Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds, Husbandry, and Diets
2.2. Conditions for Stocking and Cooking Samples
- Traditional diet—code CONTROL;
- Live larval integrated diet—code LL;
- Dry larval integrated diet—code LD;
- Sustainable diet—code ST.
2.3. Preparation of GC-MS Samples
GC-MS Analysis Conditions
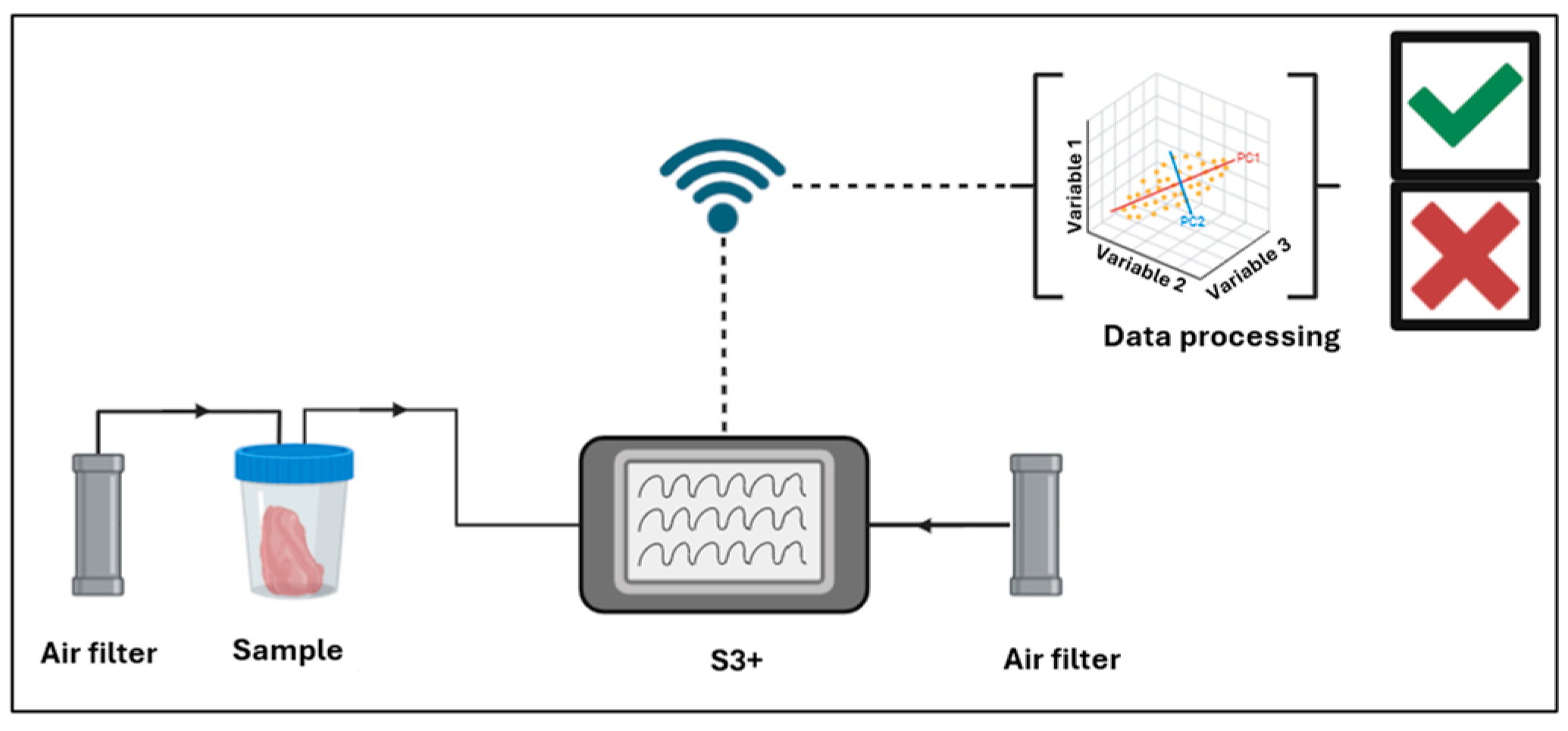
2.4. S3+ Samples Preparation
2.4.1. Calibration of MOX Sensor Arrays
2.4.2. S3+ Setup
2.5. Post-Run Analysis
3. Results and Discussion
3.1. Poultry
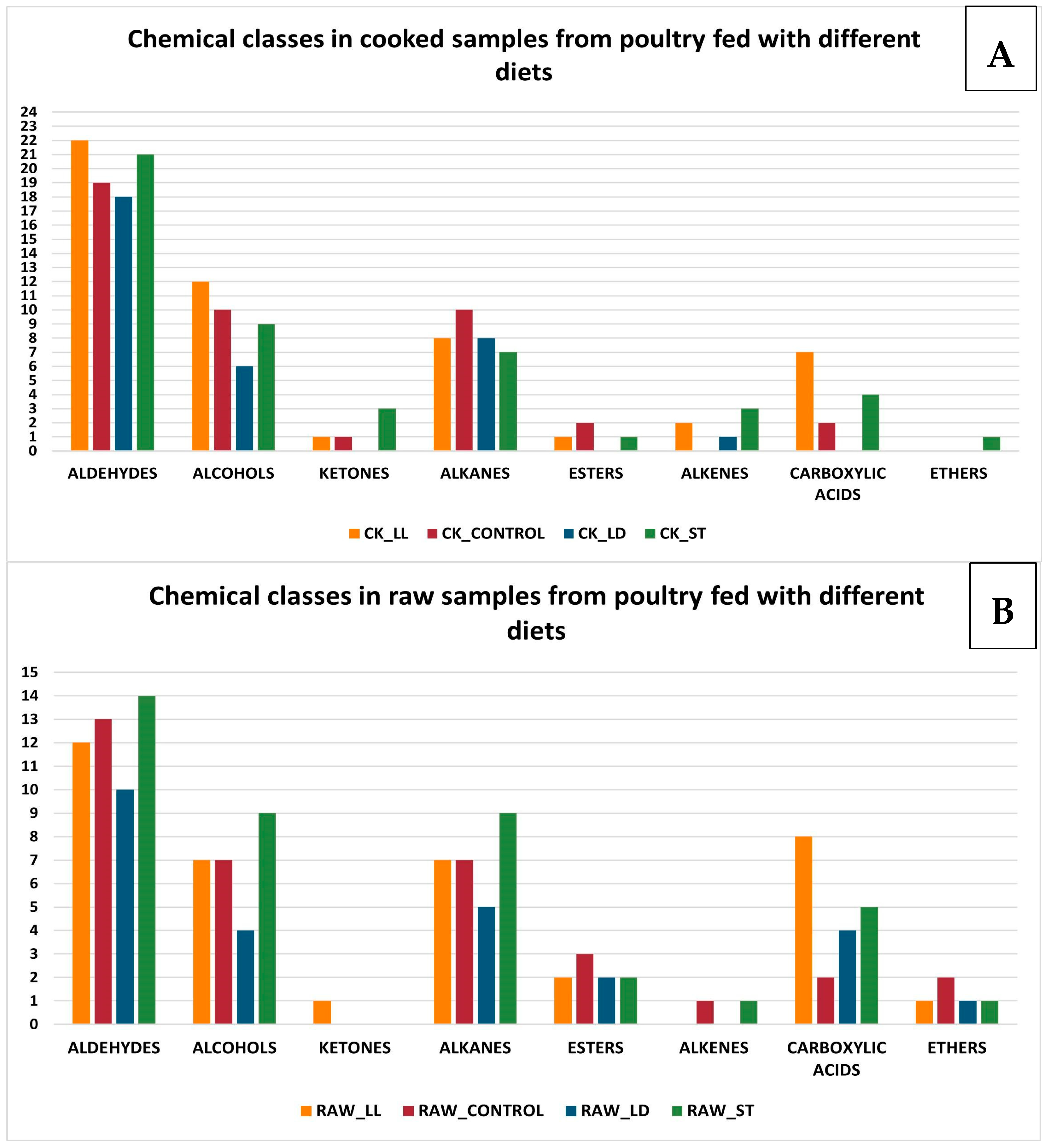
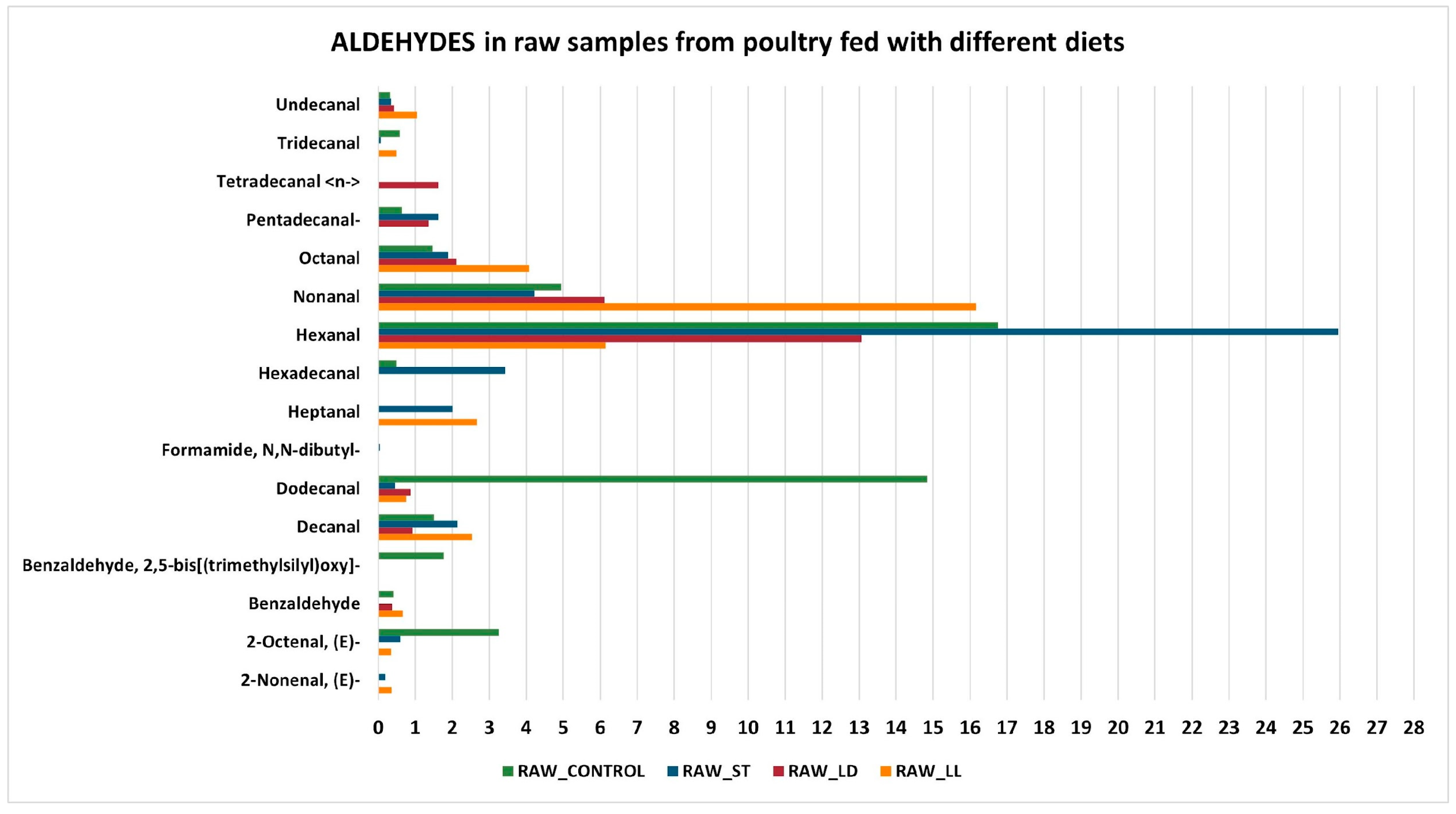
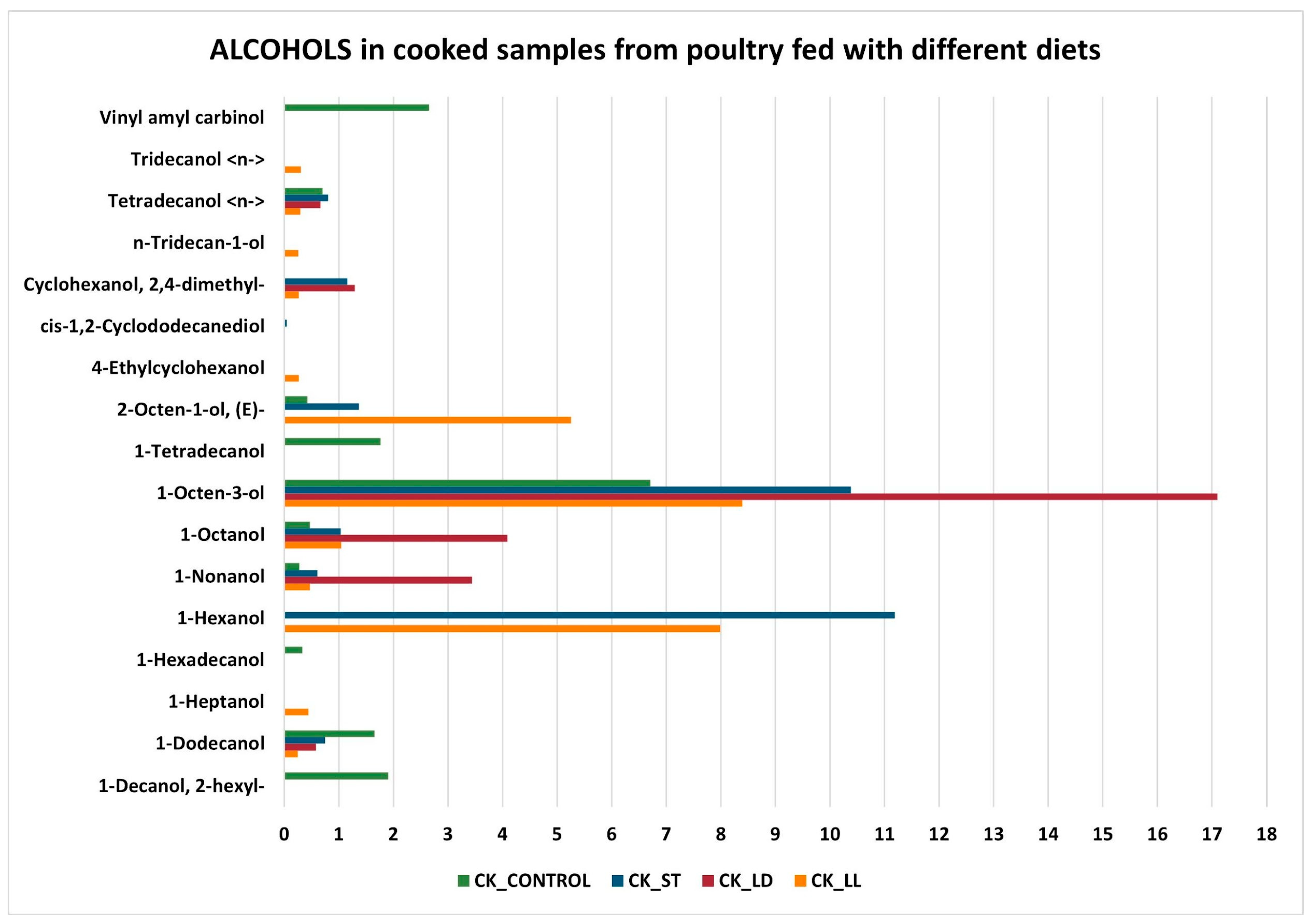
