Continuous Temperature Telemonitoring of Patients with COVID-19 and Other Infectious Diseases Treated in Hospital-at-Home: Viture® System Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Ethics
- To be greater than 18 years old;
- To be able to legally give informed consent;
- To have no limitations to the use of the Viture® device due to physical reasons or interference with other devices.
- Monitoring for less than 2 days;
- Less than 4 valid control spot temperature measurements (STMs).
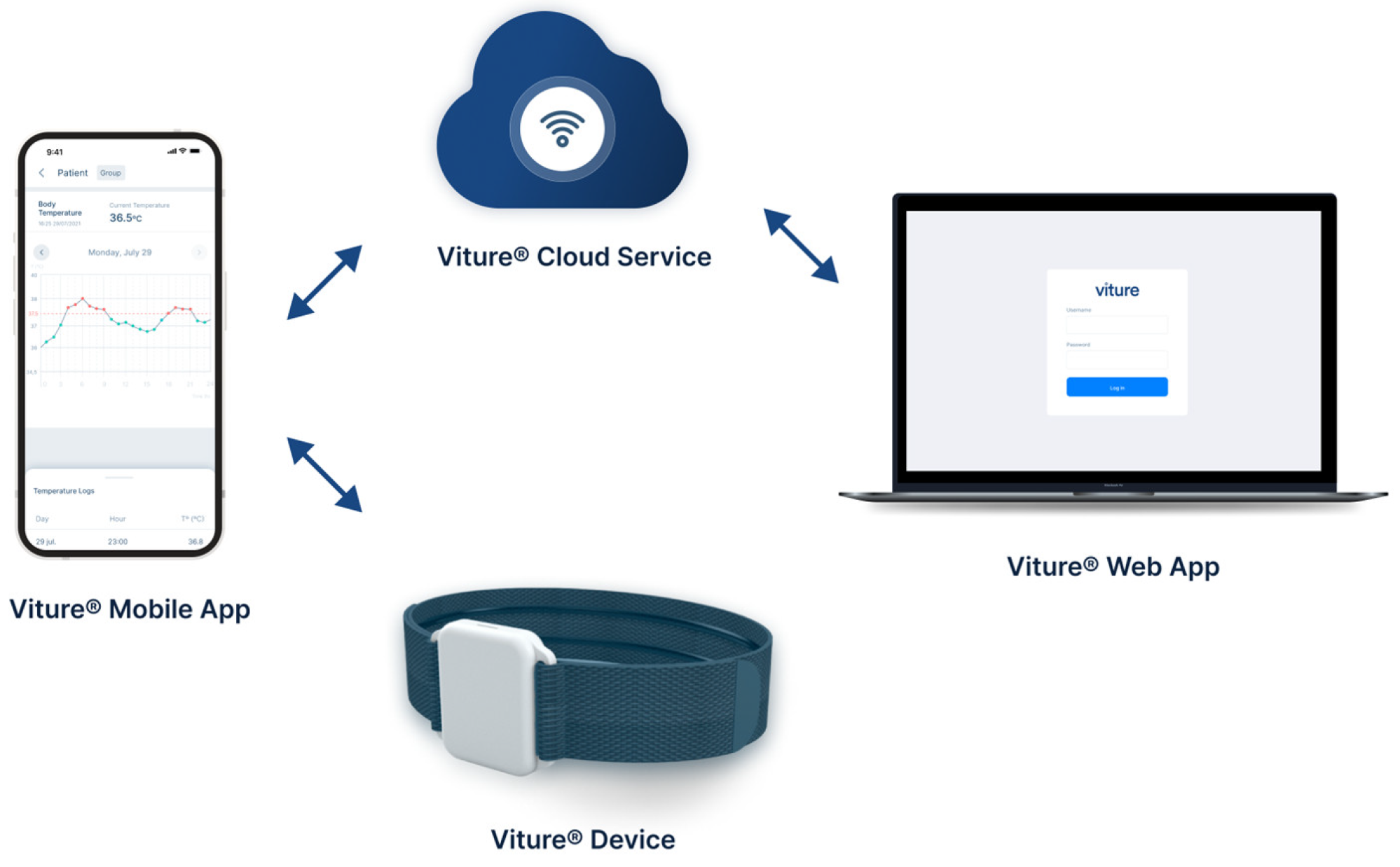
2.3. Description of the Viture® System
2.4. Protocol
- A Viture® device;
- A digital reference thermometer for control STMs (Morris MT-401);
- A smartphone with the Viture® mobile app installed;
- A device user manual in quick guide format;
- A home data collection notebook (HDCN).
