkova, R. Introduction Aquaphotomics: Dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J. Near Infrared Spectrosc. 2009, 17, 303–313. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of Aquaphotomics and Its Chemometrics Approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Pollner, B. Aquaphotomics-Software R-Package “aquap2“. In Proceedings of the Understanding Water in Biology 2nd International Symposium, Kobe, Japan, 26–29 November 2016; pp. 26–29. [Google Scholar]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020, 60, 104763. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Aguirre, D.; Corradini, M.G.; Mawson, R.; Barbosa-Cánovas, G.V. Modeling the inactivation of Listeria innocua in raw whole milk treated under thermo-sonication. Innov. Food Sci. Emerg. Technol. 2009, 10, 172–178. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, H.; Cui, Z.; Li, H.; Zhou, W.; Liu, Z.; Zhang, H.; Manoli, T.; Mo, H.; Hu, L. Ultrasound-assisted blue light killing Vibrio parahaemolyticus to improve salmon preservation. Ultrason. Sonochem. 2023, 95, 106389. [Google Scholar] [CrossRef] [PubMed]
- Gera, N.; Doores, S. Kinetics and Mechanism of Bacterial Inactivation by Ultrasound Waves and Sonoprotective Effect of Milk Components. J. Food Sci. 2011, 76, M111–M119. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T. Principal Component Analysis (PCA). In Computer Vision: A Reference Guide; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–4. ISBN 978-3-030-03243-2. [Google Scholar]