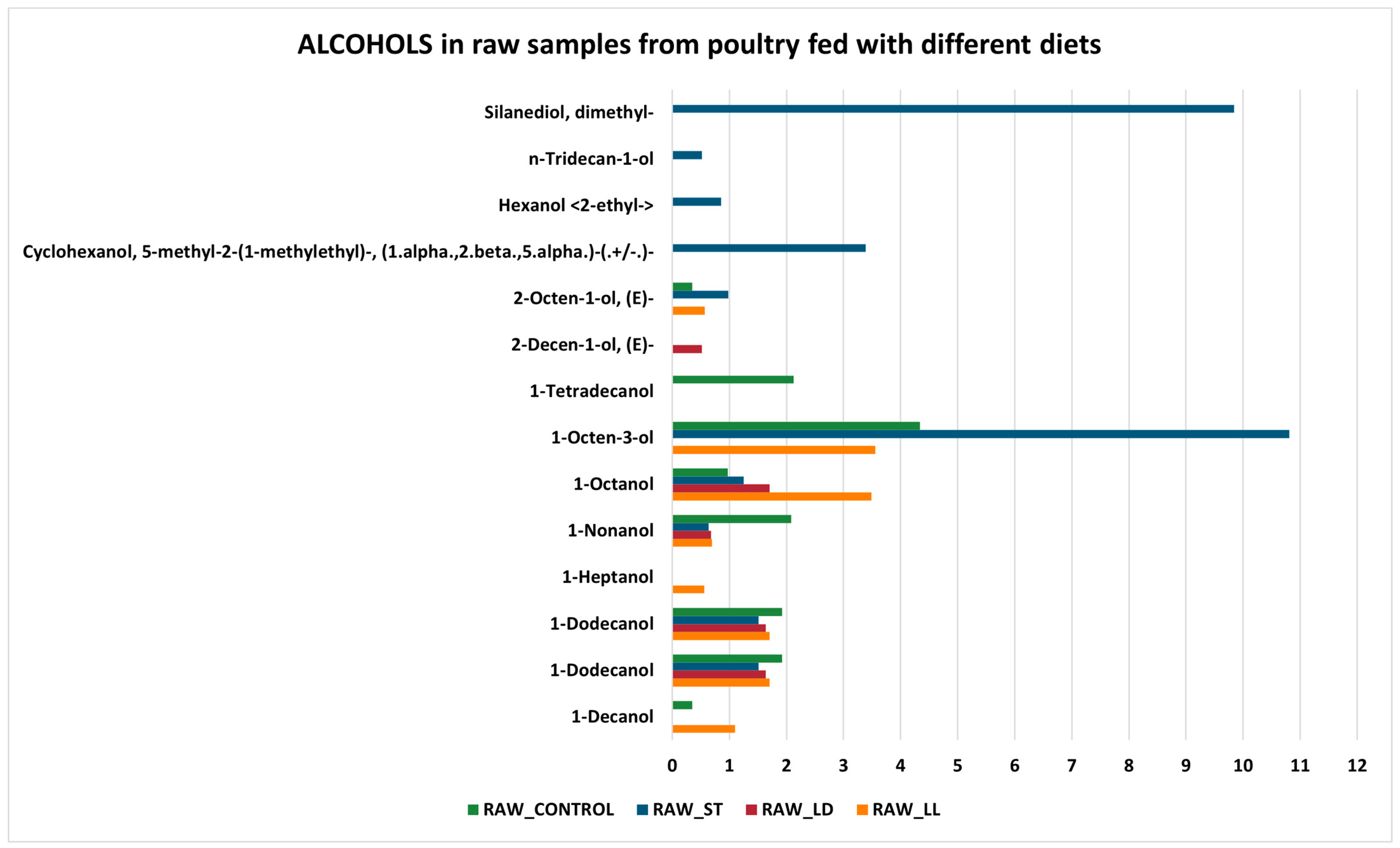
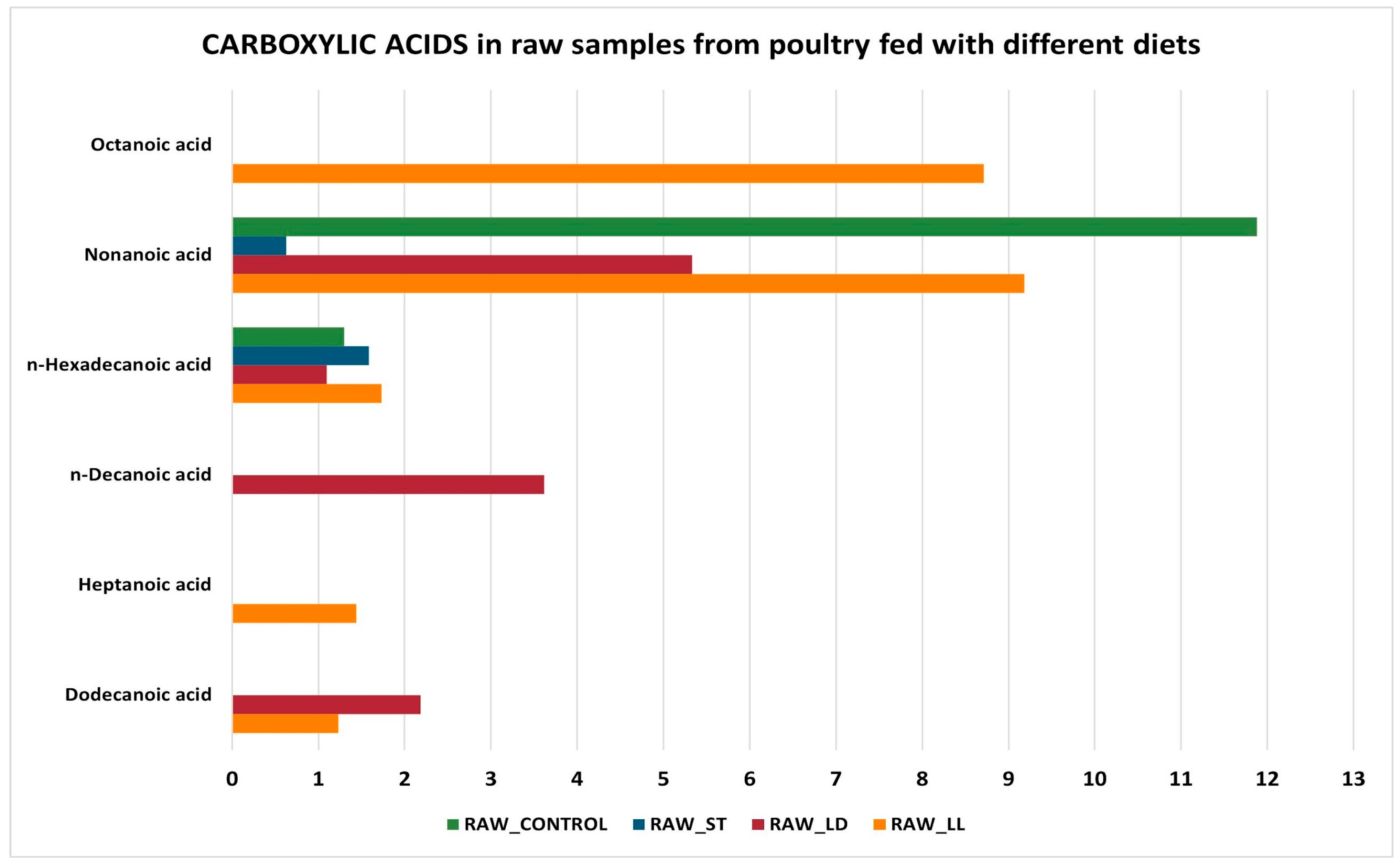
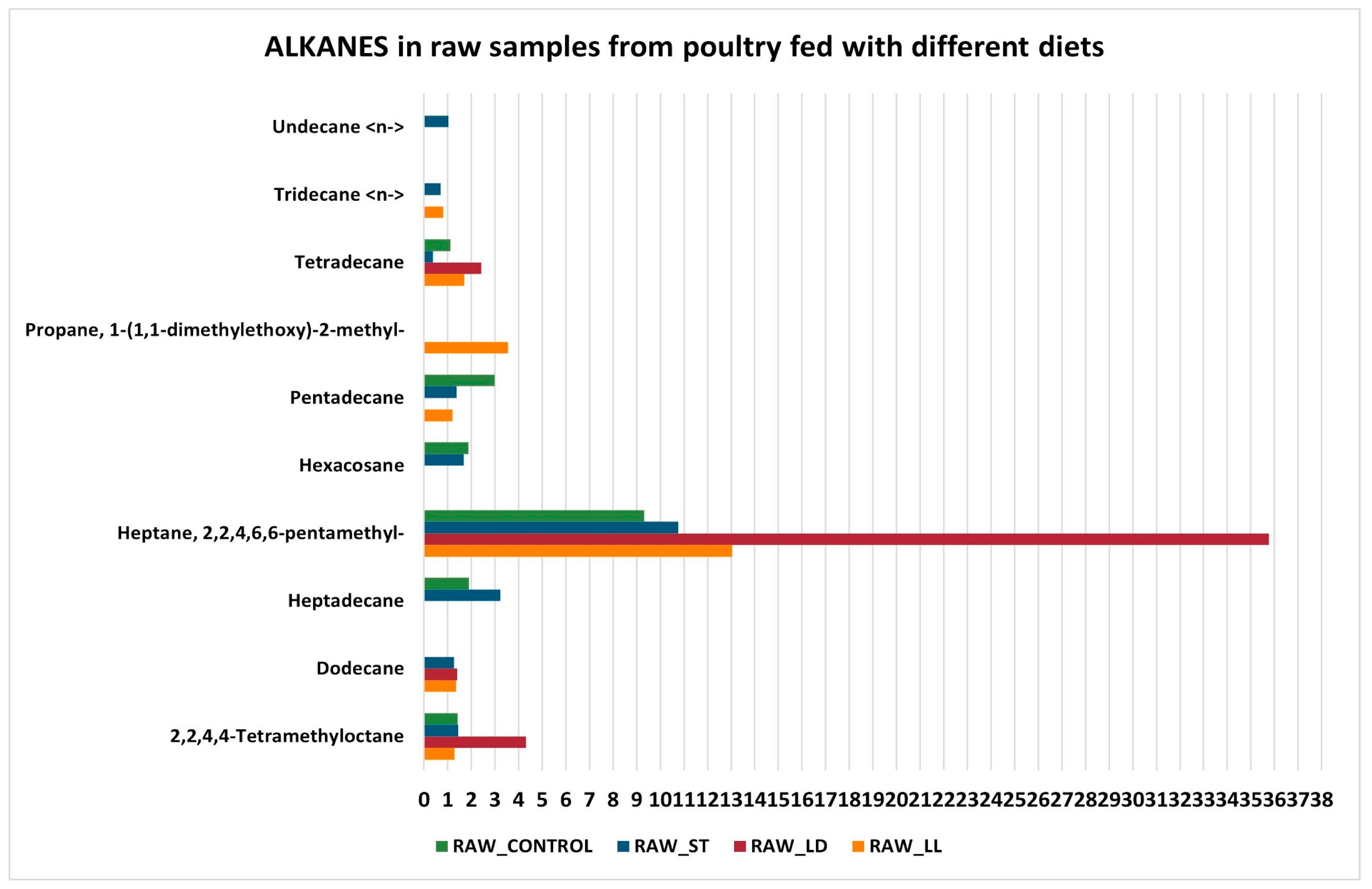
3.2. GC-MS Detection Results
- Protein Denaturation: cooking causes the denaturation of proteins, which alters the structure of protein molecules, leading to the formation of new volatile compounds.