2.5. Data Collection and Analysis
2.6. Statistical Analysis
2.7. Analysis of Febrile Episodes
3. Results
3.1. Data Filtering
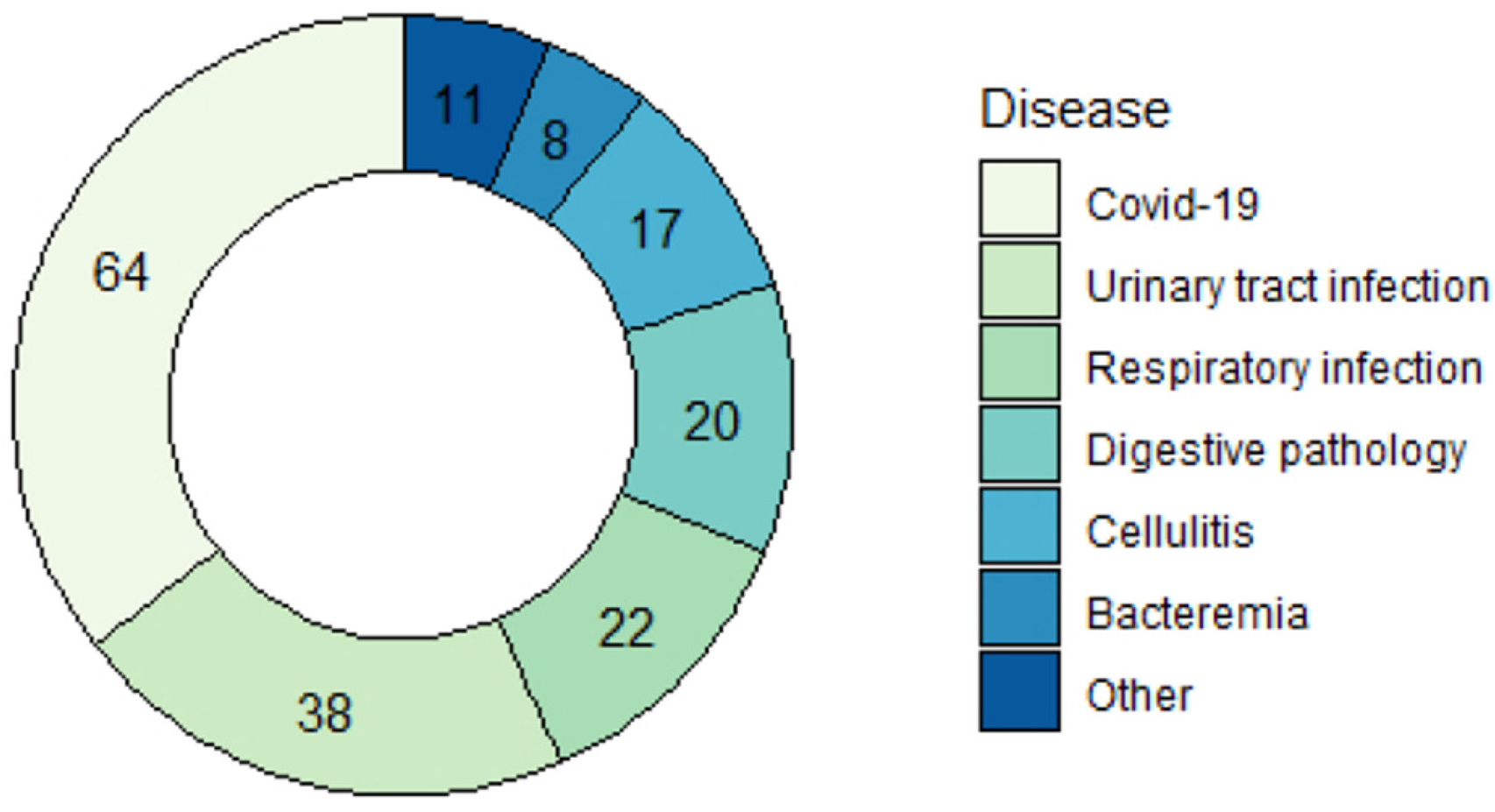
3.2. Patient Characteristics
3.3. Statistical Analysis
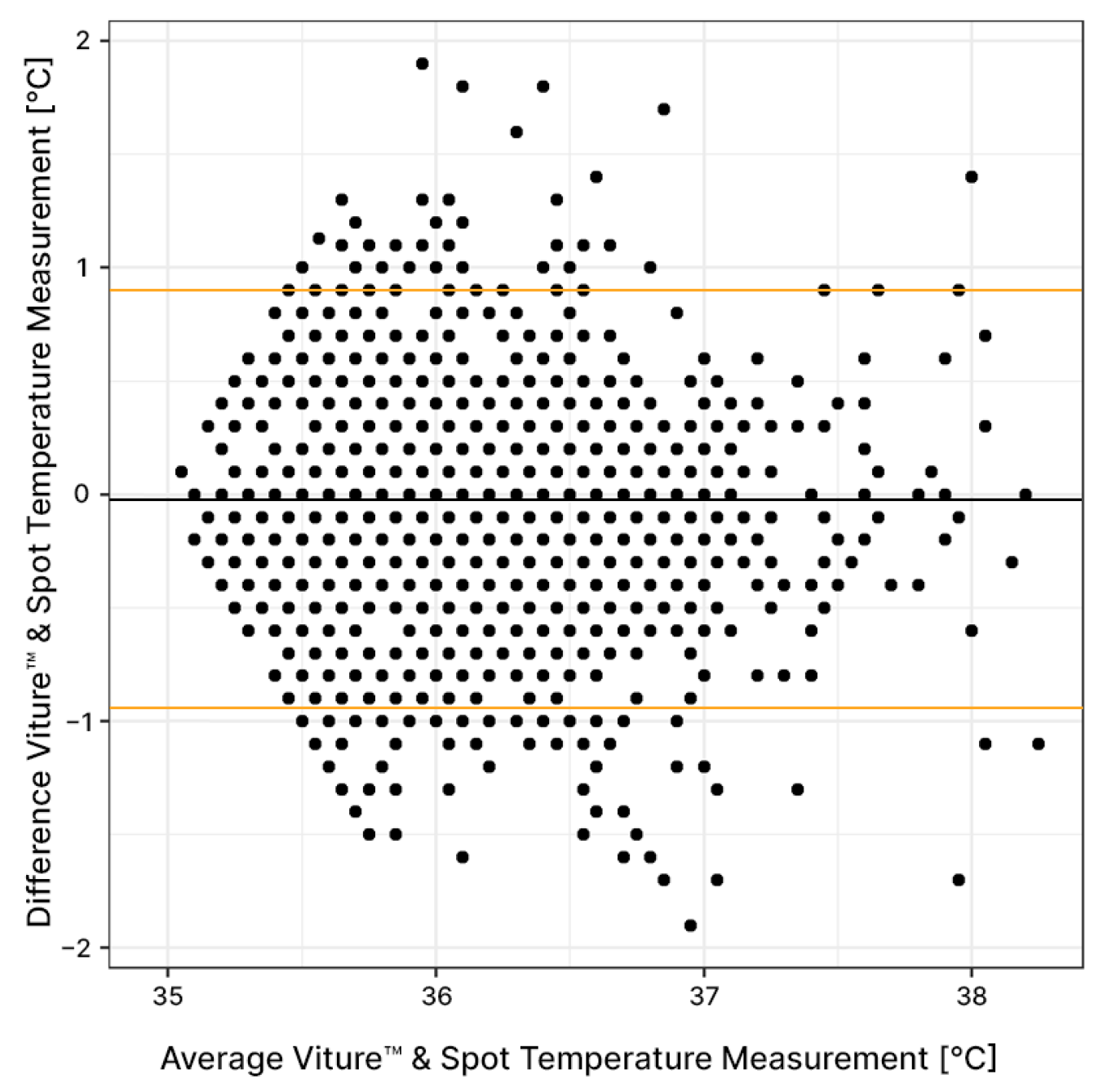
3.3.1. Average Agreement
3.3.2. Agreement between Individuals
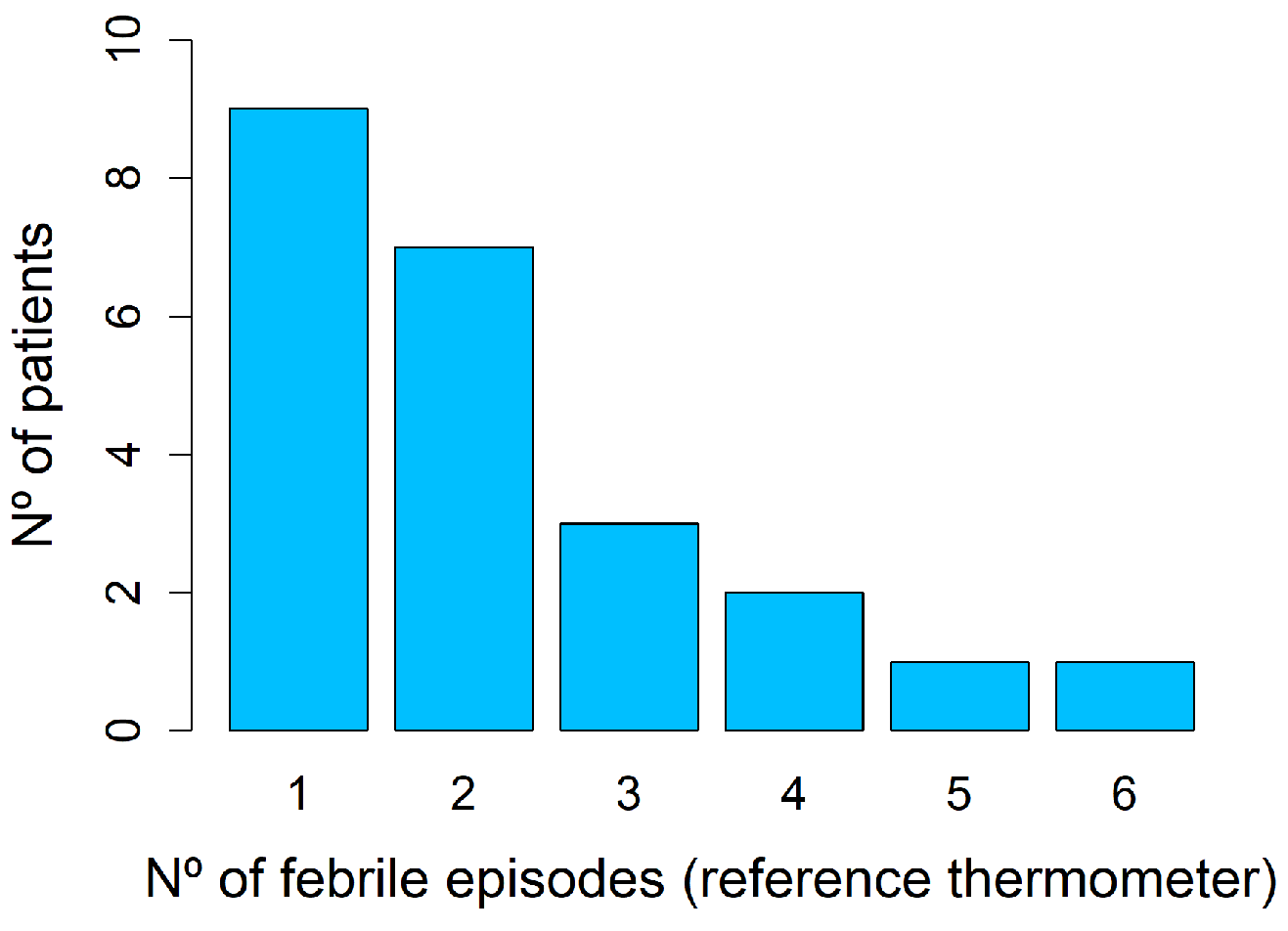
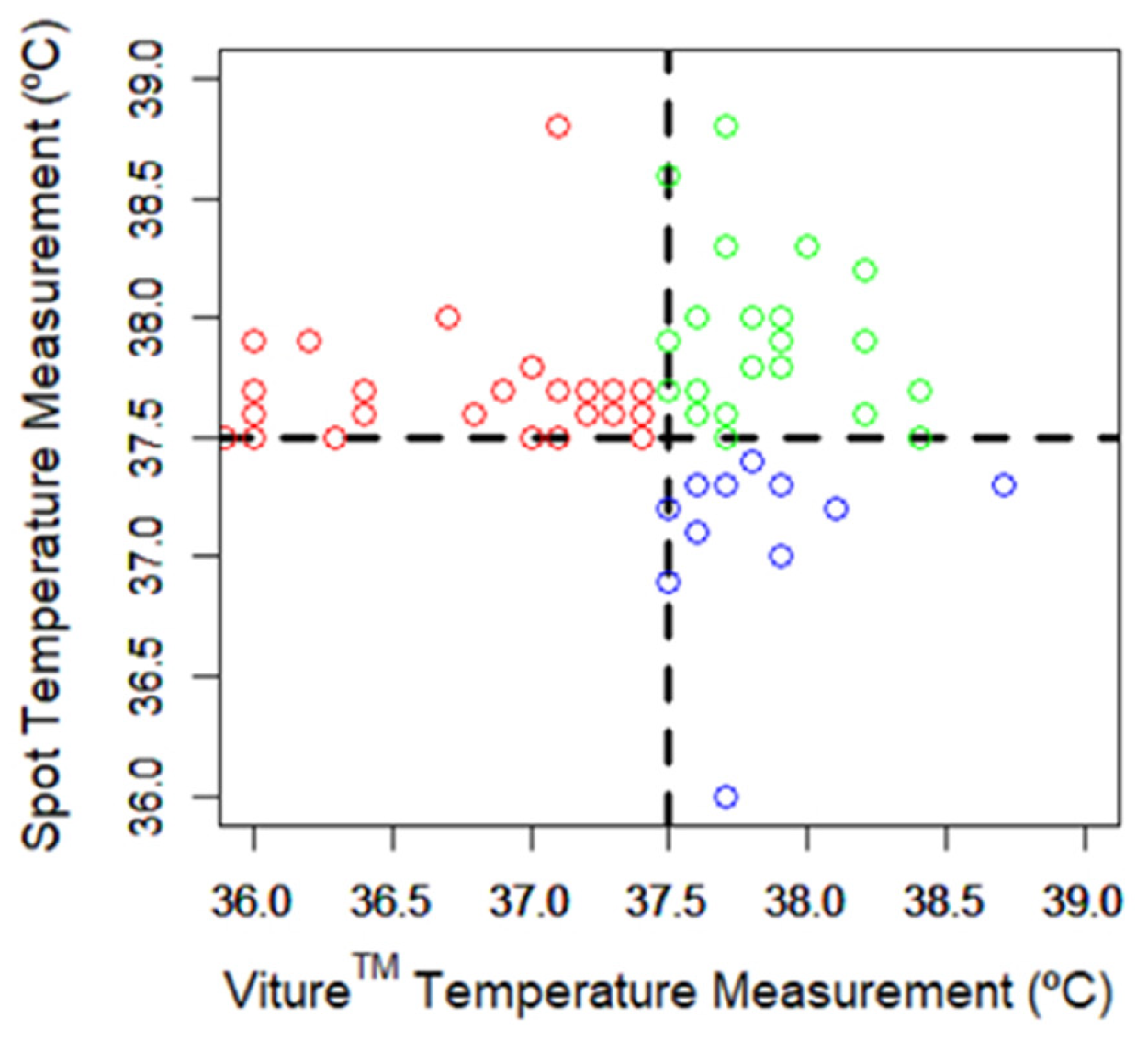
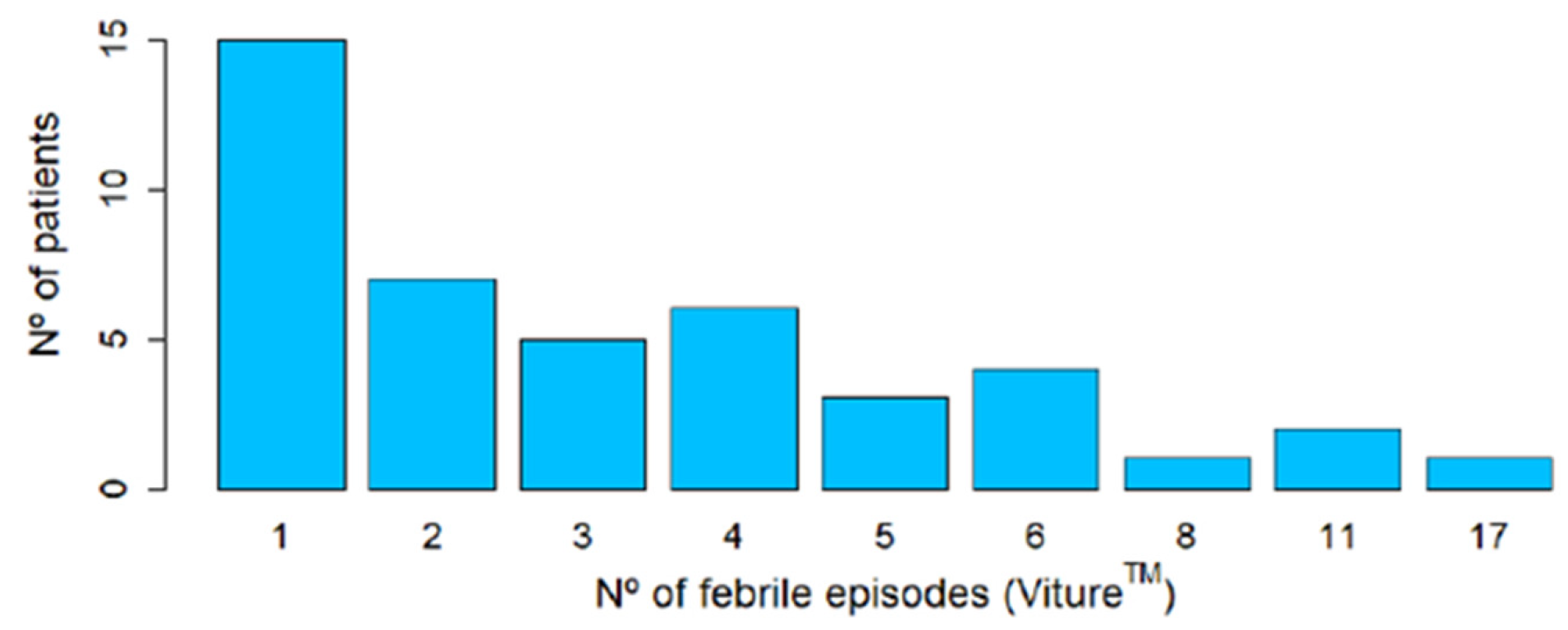
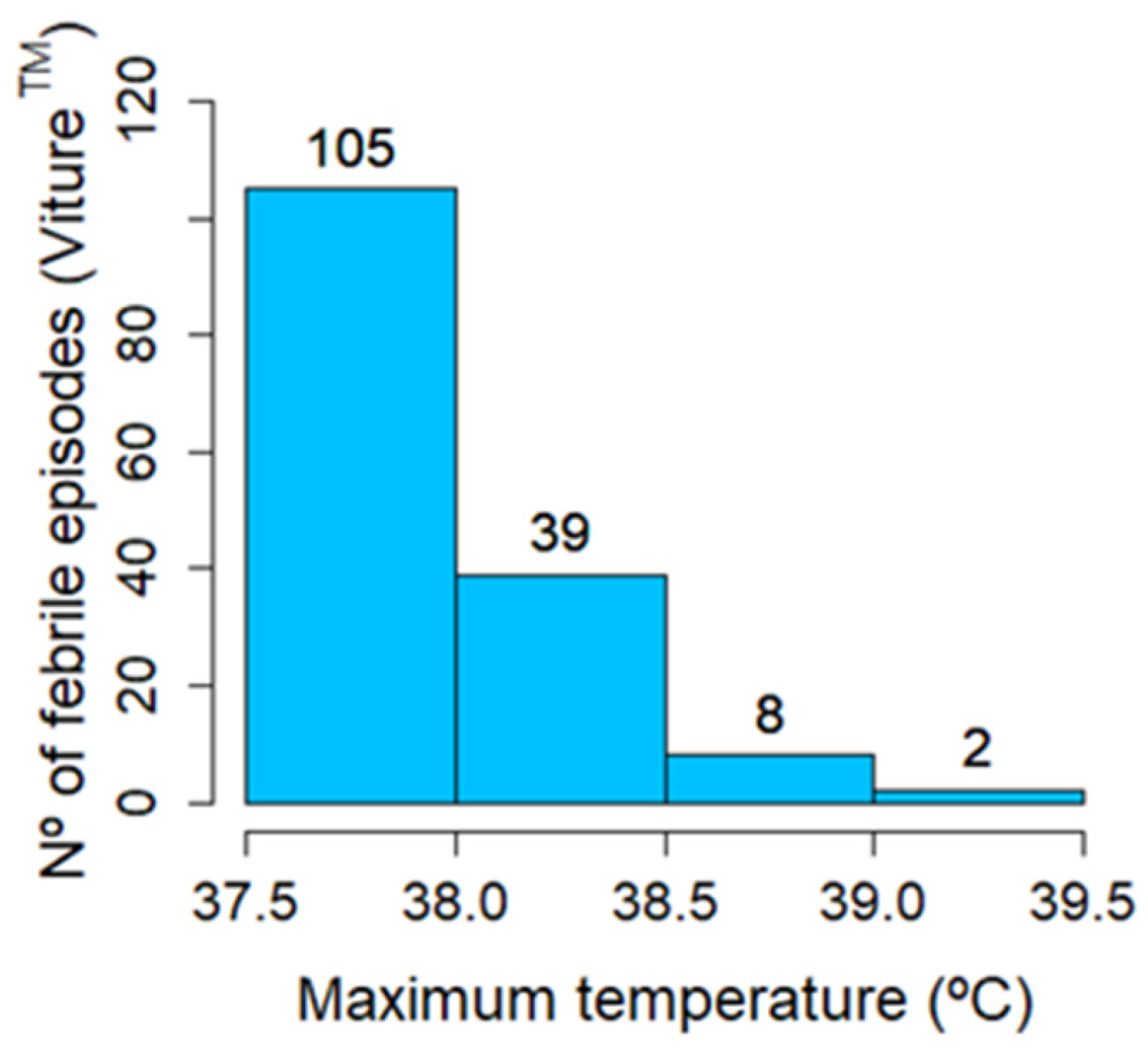
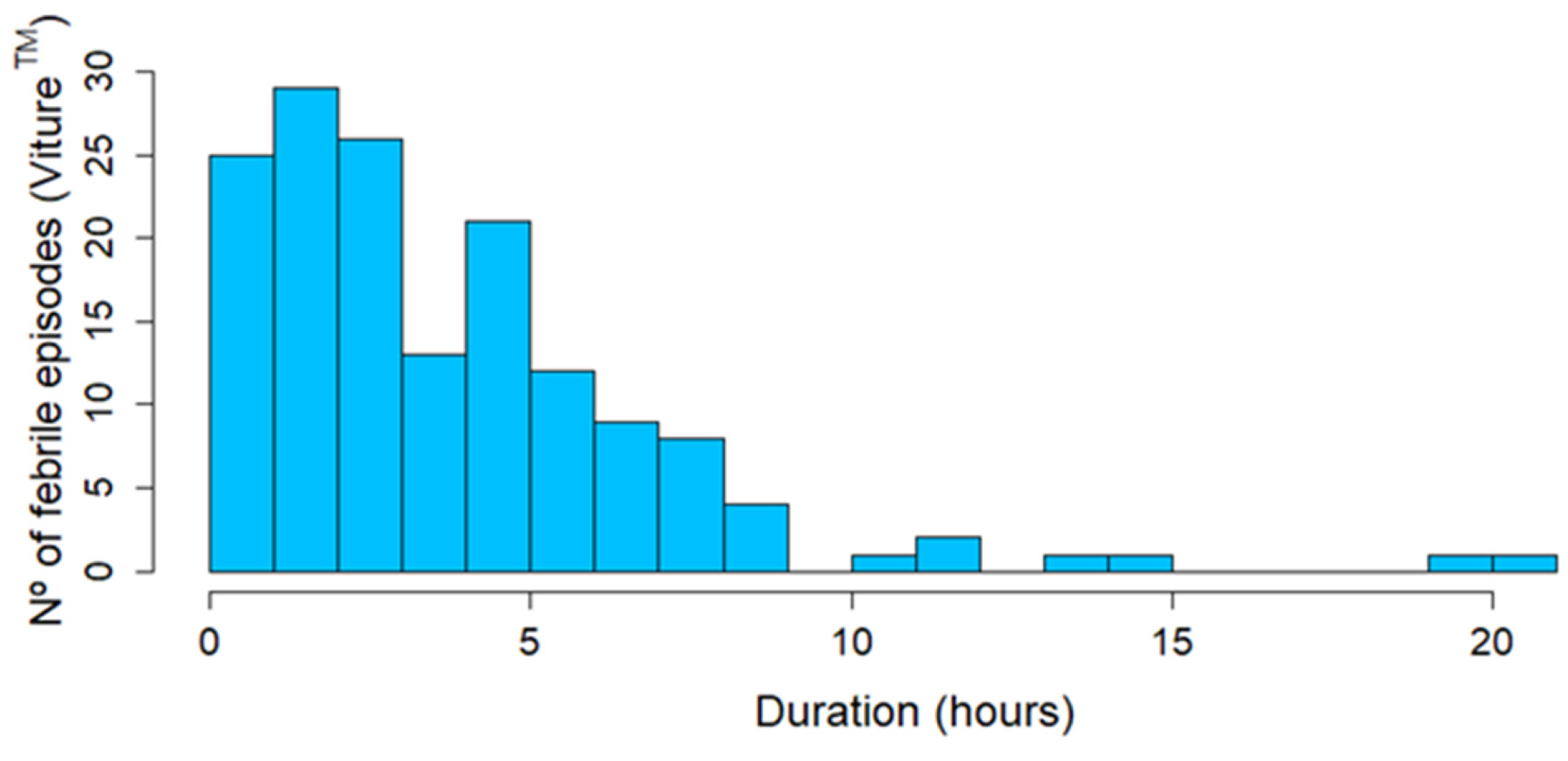
3.4. Analysis of Febrile Episodes
3.4.1. Reference Thermometer Spot Measurements
3.4.2. Comparison between Spot and Viture® Measurements
3.4.3. Viture® Continuous Temperature Measurements
3.5. Incidents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepperd, S.; Iliffe, S. Hospital at home versus in-patient hospital care. Cochrane Database Syst. Rev. 2005, 3, CD000356. [Google Scholar] [CrossRef]
- Mas, M.À.; Santaeugènia, S.J.; Tarazona-Santabalbina, F.J.; Gámez, S.; Inzitari, M. Effectiveness of a Hospital-at-Home Integrated Care Program as Alternative Resource for Medical Crises Care in Older Adults with Complex Chronic Conditions. J. Am. Med. Dir. Assoc. 2018, 19, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Shepperd, S.; Doll, H.; Angus, R.M.; Clarke, M.J.; Iliffe, S.; Kalra, L.; Ricauda, N.A.; Tibaldi, V.; Wilson, A.D. Avoiding hospital admission through provision of