Using Dynamic Laser Speckle Imaging for Plant Breeding: A Case Study of Water Stress in Sunflowers
Abstract
:1. Introduction
2. Materials and Methods
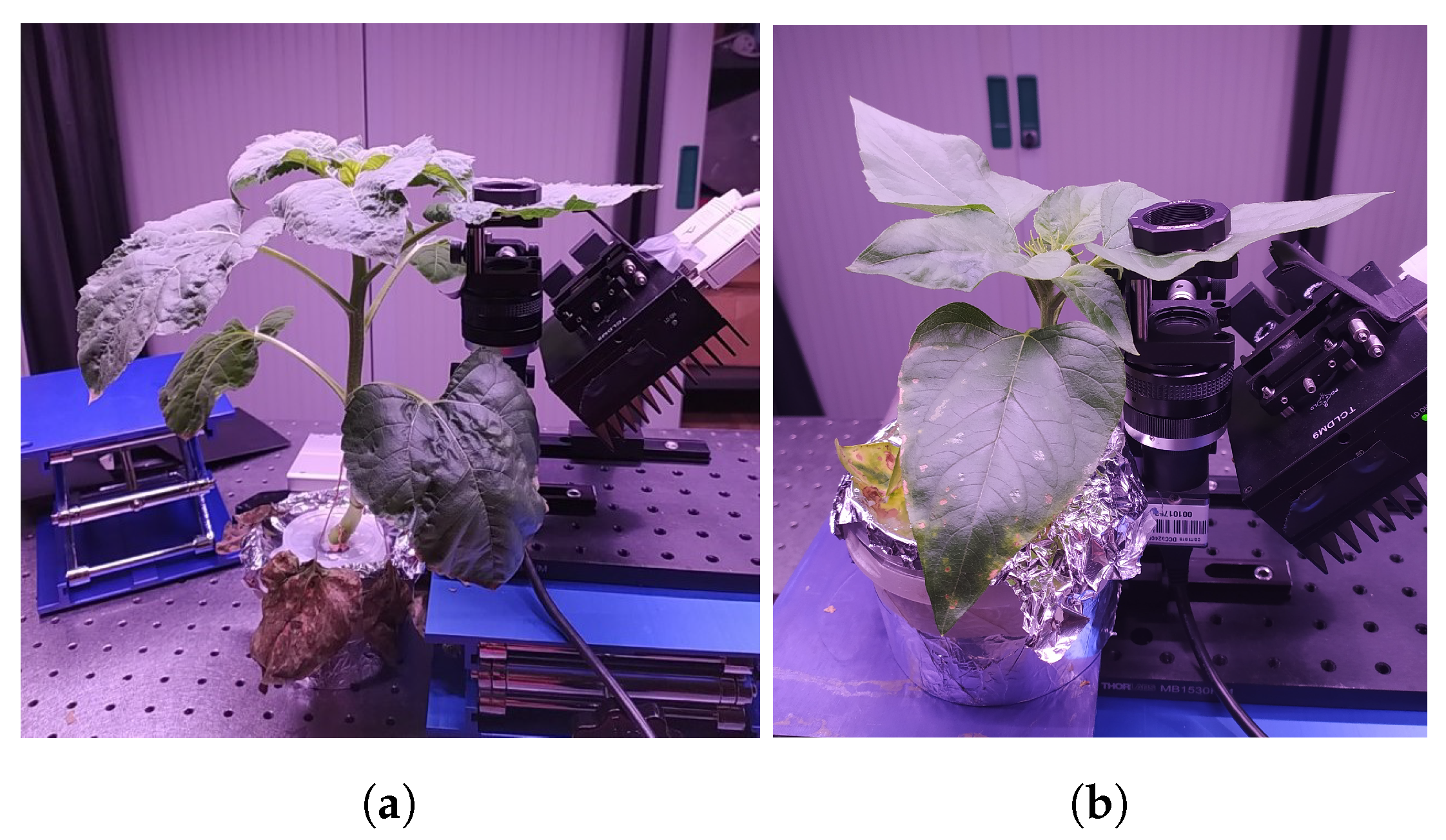
2.1. Experimental Design
2.2. Biospeckle Acquisitions