- Maillard Reactions: this set of chemical reactions occurs between amino acids and reducing sugars at elevated temperatures, producing a wide range of volatile compounds responsible for the flavor and aroma of cooked food.
- Related to the Maillard reaction is a subset of chemical processes called Strecker degradation, which plays a critical role in meat flavor generation and, in general, in producing aroma-active volatiles in processed foods. The Strecker degradation converts an α-amino acid into an aldehyde containing the side chain by way of an imine intermediate, directing the Maillard reaction from chromogenic pathways toward more aromagenic pathways [45]. Depending on the parent amino acid, the Strecker aldehydes normally have low-odor threshold values with characteristic aroma properties.
- Lipid Oxidation: lipids present in the meat oxidize during cooking, producing aldehydes, alcohols, and ketones in the initial stages, which subsequently transform into acids and other compounds [46].
- Reduction in aldehydes, alcohols, and ketones: aldehydes can further oxidize during cooking, transforming into carboxylic acids. Alcohols, in turn, can oxidize into aldehydes and ketones, which can subsequently transform into carboxylic acids. Ketones can undergo further oxidation reactions, becoming carboxylic acids.
- Increase in esters, carboxylic acids, and ethers: esters can form through reactions between alcohols and acids, facilitated by the presence of heat. Carboxylic acids increase due to the oxidation of aldehydes, alcohols, and ketones. Ethers can form through condensation reactions between alcohols, especially under high heat conditions.
- Unchanged alkanes and alkenes: these hydrocarbon compounds are relatively stable and do not readily participate in the chemical reactions that occur during cooking. Therefore, their concentrations tend to remain unchanged.
- -
- Thermal decomposition: during cooking, fatty acids can undergo thermal decomposition, leading to the formation of volatile compounds or the degradation of shorter-chain fatty acids. This can reduce the concentration of nonanoic acid and octanoic acid in cooked meat.
- -