hospital care at home: A systematic review and meta-analysis of individual patient data. CMAJ 2009, 180, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Leff, B.; Burton, L.; Mader, S.L.; Naughton, B.; Burl, J.; Inouye, S.K.; Greenough, W.B., III; Guido, S.; Langston, C.; Frick, K.D.; et al. Hospital at home: Feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann. Intern. Med. 2005, 143, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.Q.; Lim, C.W.; Lai, Y.F. Comparison of Hospital-at-Home models: A systematic review of reviews. BMJ Open 2021, 11, e043285. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Garcia, L.; Benito, N.; Cervera, C.; Sala, M.; Olivera, A.; Soriano, A.; Robau, M.; Gatell, J.M.; Miro, J.M. Specialized home care for infectious disease. Experience from 1995 to 2002. Enferm. Infecc. Microbiol. Clin. 2007, 25, 429–436. [Google Scholar] [CrossRef]
- Sriskandarajah, S.; Hobbs, J.; Roughead, E.; Ryan, M.; Reynolds, K. Safety and effectiveness of ‘hospital in the home’ and ‘outpatient parenteral antimicrobial therapy’ in different age groups: A systematic review of observational studies. Int. J. Clin. Pract. 2018, 72, e13216. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, A.F.; Wai, A.O.; Frighetto, L.; Marra, C.A.; Ferreira, B.M.; Chase, M.L.; Nicol, R.E.; Leong, C.A.; Tomlinson, S.; Jewesson, P.J. Quality-of-life assessment in an outpatient parenteral antibiotic program. Ann. Pharmacother. 2002, 36, 1851–1855. [Google Scholar] [CrossRef]