- Muncan, J.; Tsenkova, R. Aquaphotomics-From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 2742. [Google Scholar] [CrossRef] [PubMed]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course notes on the Interpretation of Infrared and Raman Spectra; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Szigedi, T. Módszerfejlesztés Fourier-Transzformációs Közeli Infravörös Technika (FT-NIR) Alkalmazási Körének Kibővítése Élelmiszeripari Mintákon; Corvinus University Budapest: Budapest, Hungary, 2014. [Google Scholar]
- Kang, D.; Jiang, Y.; Xing, L.; Zhou, G.; Zhang, W. Inactivation of Escherichia coli O157:H7 and Bacillus cereus by power ultrasound during the curing processing in brining liquid and beef. Food Res. Int. 2017, 102, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Uysal, R.S.; Boyacı, İ.H.; Soykut, E.A.; Ertaş, N. Effects of heat treatment parameters on liquid whole egg proteins. Food Chem. 2017, 216, 201–208. [Google Scholar] [CrossRef]
- Zupanc, M.; Pandur, Ž.; Stepišnik Perdih, T.; Stopar, D.; Petkovšek, M.; Dular, M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019, 57, 147–165. [Google Scholar] [CrossRef]
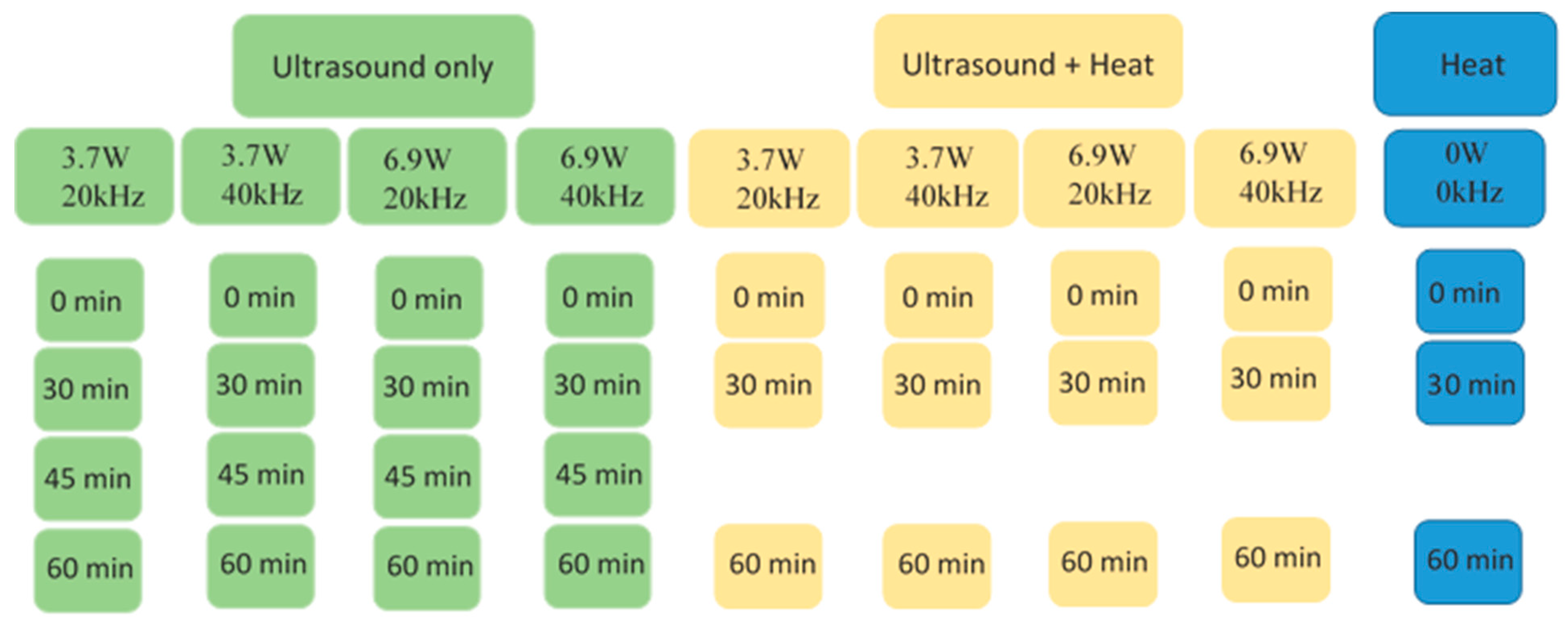
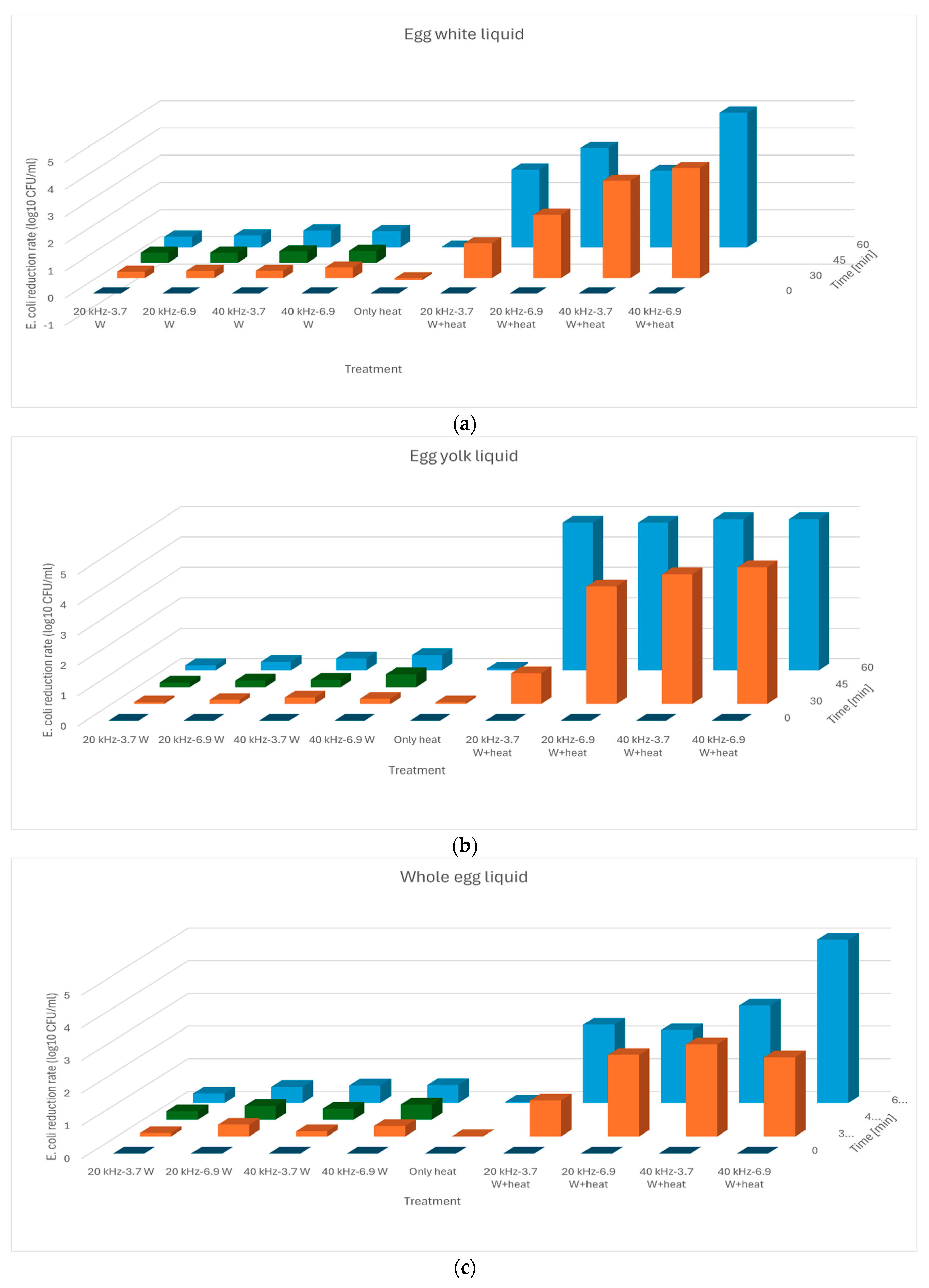

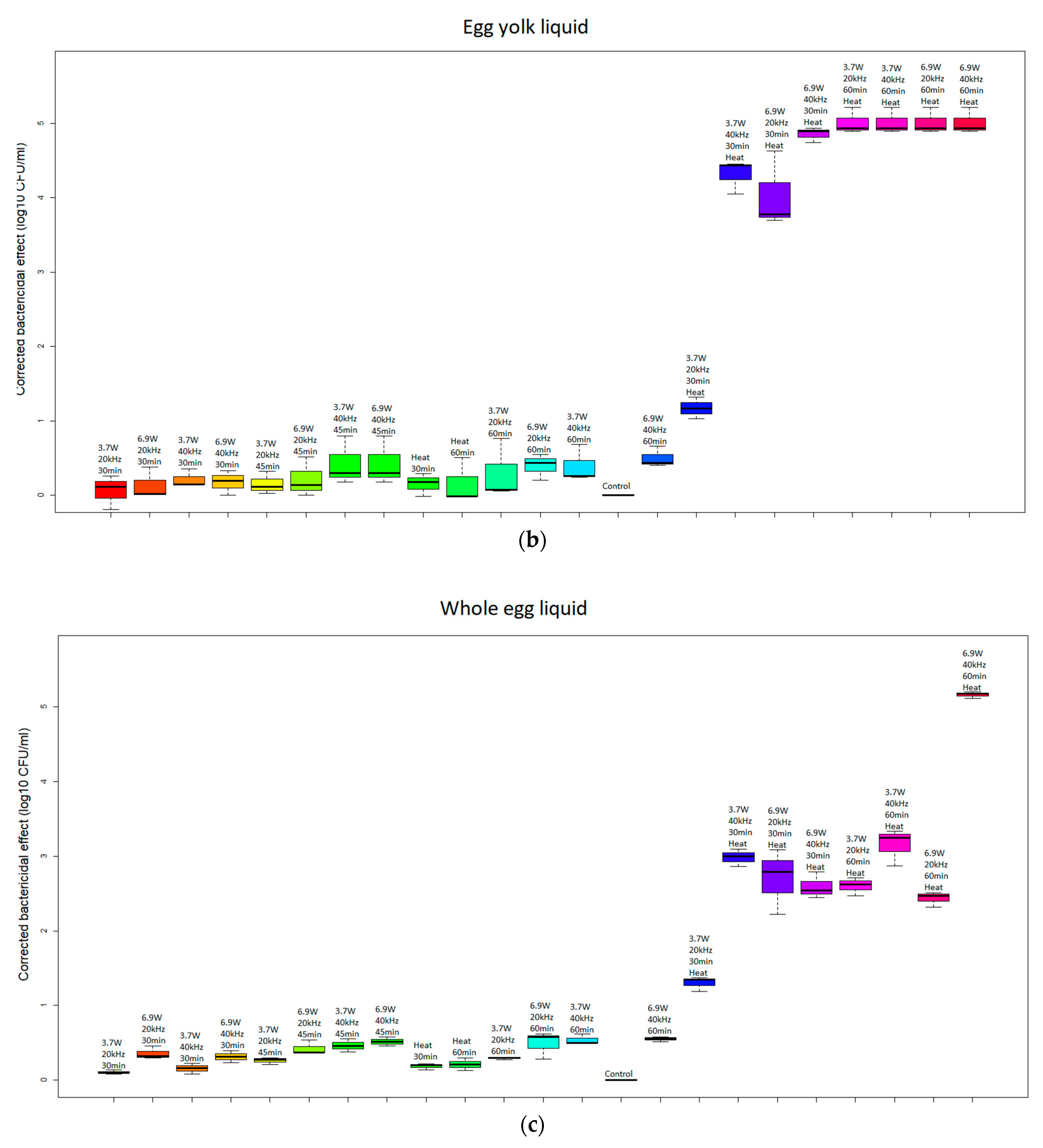





| Group | Absorbed Power [W] | Frequency [kHz] | Time [min] | Temperature [°C] |
|---|---|---|---|---|
| A | 3.7 | 20 | 30 | 18 |
| B | 3.7 | 40 | 30 | 18 |
| C | 6.9 | 20 | 30 | 18 |
| D | 6.9 | 40 | 30 | 18 |
| E | 3.7 | 20 | 60 | 18 |
| F | 3.7 | 40 | 60 | 18 |
| G | 6.9 | 20 | 60 | 18 |
| H | 6.9 | 40 | 60 | 18 |