2.3. Data Analysis
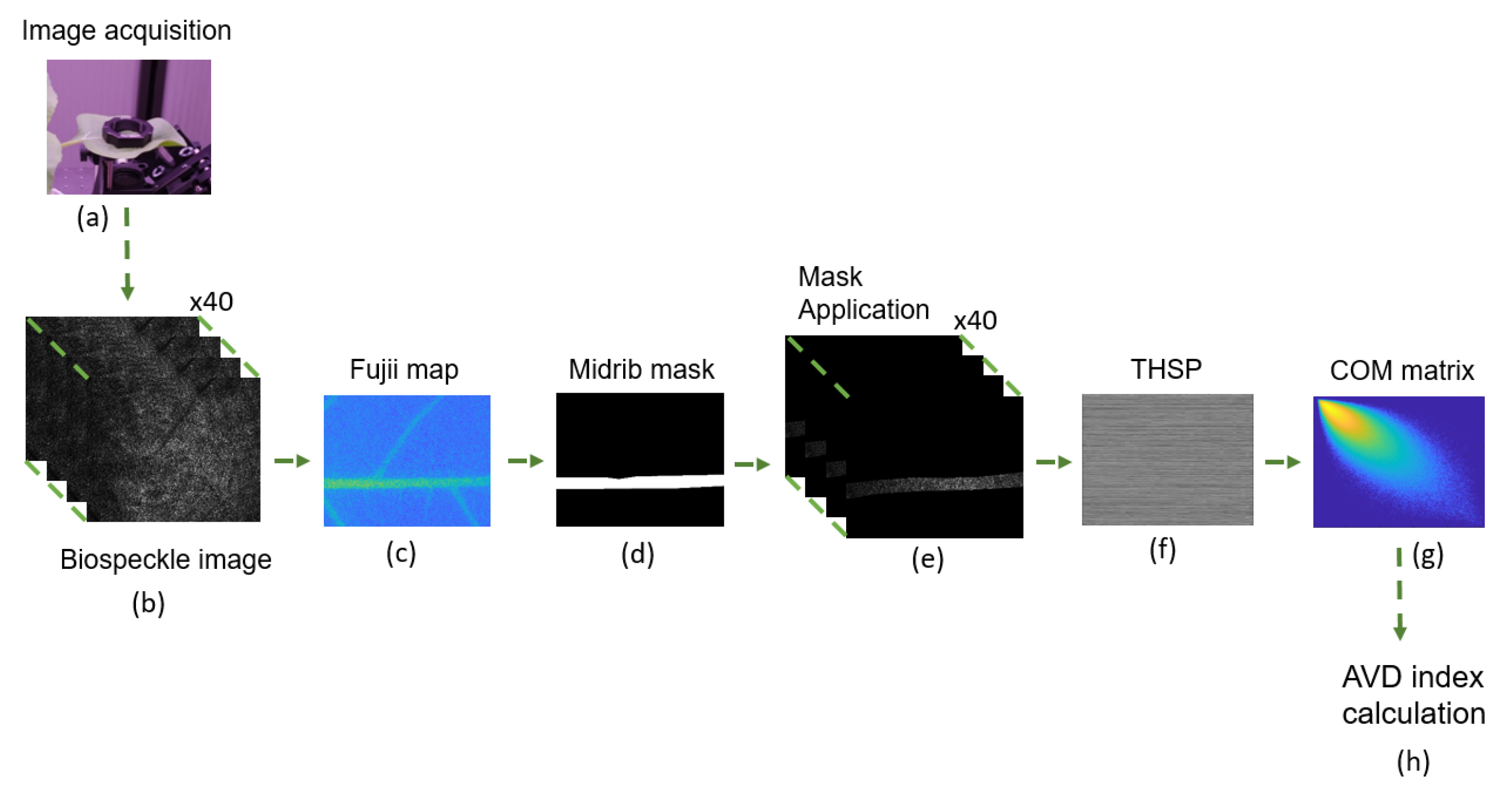
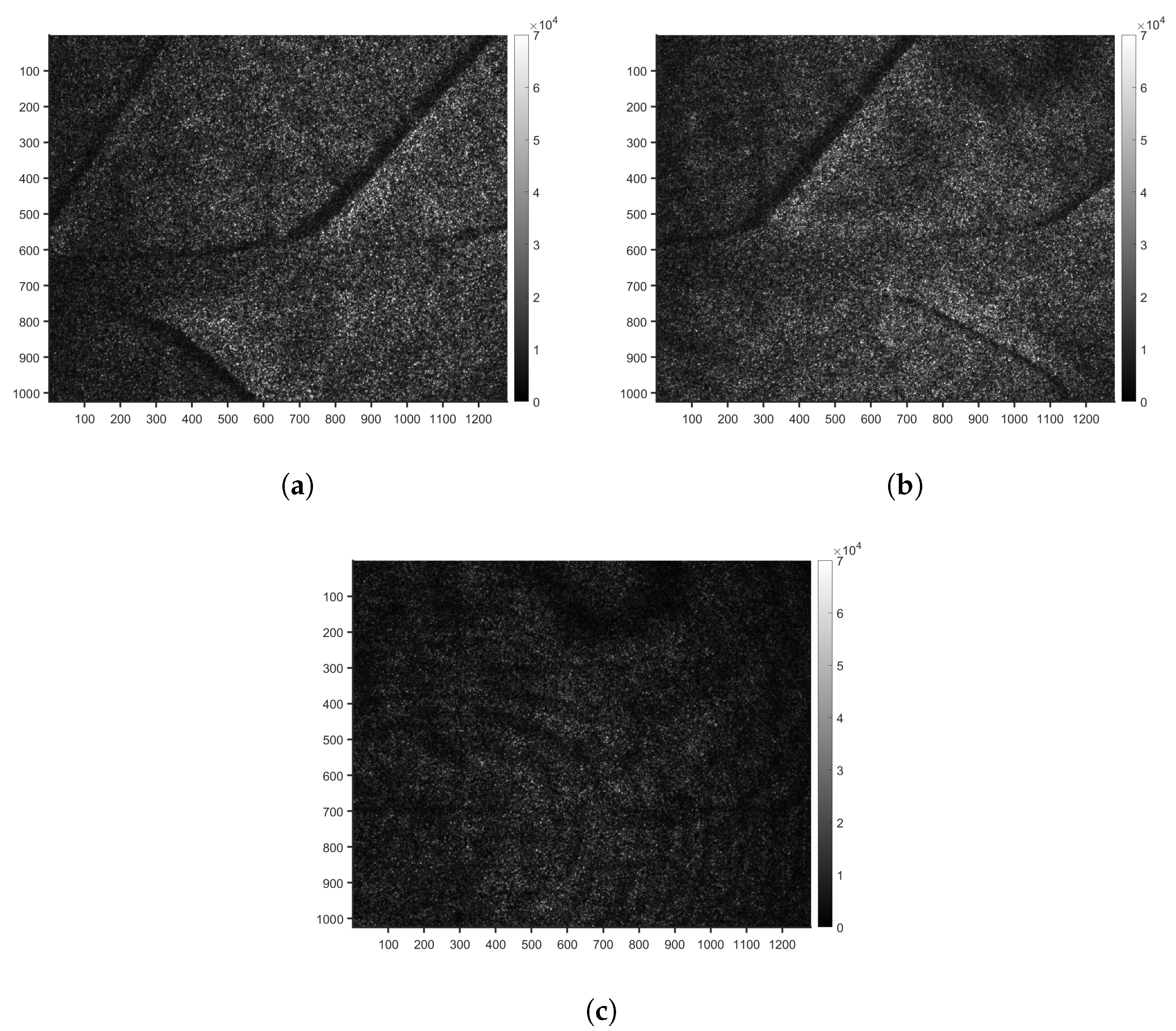