- Oxidation: cooking, especially at high temperatures, can accelerate the oxidation of fatty acids. Nonanoic and octanoic acids can oxidize and transform into other compounds, such as aldehydes, ketones, and shorter acids, thereby reducing their concentration in cooked meat.
- -
- Evaporation: some short- and medium-chain fatty acids can evaporate during cooking. Octanoic acid and nonanoic acid have relatively low boiling points compared to longer-chain fatty acids, which can lead to their partial loss as volatile compounds during cooking.
- -
- Maillard reactions: during cooking, especially at high temperatures, Maillard reactions occur, which are complex chemical reactions between amino acids and reducing sugars. These reactions can influence the lipid profile of the meat, leading to the formation of new compounds and the reduction of the originally present fatty acids.
- -
- Lipolysis: in the case of raw meat, enzymatic processes such as lipolysis can be active, leading to the release of free fatty acids like nonanoic acid and octanoic acid. Cooking inactivates these enzymes, interrupting the lipolysis process and thereby reducing the formation of free fatty acids.
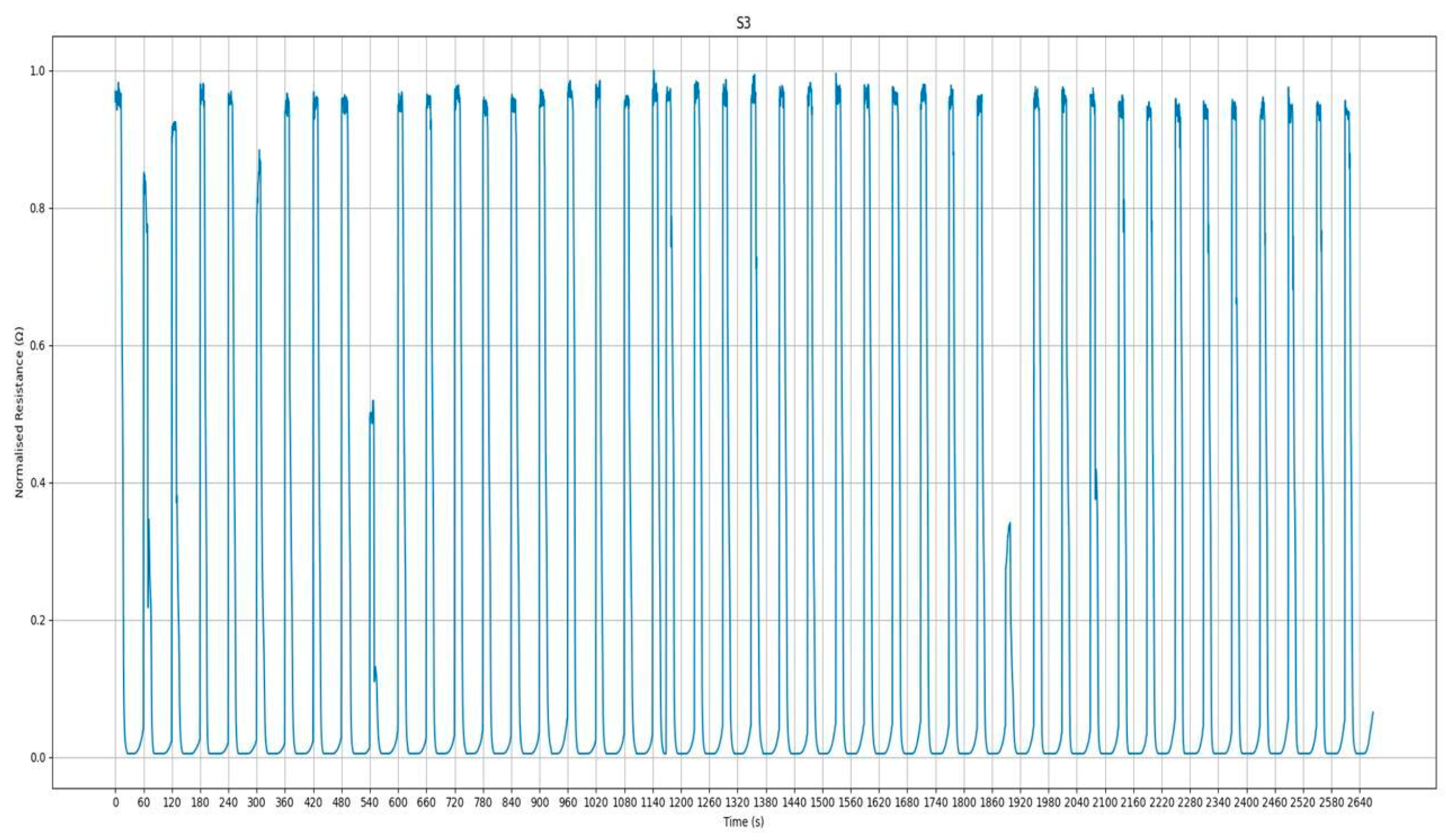
3.3. S3+ Detection Results
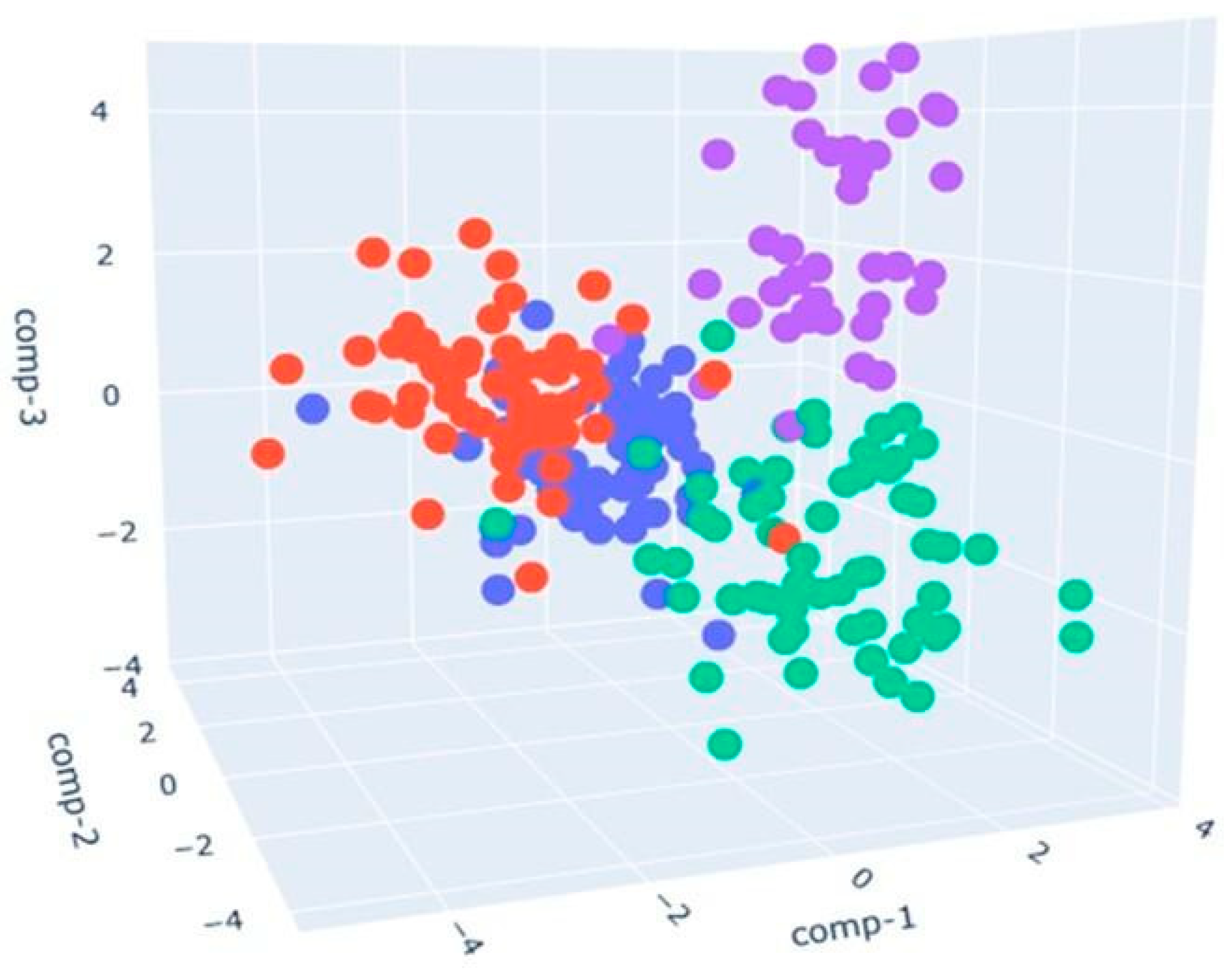
- The blue points represent the control samples (CK_CONTROL). The points appear clustered in a specific area, indicating that the control samples tend to have similar olfactory profiles.
- The red points indicate the samples with a sustainable diet (CK_ST). These points are located in an area distinct from the area of the blue dots, indicating significant differences in olfactory profiles compared to the control samples. The separate position suggests that a sustainable diet has a measurable and distinct impact on the olfactory profile of chicken meat.
- The green points represent the samples fed with a dry larval diet (CK_LD), and they are mainly concentrated in the center of the chart.
- The purple points correspond to the samples fed with a live larval diet (CK_LL). Purple dots are placed in their region of the LDA analysis space. Their separation from other groups implies that the diet based on live larvae has a complex and characteristic volatilome.
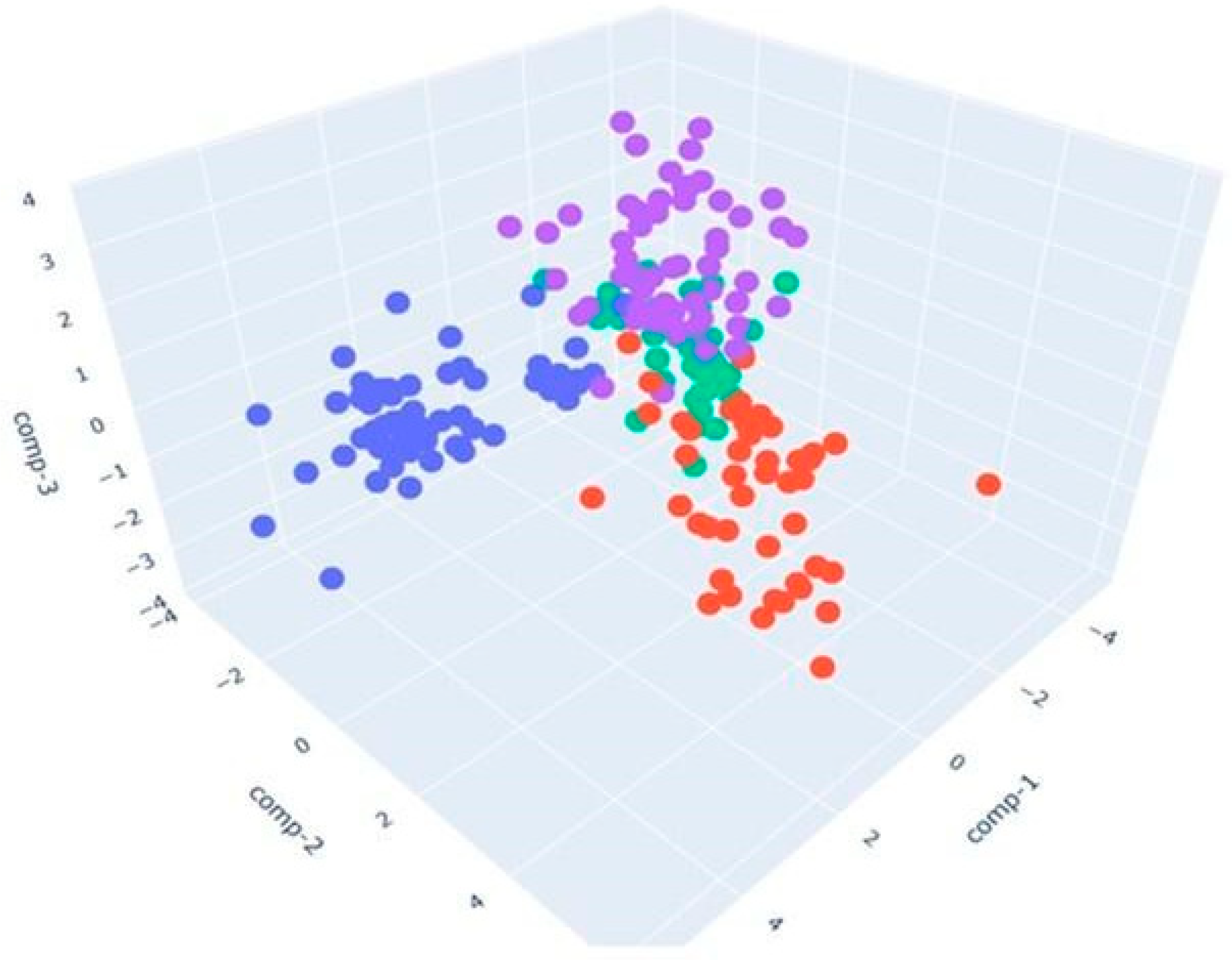
- The blue points represent the control samples (RAW_CONTROL) and are mainly concentrated in the center of the chart.