- Nogues, X.; Sanchez-Martinez, F.; Castells, X.; Diez-Perez, A.; Sabate, R.A.; Petit, I.; Brase, A.; Horcajada, J.P.; Güerri-Fernández, R.; Pascual, J. Hospital-at-Home Expands Hospital Capacity During COVID-19 Pandemic. J. Am. Med. Dir. Assoc. 2021, 22, 939–942. [Google Scholar] [CrossRef]
- Siu, A.L.; Zimbroff, R.M.; Federman, A.D.; DeCherrie, L.V.; Garrido, M.; Morano, B.; Lubetsky, S.; Catalan, E.; Leff, B. The effect of adapting Hospital at Home to facilitate implementation and sustainment on program drift or voltage drop. BMC Health Serv. Res. 2019, 19, 264. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; McAlister, F.A.; Stewart, S.; Cleland, J.G. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur. J. Heart Fail. 2011, 13, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.S.; Soma, M.; Pulluri, D.; Nemali, N.T.; Brooks, M. The effectiveness of telemedicine in the management of chronic heart disease—A systematic review. JRSM Open 2017, 8, 2054270416681747. [Google Scholar] [CrossRef] [PubMed]
- Eron, L.; King, P.; Marineau, M.; Yonehara, C. Treating acute infections by telemedicine in the home. Clin. Infect. Dis. 2004, 39, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Bal-Alvarado, M.; Guerra, F.S.; Ares-Rico, R.; Suárez-Gil, R.; Rodríguez-Álvarez, A.; Pérez-López, A.; Casariego-Vales, E.; TELEA COVID-19 Team; Rial, Á.F.; et al. Monitoring of COVID-19 patients by telemedicine with telemonitoring. Rev. Clin. Esp. 2020, 220, 472–479. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P.; McGovern, K.M.; Perreard, I.M.; Huang, V.; Moss, L.B.; Blike, G.T. Inpatient Respiratory Arrest Associated with Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J. Patient Saf. 2021, 17, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Pronovost, P.J.; Cole, M.D.; Hughes, R.M. Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit. JAMA 2022, 327, 1125–1126. [Google Scholar] [CrossRef]
- Haveman, M.E.; van Rossum, M.C.; Vaseur, R.M.; van der Riet, C.; Schuurmann, R.C.; Hermens, H.J.; de Vries, J.P.P.; Tabak, M. Continuous Monitoring of Vital Signs with Wearable Sensors During Daily Life Activities: Validation Study. JMIR Form. Res. 2022, 6, e30863. [Google Scholar] [CrossRef]
- Jiang, W.; Majumder, S.; Kumar, S.; Subramaniam, S.; Li, X.; Khedri, R.; Mondal, T.; Abolghasemian, M.; Satia, I.; Deen, M.J. A Wearable Tele-Health System towards Monitoring COVID-19 and Chronic Diseases. IEEE Rev. Biomed. Eng. 2022, 15, 61–84. [Google Scholar] [CrossRef]
- Flora, C.; Tyler, J.; Mayer, C.; Warner, D.E.; Khan, S.N.; Gupta, V.; Lindstrom, R.; Mazzoli, A.; Rozwadowski, M.; Braun, T.M.; et al. High-frequency temperature monitoring for early detection of febrile adverse events in patients with cancer. Cancer Cell 2021, 39, 1167–1168. [Google Scholar] [CrossRef]