| I | 3.7 | 20 | 30 | 55 |
| J | 3.7 | 40 | 30 | 55 |
| K | 6.9 | 20 | 30 | 55 |
| L | 6.9 | 40 | 30 | 55 |
| M | 3.7 | 20 | 60 | 55 |
| N | 3.7 | 40 | 60 | 55 |
| O | 6.9 | 20 | 60 | 55 |
| P | 6.9 | 40 | 60 | 55 |
| Heat | 0 | 0 | 60 | 55 |
| Control | - | - | - | - |
| Treatment | Egg White Liquid | Egg Yolk Liquid | Whole Egg Liquid |
|---|---|---|---|
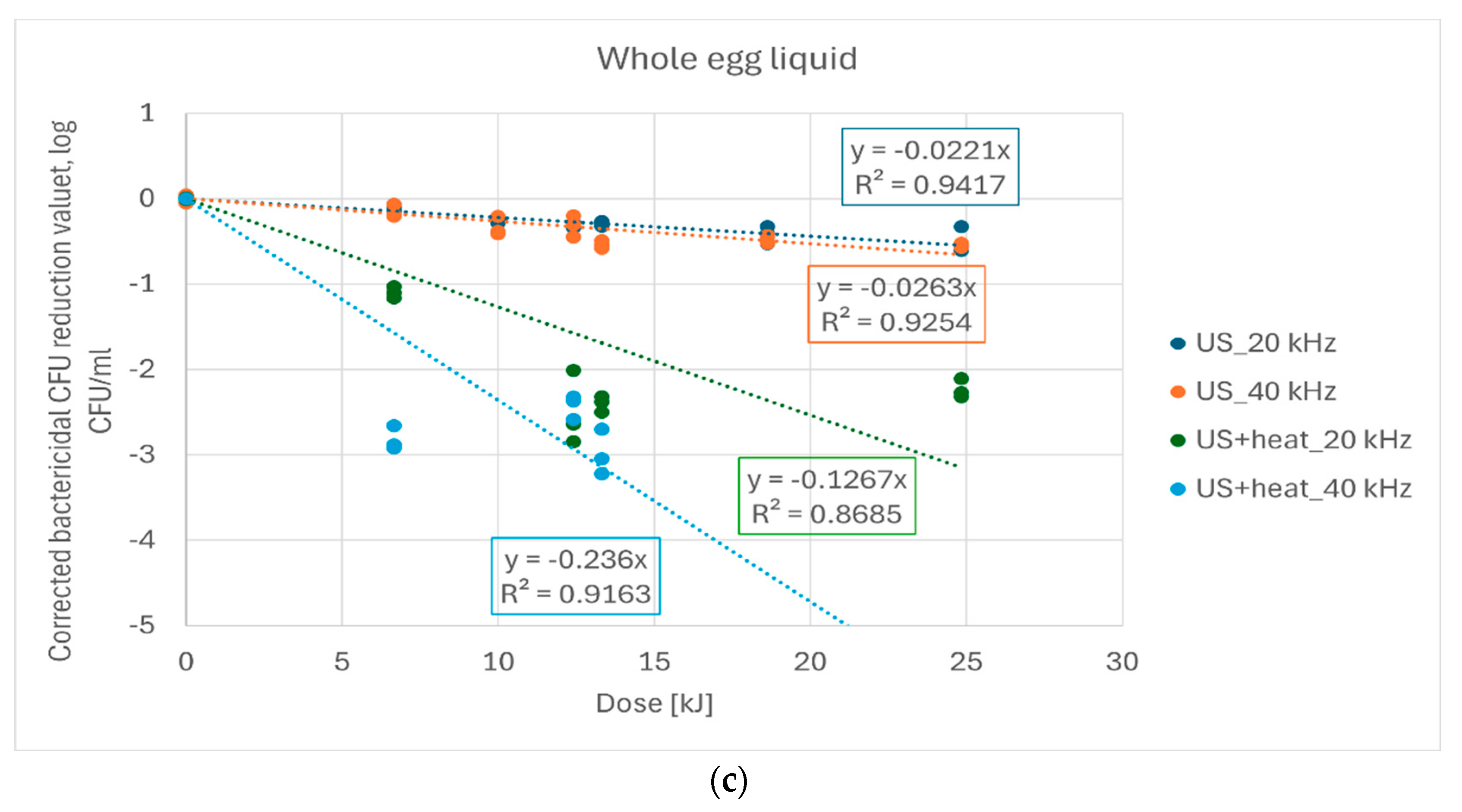
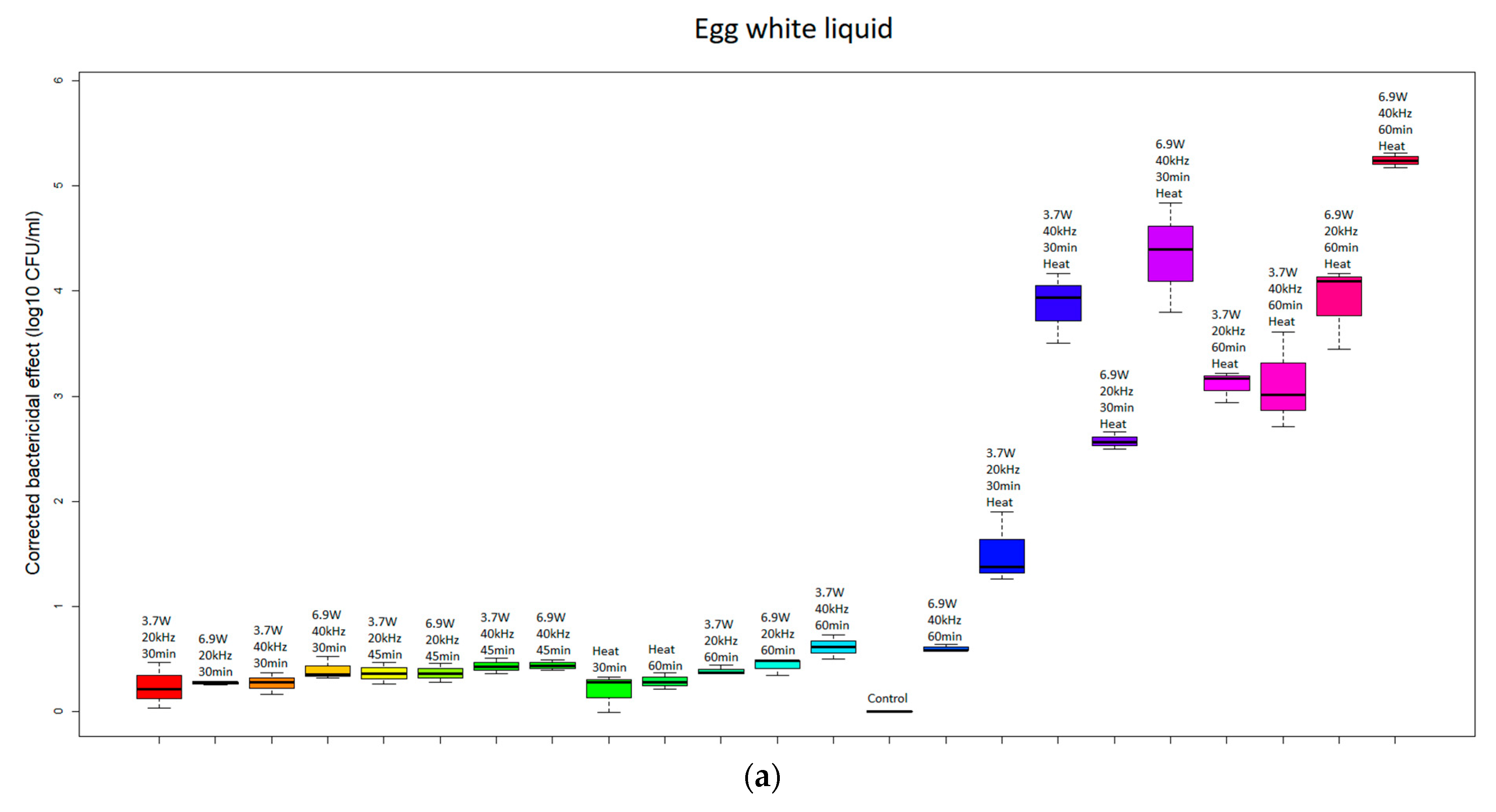
| US, 20 kHz | −0.0219 a | −0.0149 a | −0.0220 a |
| US, 40 kHz | −0.0292 b | −0.0220 b | −0.0262 a |
| US+heat, 20 kHz | −0.1674 c | −0.2766 c | −0.1266 b |
| US+heat, 40 kHz | −0.2972 d | −0.4914 d | −0.2359 c |
| 3.7 W | 6.9 W | ||
|---|---|---|---|
| Type | Setup | Needed treatment time, min | |
| Egg white | US 20 kHz | 1023.8 | 549.0 |
| US 40 kHz | 768.7 | 412.2 | |
| US+heat 20 kHz | 134.5 | 72.1 | |
| US+heat 40 kHz | 75.8 | 40.6 | |
| Egg yolk | US 20 kHz | 1501.5 | 805.2 |
| US 40 kHz | 1023.8 | 549.0 | |
| US+heat 20 kHz | 81.4 | 43.6 | |
| US+heat 40 kHz | 45.8 | 24.6 | |
| Whole egg liquid | US 20 kHz | 1019.1 | 546.5 |
| US 40 kHz | 856.4 | 459.2 | |
| US+heat 20 kHz | 177.8 | 95.3 | |
| US+heat 40 kHz | 95.4 | 51.2 | |
| WAMACS | Wavelength | |
|---|---|---|
| C1 | 1336–1348 | H2O asymmetric stretching vibration |
| C2 | 1360–1366 | Water solvation shell |
| C3 | 1370–1376 | H2O symmetrical stretching vibration and H2O asymmetric stretching vibration |
| C4 | 1380–1388 | OH-(H2O)1,4: Water solvation shell O2-(H2O)4: Hydrated superoxide clusters H2O symmetrical stretching vibration |