- The red points indicate the samples with a sustainable diet (RAW_ST). These points are also concentrated in the center, partially overlapping with the blue control samples.
- The green points represent the samples fed with a dry larval diet (RAW_LD), primarily distributed on the lower right side of the chart.
- The purple points correspond to the samples fed with a live larval diet (RAW_LL), mainly distributed on the upper right side of the chart.
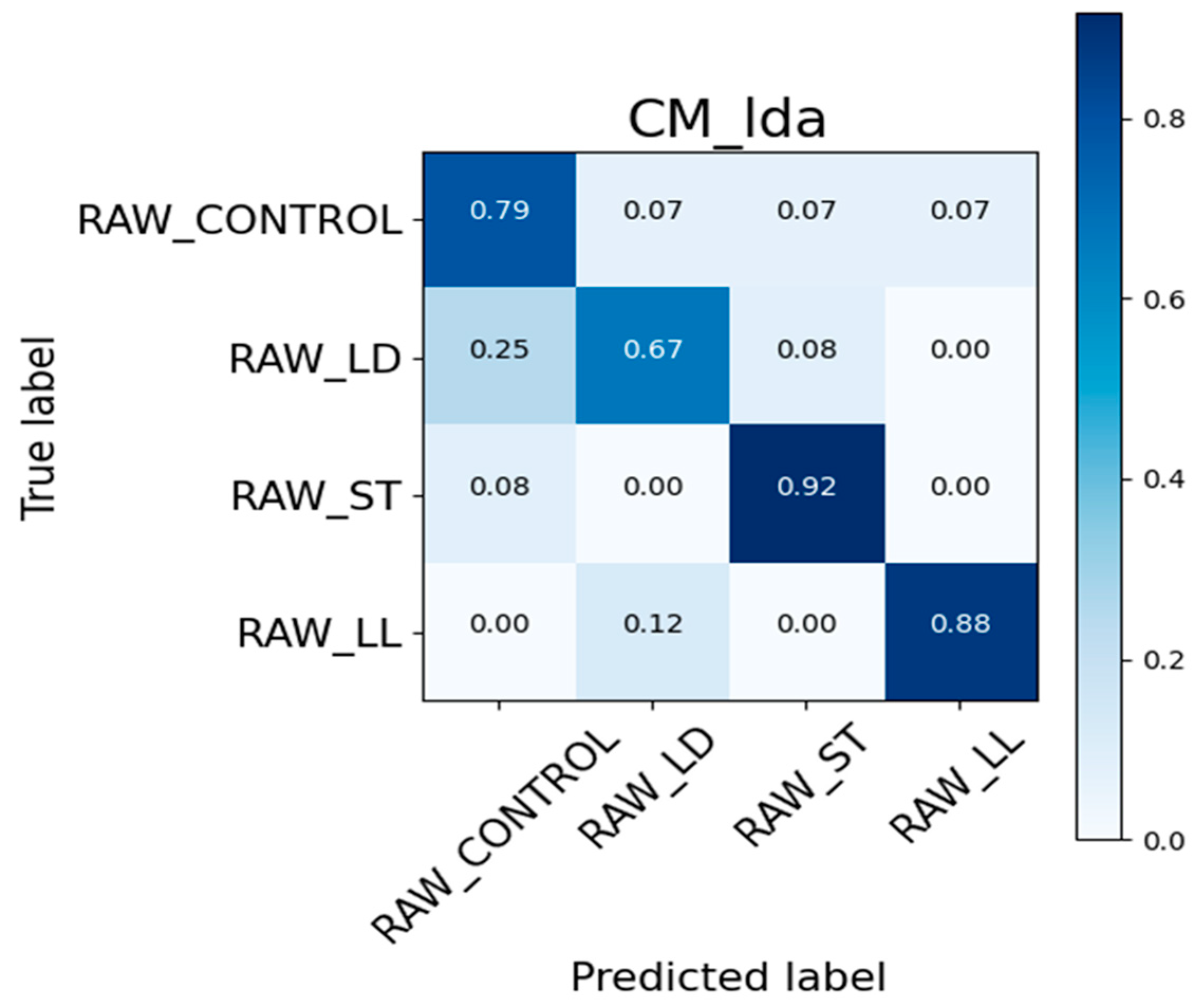
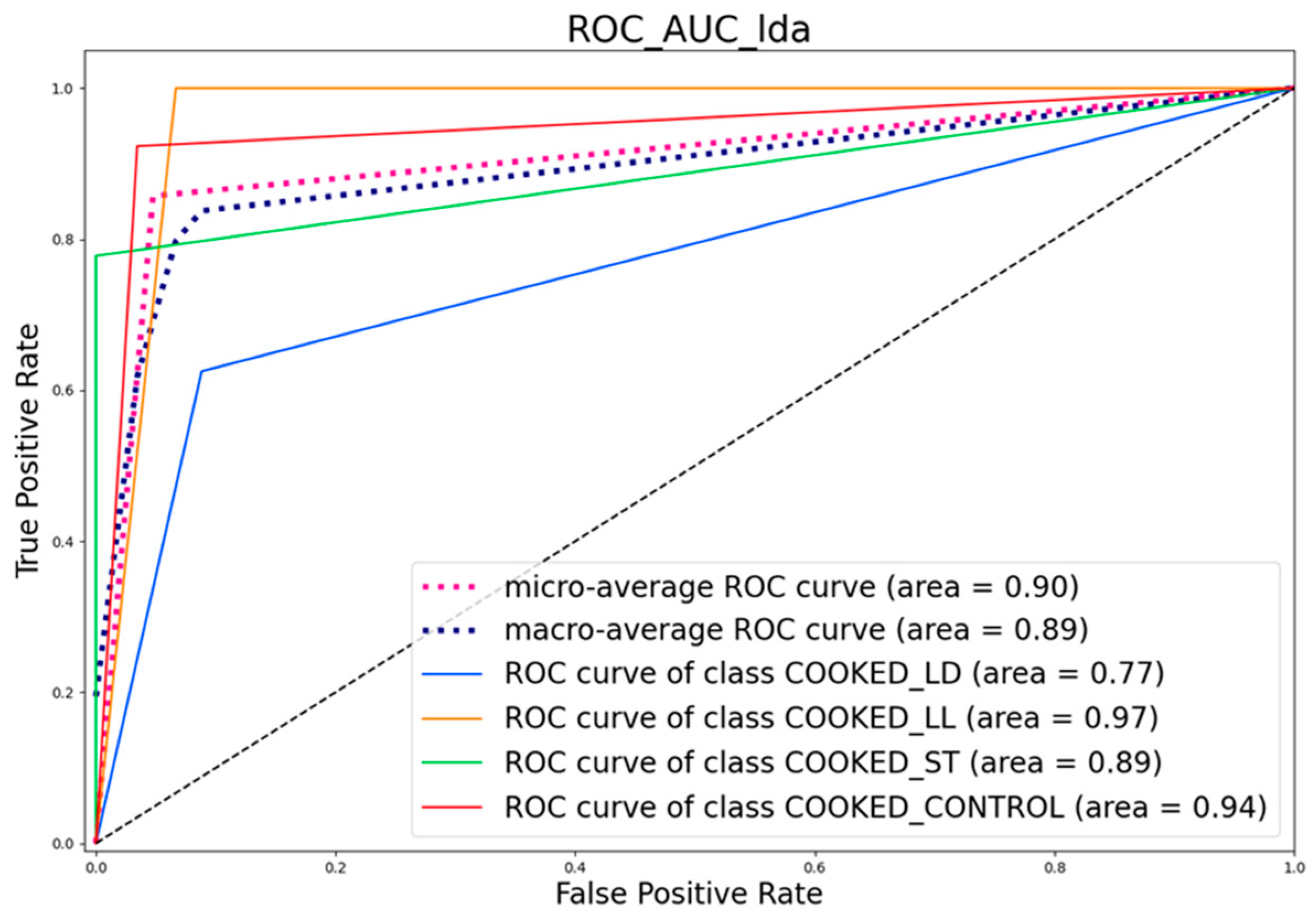
3.4. Confusion Matrix and ROC Curve Images
3.4.1. Confusion Matrix
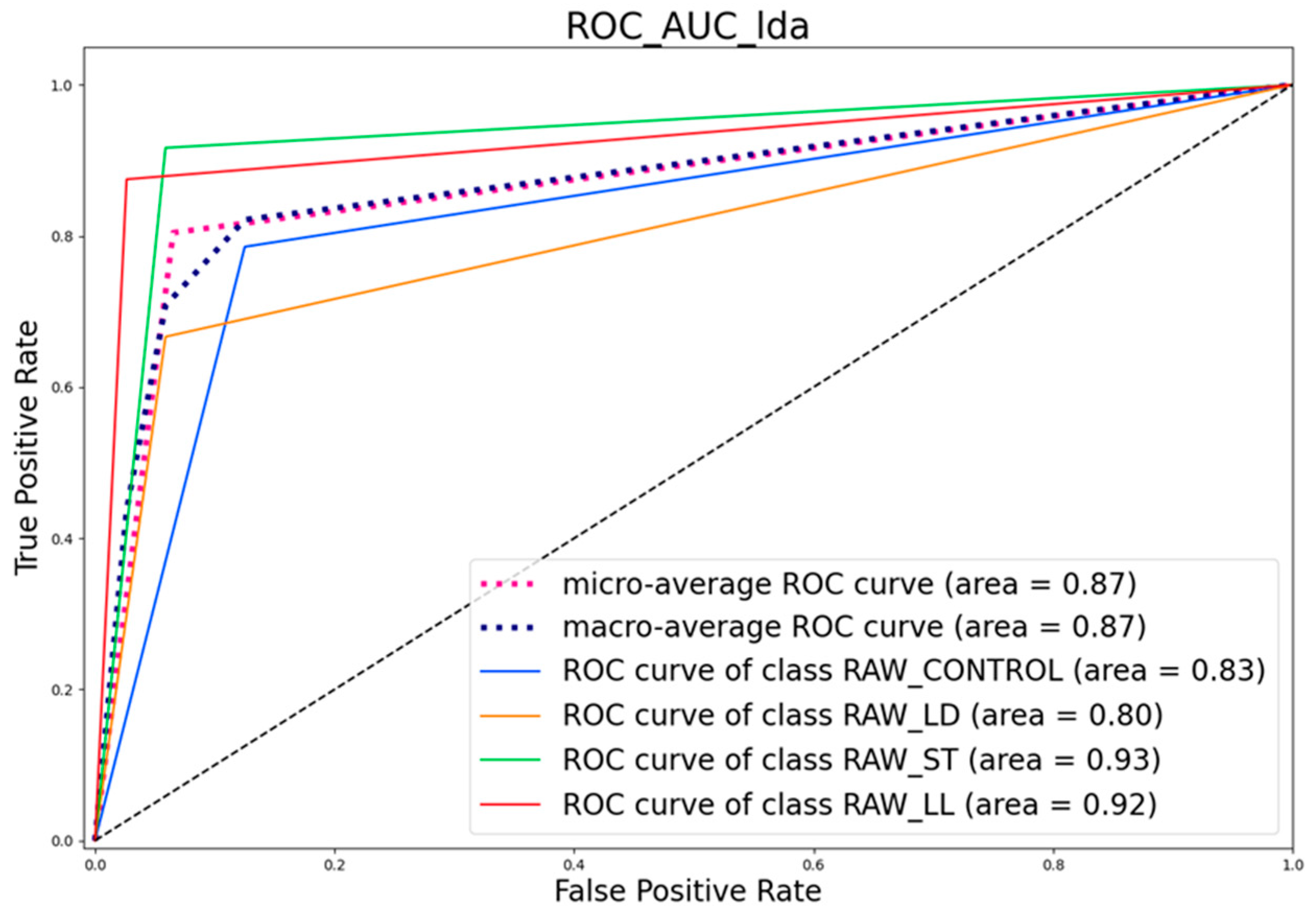
3.4.2. ROC Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Kuang, H.; Tang, C.; Song, J. Edible insects as ingredients in food products: Nutrition, functional properties, allergenicity of insect proteins, and processing modifications. Crit. Rev. Food Sci. Nutr. 2023, 21, 1–23. [Google Scholar] [CrossRef]
- Akintan, O.; Gebremedhin, K.G.; Uyeh, D.D. Animal Feed Formulation—Connecting Technologies to Build a Resilient and Sustainable System. Animals 2024, 14, 1497. [Google Scholar] [CrossRef] [PubMed]
- Baéza, E.; Guillier, L.; Petracci, M. Review: Production factors affecting poultry carcass and meat quality attributes. Animal 2022, 16 (Suppl. S1), 100331. [Google Scholar] [CrossRef]
- De Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The potential of insects as food sources—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. An Array of MOX Sensors and ANNs to Assess Grated Parmigiano Reggiano Cheese Packs’ Compliance with CFPR Guidelines. Biosensors 2020, 10, 47. [Google Scholar] [CrossRef]
- Poeta, E.; Liboà, A.; Mistrali, S.; Núñez-Carmona, E.; Sberveglieri, V. Nanotechnology and E-Sensing for Food Chain Quality and Safety. Sensors 2023, 23, 8429. [Google Scholar] [CrossRef]
- Kumar, A.; Castro, M.; Feller, J.F. Review on Sensor Array-Based Analytical Technologies for Quality Control of Food and Beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Ying, X.; Shi, C.; Jia, Z.; Jia, B. Applications of Gas Sensing in Food Quality Detection: A Review. Foods 2023, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, J.; Zhang, R.; Mao, Y.; Tian, S. Freshness Evaluation of Three Kinds of Meats Based on the Electronic Nose. Sensors 2019, 19, 605. [Google Scholar] [CrossRef]
- El Barbri, N.; Llobet, E.; El Bari, N.; Correig, X.; Bouchikhi, B. Electronic Nose Based on Metal Oxide Semiconductor Sensors as an Alternative Technique for the Spoilage Classification of Red Meat. Sensors 2008, 8, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Xuezhen, H.; Wang, J.; Zheng, H. Discrimination and prediction of multiple beef freshness indexes based on electronic nose. Sens. Actuators B Chem. 2012, 161, 381–389. [Google Scholar]
- Hong, X.; Wang, J. Discrimination and Prediction of Pork Freshness by E-nose. Comput. Comput. Technol. Agric. V IFIP Adv. Inf. Commun. Technol. 2012, 1–14. [Google Scholar] [CrossRef]
- Zambotti, G.; Capuano, R.; Pasqualetti, V.; Soprani, M.; Gobbi, E.; Di Natale, C.; Ponzoni, A. Monitoring Fish Freshness in Real Time under Realistic Conditions through a Single Metal Oxide Gas Sensor. Sensors 2022, 22, 5888. [Google Scholar] [CrossRef]
- Samuel, M.Y.; Kim, J.Y.; Kim, B.S. Prediction of egg freshness during storage using electronic nose. Poult. Sci. 2017, 96, 3733–3746. [Google Scholar]
- Machungo, C.W.; Berna, A.Z.; McNevin, D.; Wang, R.; Harvey, J.; Trowell, S. Evaluation of performance of metal oxide electronic nose for detection of aflatoxin in artificially and naturally contaminated maize. Sens. Actuators B Chem. 2023, 381, 133446. [Google Scholar] [CrossRef]
- Rutolo, M.F.; Clarkson, J.P.; Covington, J.A. The use of an electronic nose to detect early signs of soft-rot infection in potatoes. Biosyst. Eng. 2018, 167, 137–143. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. Application of a Novel S3 Nanowire Gas Sensor Device in Parallel with GC-MS for the Identification of Rind Percentage of Grated Parmigiano Reggiano. Sensors 2018, 18, 1617. [Google Scholar] [CrossRef] [PubMed]
- Liboà, A.; Genzardi, D.; Núñez-Carmona, E.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Sberveglieri, V. Different Diacetyl Perception Detected through MOX Sensors in Real-Time Analysis of Beer Samples. Chemosensors 2023, 11, 147. [Google Scholar] [CrossRef]