- Varela, M.; Cuesta, D.; Madrid, J.A.; Churruca, J.; Miro, P.; Ruiz, R.; Martinez, C. Holter monitoring of central and peripheral temperature: Possible uses and feasibility study in outpatient settings. J. Clin. Monit. Comput. 2009, 23, 209–216. [Google Scholar] [CrossRef]
- Ajčević, M.; Buoite Stella, A.; Furlanis, G.; Caruso, P.; Naccarato, M.; Accardo, A.; Manganotti, P. A Novel Non-Invasive Thermometer for Continuous Core Body Temperature: Comparison with Tympanic Temperature in an Acute Stroke Clinical Setting. Sensors 2022, 22, 4760. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Eckmann, J.; Strohmayer, K.; Koele, W.; Federspiel, M.; Schenk, M.; Weiss, G.; Krause, R. Investigation of Non-invasive Continuous Body Temperature Measurements in a Clinical Setting Using an Adhesive Axillary Thermometer (SteadyTemp(R)). Front. Digit. Health 2021, 3, 794274. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Gao, M.; Wang, Y.; Bai, Y.; Xu, R.; Gong, R. Evaluation of a wearable wireless device with artificial intelligence, iThermonitor WT705, for continuous temperature monitoring for patients in surgical wards: A prospective comparative study. BMJ Open 2020, 10, e039474. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.; Nallathambi, G.; Moghadam, R.; Aga, A. Fully Disposable Wireless Patch Sensor for Continuous Remote Patient Monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: New York, NY, USA, 2018. [Google Scholar]