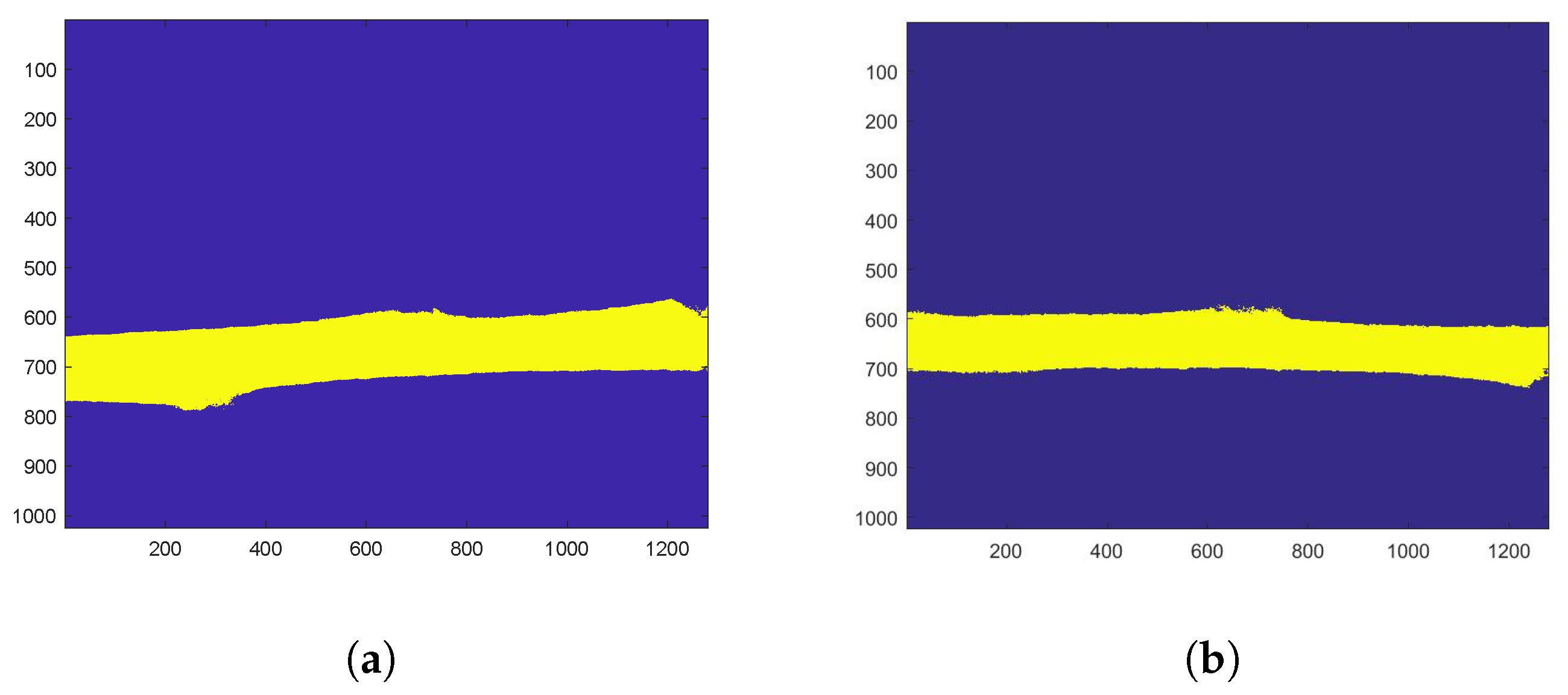
2.3.1. Activity Maps to Identify a Region of Interest
2.3.2. AVD Indicator Computation