| C5 | 1398–1418 | Water confined in a local field of ions (trapped water) S0: Free water Water with free OH− |
| C6 | 1421–1430 | Water hydration band |
| C7 | 1432–1444 | Water molecules with 1 hydrogen bond |
| C8 | 1448–1454 | Water solvation shell |
| C9 | 1458–1468 | Water molecules with 2 hydrogen bonds |
| C10 | 1472–1482 | Water molecules with 3 hydrogen bonds |
| C11 | 1482–1495 | Water molecules with 4 hydrogen bonds |
| C12 | 1506–1516 | H2O symmetrical stretching vibration Strongly bound water |
| Egg White Liquid | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | A_US | B_US | C_US | D_US | E_US | F_US | G_US | H_US | L_US+H | P_US+H | O_US+H | K_US+H | J_US+H | N_US+H | I_US+H | M_US+H | Heat | |
| Control | 33.38 | 29.12 | 16.62 | 0.87 | 8.36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A_US | 16.62 | 45.88 | 0 | 7.03 | 16.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B_US | 25 | 0 | 45.88 | 5.26 | 12.48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_US | 25 | 25 | 33.38 | 86.84 | 20.85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D_US | 0 | 0 | 4.12 | 0 | 20.85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E_US | 0 | 0 | 0 | 0 | 0 | 70.88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F_US | 0 | 0 | 0 | 0 | 4.12 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_US | 0 | 0 | 0 | 0 | 0 | 29.12 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H_US | 0 | 0 | 0 | 0 | 8.36 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O_US+H | 0 | 0 | 0 | 0 | 8.36 