- Verma, N.; Haji-Abolhassani, I.; Ganesh, S.; Vera-Aguilera, J.; Paludo, J.; Heitz, R.; Markovic, S.N.; Kulig, K.; Ghoreyshi, A. A Novel Wearable Device for Continuous Temperature Monitoring & Fever Detection. IEEE J. Transl. Eng. Health Med. 2021, 9, 2700407. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, D.; Spielhagen, P.; Siegmann, A.; Gercekcioglu, A.; Gorgass, J.; Henze, S.; Kolar, Y.; Koneberg, F.; Kukkonen, S.; McGowan, H.; et al. Remote monitoring of COVID-19 positive high-risk patients in domestic isolation: A feasibility study. PLoS ONE 2021, 16, e0257095. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.G.; Yo, J.H.; Karschimkus, C.; Volpato, F.; Christov, S.; Smith, E.R.; Hewitson, T.D.; Worth, L.J.; Champion De Crespigny, P. Monitoring skin temperature at the wrist in hospitalised patients may assist in the detection of infection. Intern. Med. J. 2020, 50, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Smarr, B.L.; Aschbacher, K.; Fisher, S.M.; Chowdhary, A.; Dilchert, S.; Puldon, K.; Rao, A.; Hecht, F.M.; Mason, A.E. Feasibility of continuous fever monitoring using wearable devices. Sci. Rep. 2020, 10, 21640. [Google Scholar] [CrossRef] [PubMed]
- Hospital Universitario de Navarra & Navarrabiomed. (ClinicalTrials.gov, 2023). Available online: https://clinicaltrials.gov/study/NCT05959928 (accessed on 1 August 2024).