2.3.3. Signal Analysis
3. Results
3.1. Identification of a Homogeneous Area of Interest
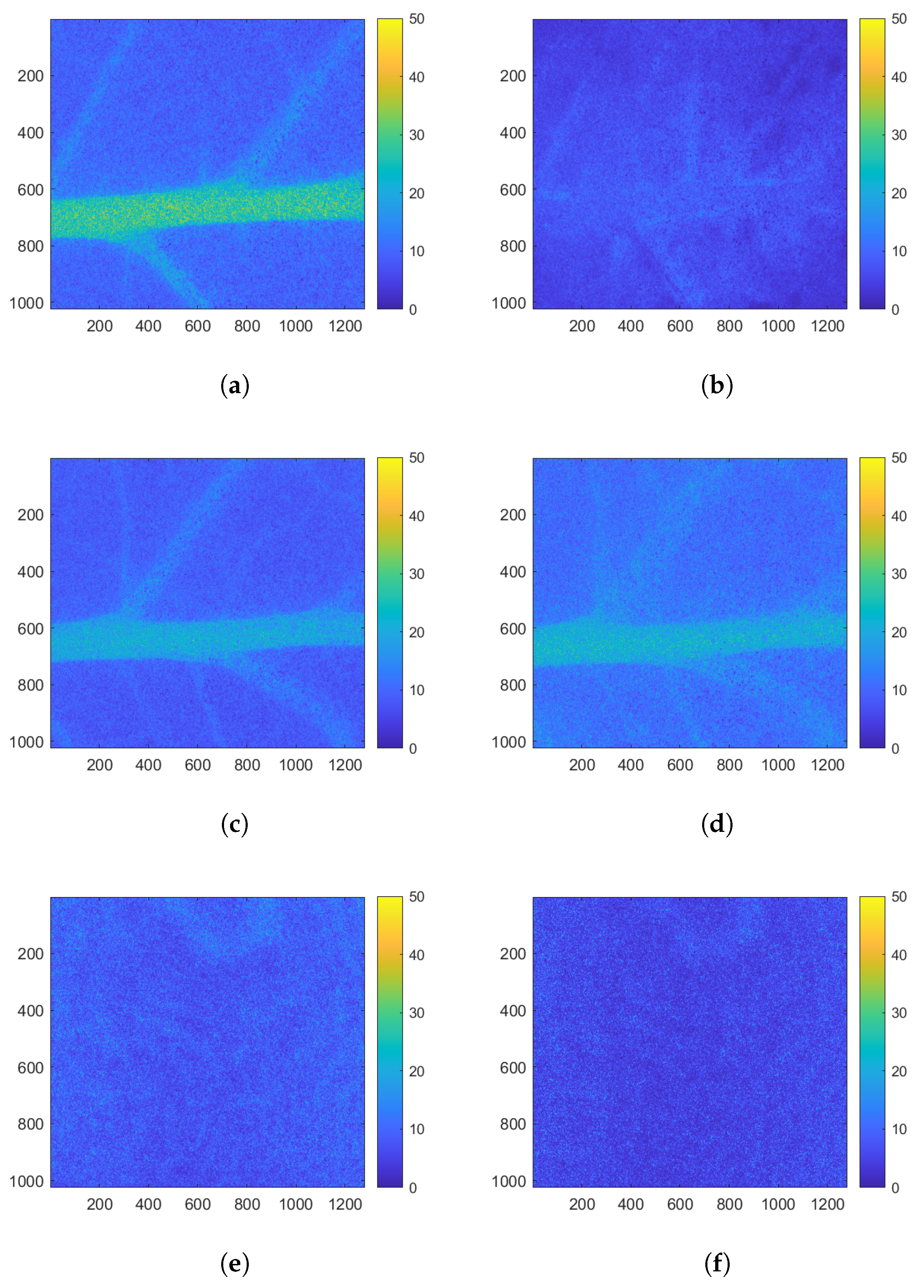