- Santos, J.P.; García, M.; Aleixandre, M.; Horrillo, M.C.; Gutiérrez, J.; Sayago, I.; Fernández, M.J.; Arés, L. Electronic nose for the identification of pig feeding and ripening time in Iberian hams. Meat Sci. 2004, 66, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Innovative Sensor Approach to Follow Campylobacter jejuni Development. Biosensors 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Abbatangelo, M.; Zappa, D.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanostructured MOS Sensor for the Detection, Follow up, and Threshold Pursuing of Campylobacter Jejuni Development in Milk Samples. Sensors 2020, 20, 2009. [Google Scholar] [CrossRef]
- Sánchez del Pulgar, J.; Soukoulis, C.; Carrapiso, A.I.; Cappellin, L.; Granitto, P.; Aprea, E.; Romano, A.; Gasperi, F.; Biasioli, F. Effect of the pig rearing system on the final volatile profile of Iberian dry-cured ham as detected by PTR-ToF-MS. Meat Sci. 2013, 93, 420–428. [Google Scholar] [CrossRef]
- Voss, H.G.J.; Stevan, S.L.; Ayub, R.A. Peach growth cycle monitoring using an electronic nose. Comput. Electron. Agric. 2019, 163, 104858. [Google Scholar] [CrossRef]
- Rahimzadeh, H.; Sadeghi, M.; Ghasemi-Varnamkhasti, M.; Mireei, S.A.; Tohidi, M. On the feasibility of metal oxide gas sensor based electronic nose software modification to characterize rice ageing during storage. J. Food Eng. 2019, 245, 1–10. [Google Scholar] [CrossRef]
- -Mariotti, R.; Núñez-Carmona, E.; Genzardi, D.; Pandolfi, S.; Sberveglieri, V.; Mousavi, S. Volatile Olfactory Profiles of Umbrian Extra Virgin Olive Oils and Their Discrimination through MOX Chemical Sensors. Sensors 2022, 22, 7164. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Carmona, N.; Duina, G.; Sberveglieri, V.; Núñez-Carmona, E. Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules 2019, 24, 1457. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Fiorilla, E.; Gariglio, M.; Martinez-Miro, S.; Rosique, C.; Madrid, J.; Montalban, A.; Biasato, I.; Bongiorno, V.; Cappone, E.E.; Soglia, D.; et al. Improving sustainability in autochthonous slow-growing chicken farming: Exploring new frontiers through the use of alternative dietary proteins. J. Clean. Prod. 2024, 434, 140041. [Google Scholar] [CrossRef]
- Bellezza Oddon, S.; Biasato, I.; Imarisio, A.; Pipan, M.; Dekleva, D.; Colombino, E.; Capucchio, M.T.; Meneguz, M.; Bergagna, S.; Barbero, R.; et al. Black soldier fly and yellow mealworm live larvae for broiler chickens: Effects on bird performance and health status. J. Anim. Physiol. Anim. Nutr. 2021, 105, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://faolex.fao.org/docs/pdf/eur90989.pdf (accessed on 23 July 2024).
- Shunping, Z.; Changsheng, X.; Huayao, L.; Zikui, B.; Xianping, X.; Dawen, Z. A reaction model of metal oxide gas sensors and a recognition method by pattern matching. Sens. Actuators B Chem. 2009, 135, 552–559. [Google Scholar]
- Guozhu, Z.; Changsheng, X.; Shunping, Z.; Jianwei, Z.; Tao, L.; Dawen, Z. Temperature-Programmed Technique Accompanied with High-Throughput Methodology for Rapidly Searching the Optimal Operating Temperature of MOX Gas Sensors. ACS Comb. Sci. 2014, 16, 459–465. [Google Scholar]
- Masson, N.; Piedrahita, R.; Hannigan, M. Approach for quantification of metal oxide type semiconductor gas sensors used for ambient air quality monitoring. Sens. Actuators B Chem. 2015, 208, 339–345. [Google Scholar]
- Genzardi, D.; Greco, G.; Núñez-Carmona, E.; Sberveglieri, V. Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors. Sensors 2022, 22, 6247. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, P.; Pardalos, P.M.; Trafalis, T.B. Linear Discriminant Analysis. In Robust Data Mining, SpringerBriefs in Optimization; Springer: New York, NY, USA, 2013. [Google Scholar]
- Sharma, A.; Paliwal, K.K. Linear discriminant analysis for the small sample size problem: An overview. Int. J. Mach. Learn. Cybern. 2015, 6, 443–454. [Google Scholar] [CrossRef]
- Lin, W.; Wu, Z.; Lin, L.; Wen, A.; Li, J. An Ensemble Random Forest Algorithm for Insurance Big Data Analysis. IEEE Access 2017, 5, 16568–16575. [Google Scholar] [CrossRef]