- Caldwell, A.R. SimplyAgree: An R package and jamovi Module for Simplifying Agreement and Reliability Analyses. J. Open Source Softw. 2022, 7, 4148. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Yuan, S.; Jiang, S.C.; Li, Z.L. Iterative Monitoring of Temperatures in Confinement for Early Screening of SARS-CoV-2 Infections. Front. Med. 2020, 7, 564377. [Google Scholar] [CrossRef] [PubMed]
- Sund-Levander, M.; Grodzinsky, E. Time for a change to assess and evaluate body temperature in clinical practice. Int. J. Nurs. Pract. 2009, 15, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lodha, R.; Mukerji, N.; Sinha, N.; Pandey, R.M.; Jain, Y. Is axillary temperature an appropriate surrogate for core temperature? Indian J. Pediatr. 2000, 67, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, E.S.; McCraw, J.; Ganesh, S.; Lang, S.; Mariotti, M.; Evered, M.; Ghoreyshi, A.; Williamson, J.; Zamora, Z. Feasibility, Acceptability, and Performance of a Continuous Temperature Monitor in Older Adults and Staff in Congregate-Living Facilities. J. Am. Med. Dir. Assoc. 2022, 23, 1729–1735.e1. [Google Scholar] [CrossRef] [PubMed]
- Khatsenko, K.; Khin, Y.; Maibach, H. Allergic Contact Dermatitis to Components of Wearable Adhesive Health Devices. Dermatitis 2020, 31, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.P.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Eldaly, A.S.; Haider, C.R.; Felton, C.L.; Paulson, M.R.; Maniaci, M.J.; et al. Benefits and Challenges of Remote Patient Monitoring as Perceived by Health Care Practitioners: A Systematic Review. Perm. J. 2023, 27, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Kallinich, T.; Gattorno, M.; Grattan, C.E.; De Koning, H.D.; Traidl-Hoffmann, C.; Feist, E.; Krause, K.; Lipsker, D.; Navarini, A.A.; Maurer, M.; et al. Unexplained recurrent fever: When is autoinflammation the explanation? Allergy 2013, 68, 285–296. [Google Scholar] [CrossRef]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesma-Sánchez, L.; Ruiz-Castellano, M.; Romero-Roldán, A.; Álvarez-García, L.; Morrás-Gómez, M.; Tabar-Liberal, I.; Pulido-Fontes, M.; Salmón-García, B. Continuous Temperature Telemonitoring of Patients with COVID-19 and Other Infectious Diseases Treated in Hospital-at-Home: Viture® System Validation. Sensors 2024, 24, 5027. https://doi.org/10.3390/s24155027
Sesma-Sánchez L, Ruiz-Castellano M, Romero-Roldán A, Álvarez-García L, Morrás-Gómez M, Tabar-Liberal I, Pulido-Fontes M, Salmón-García B. Continuous Temperature Telemonitoring of Patients with COVID-19 and Other Infectious Diseases Treated in Hospital-at-Home: Viture® System Validation. Sensors. 2024; 24(15):5027. https://doi.org/10.3390/s24155027
Chicago/Turabian StyleSesma-Sánchez, Laura, María Ruiz-Castellano, Ainara Romero-Roldán, Laura Álvarez-García, Marta Morrás-Gómez, Idoia Tabar-Liberal, Marta Pulido-Fontes, and Belén Salmón-García. 2024. "Continuous Temperature Telemonitoring of Patients with COVID-19 and Other Infectious Diseases Treated in Hospital-at-Home: Viture® System Validation" Sensors 24, no. 15: 5027. https://doi.org/10.3390/s24155027
APA StyleSesma-Sánchez, L., Ruiz-Castellano, M., Romero-Roldán, A., Álvarez-García, L., Morrás-Gómez, M., Tabar-Liberal, I., Pulido-Fontes, M., & Salmón-García, B. (2024). Continuous Temperature Telemonitoring of Patients with COVID-19 and Other Infectious Diseases Treated in Hospital-at-Home: Viture® System Validation. Sensors, 24(15), 5027. https://doi.org/10.3390/s24155027