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| K_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| J_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| N_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| I_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| M_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Heat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Egg yolk liquid | ||||||||||||||||||
| Control | A_US | B_US | C_US | D_US | E_US | F_US | G_US | H_US | L_US+H | P_US+H | O_US+H | K_US+H | J_US+H | N_US+H | I_US+H | M_US+H | Heat | |
| Control | 58.3 | 0 | 0 | 18.42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A_US | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B_US | 0 | 0 | 58.38 | 5.26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_US | 24.97 | 50 | 41.62 | 69.29 | 66.62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D_US | 8.36 | 0 | 0 | 1.76 | 25 | 4.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E_US | 0 | 0 | 0 | 0 | 0 | 45.43 | 33.33 | 0 | 4.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F_US | 0 | 0 | 0 | 0 | 0 | 40.93 | 33.33 | 4.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_US | 0 | 0 | 0 | 1.76 | 0 | 9.14 | 33.33 | 95.88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H_US | 8.36 | 0 | 0 | 3.5 | 8.38 | 0 | 0 | 0 | 95.88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94.5 | 0 | 0 | 0 | 5.5 | 0 | 0 | 0 |
| O_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| K_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| J_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| N_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.5 | 0 | 0 | 0 | 94.5 | 0 | 0 | 0 |
| I_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| M_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Heat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Whole egg liquid | ||||||||||||||||||
| Control | A_US | B_US | C_US | D_US | E_US | F_US | G_US | H_US | L_US+H | P_US+H | O_US+H | K_US+H | J_US+H | N_US+H | I_US+H | M_US+H | Heat | |
| Control | 25 | 0 | 0 | 0.87 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A_US | 16.62 | 29.12 | 12.5 | 0.87 | 8.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B_US | 0 | 4.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C_US | 58.38 | 58.38 | 75 | 94.76 | 66.62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D_US | 0 | 0 | 0 | 3.5 | 8.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E_US | 0 | 0 | 0 | 0 | 0 | 79.12 | 0 | 0 | 8.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F_US | 0 | 0 | 0 | 0 | 0 | 0 | 66.62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G_US | 0 | 8.38 | 12.5 | 0 | 16.62 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H_US | 0 | 0 | 0 | 0 | 0 | 20.88 | 33.38 | 0 | 91.62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 88.83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| O_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| K_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| J_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.17 