- Flores, M. Chapter 13—The Eating Quality of Meat: III—Flavor. In Lawrie’s Meat Science; Toldrá, F., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 383–417. [Google Scholar]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V.A. Recent Advances in the Chemistry of Strecker Degradation and Amadori Rearrangement: Implications to Aroma and Color Formation. Food Sci. Technol. Res. 2003, 9, 1–6. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Soncin, S.; Chiesa, M.L.; Panseri, S.; Biondi, P.; Cantoni, C. Determination of volatile compounds of precooked prawn (Penaeus vannamei) and cultured gilthead sea bream (Sparus aurata) stored in ice as possible spoilage markers using solid phase microextraction and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2009, 89, 436–442. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.-J.E. Volatilomics for food quality and authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [PubMed]
- Kawai, T. Fish flavor. Crit. Rev. Food Sci. Nutr. 1996, 36, 257–298. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour chemistry of chicken meat: A review. Asian-Australas. J. Anim. Sci. 2013, 26, 732–742. [Google Scholar]
- Madruga, M.S.; Elmore, J.S.; Oruna Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar]
- Tang, J.; Jin, Q.Z.; Shen, G.H.; Ho, C.T.; Chang, S.S. Isolation and identification of volatile compounds from fried chicken. J. Agric. Food Chem. 1983, 31, 1287–1292. [Google Scholar] [CrossRef]
- Kerler, J.; Grosch, W. Character impact odorants of boiled chicken: Changes during refrigerated storage and reheating. Z. Lebensm. Unters. Forsch. 1997, 205, 232–238. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Pablos, F.; Jurado, J.M.; León-Camacho, M. Authentication of fattening diet of Iberian pigs according to their volatile compounds profile from raw subcutaneous fat. Anal. Bioanal. Chem. 2011, 399, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, G.J. Discriminant Analysis and Statistical Pattern Recognition; Wiley Interscience: Hoboken, NJ, USA, 2004. [Google Scholar]




| Type of Sensor | Doping | Working Temperature (°C) |
|---|---|---|
| MOX sensor | SnO2 | 500 °C 500°C |
| MOX sensor MOX sensor | SnO2 + Pd SnO2 + Au | 500°C |
| Features | Description |
|---|---|
| Sharpe Forward 25% | Variability index equivalent to the ratio between the mean and the standard deviation calculated from the beginning of the signal to 25% of it. |
| Sharpe Back 25% | Variability index equivalent to the ratio between the mean and the standard deviation calculated from the end of the signal to 25% of it. |
| Sharpe Forward 50% | Variability index equivalent to the ratio between the mean and the standard deviation calculated from the beginning of the signal to 50% of it. |
| Sharpe back 50% | Variability index equivalent to the ratio between the mean and the standard deviation calculated from the end of the signal to 50% of it. |
| Minimum derivative | Calculation of the minimum derivative of the function in the selected interval. |
| Maximum derivative | Calculation of the maximum derivative of the function in the selected interval. |
| Integral | Calculation of the integral of the function in the selected interval. |
| ∆R | Often called excursion range, this feature represents the difference between the maximum and minimum values observed in the time series. |
| Logarithm of sum | The sum of the natural of the signal. |
| Minimum | The minimum value observed in the time series. |
| Maximum | The maximum value observed in the time series. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genzardi, D.; Núñez Carmona, E.; Poeta, E.; Gai, F.; Caruso, I.; Fiorilla, E.; Schiavone, A.; Sberveglieri, V. Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding. Sensors 2024, 24, 4921. https://doi.org/10.3390/s24154921
Genzardi D, Núñez Carmona E, Poeta E, Gai F, Caruso I, Fiorilla E, Schiavone A, Sberveglieri V. Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding. Sensors. 2024; 24(15):4921. https://doi.org/10.3390/s24154921
Chicago/Turabian StyleGenzardi, Dario, Estefanía Núñez Carmona, Elisabetta Poeta, Francesco Gai, Immacolata Caruso, Edoardo Fiorilla, Achille Schiavone, and Veronica Sberveglieri. 2024. "Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding" Sensors 24, no. 15: 4921. https://doi.org/10.3390/s24154921