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| N_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| I_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| M_US+H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Heat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, D.; Zsom, T.; Taczman-Brückner, A.; Somogyi, T.; Zsom-Muha, V.; Felföldi, J. Comparison of the Bactericidal Effect of Ultrasonic and Heat Combined with Ultrasonic Treatments on Egg Liquids and Additional Analysis of Their Effect by NIR Spectral Analysis. Sensors 2024, 24, 4547. https://doi.org/10.3390/s24144547
Nagy D, Zsom T, Taczman-Brückner A, Somogyi T, Zsom-Muha V, Felföldi J. Comparison of the Bactericidal Effect of Ultrasonic and Heat Combined with Ultrasonic Treatments on Egg Liquids and Additional Analysis of Their Effect by NIR Spectral Analysis. Sensors. 2024; 24(14):4547. https://doi.org/10.3390/s24144547
Chicago/Turabian StyleNagy, Dávid, Tamás Zsom, Andrea Taczman-Brückner, Tamás Somogyi, Viktória Zsom-Muha, and József Felföldi. 2024. "Comparison of the Bactericidal Effect of Ultrasonic and Heat Combined with Ultrasonic Treatments on Egg Liquids and Additional Analysis of Their Effect by NIR Spectral Analysis" Sensors 24, no. 14: 4547. https://doi.org/10.3390/s24144547
APA StyleNagy, D., Zsom, T., Taczman-Brückner, A., Somogyi, T., Zsom-Muha, V., & Felföldi, J. (2024). Comparison of the Bactericidal Effect of Ultrasonic and Heat Combined with Ultrasonic Treatments on Egg Liquids and Additional Analysis of Their Effect by NIR Spectral Analysis. Sensors, 24(14), 4547. https://doi.org/10.3390/s24144547