3.2. Midrib Biospeckle Activity
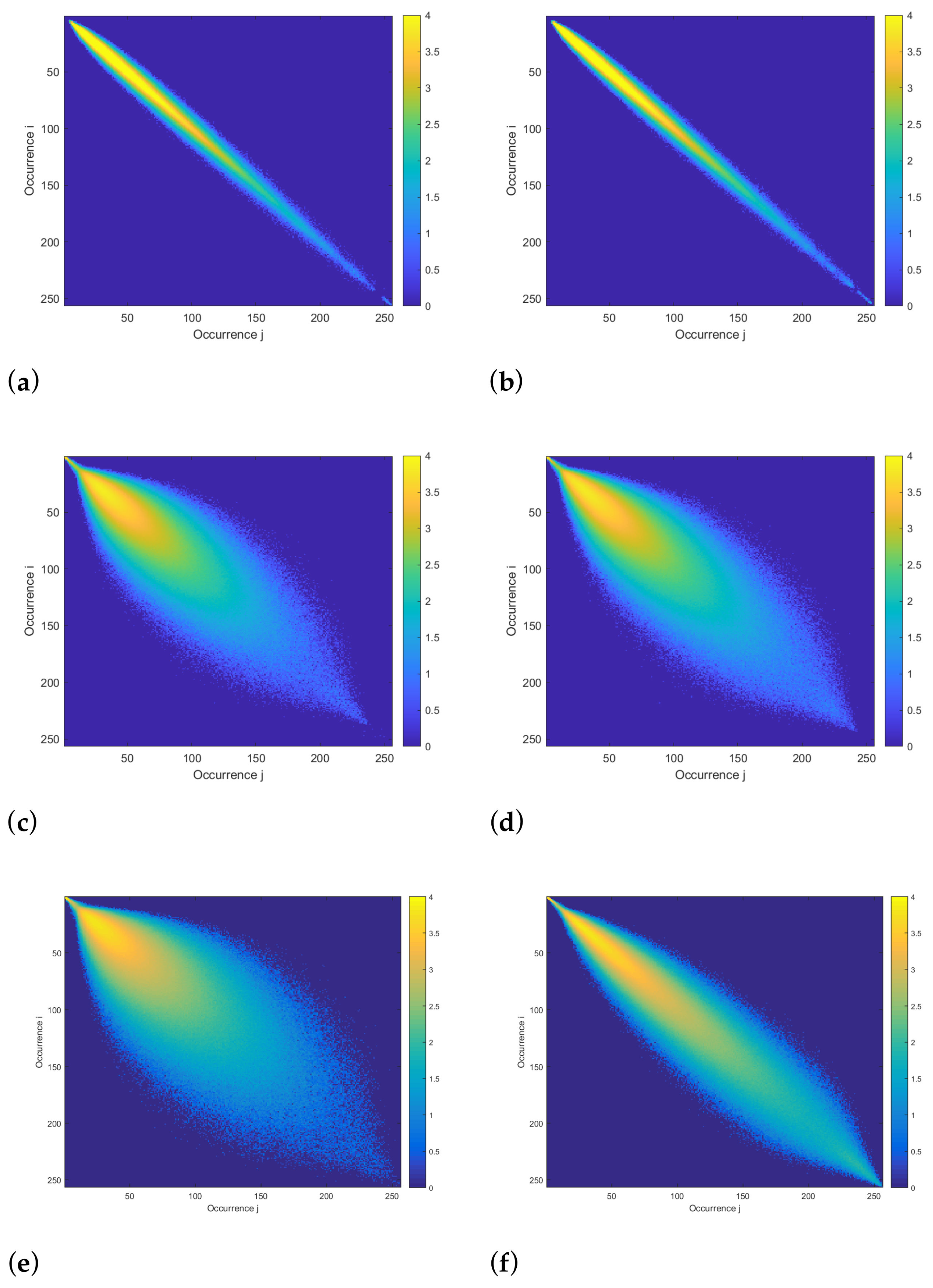
3.2.1. Co-Occurrence Matrices
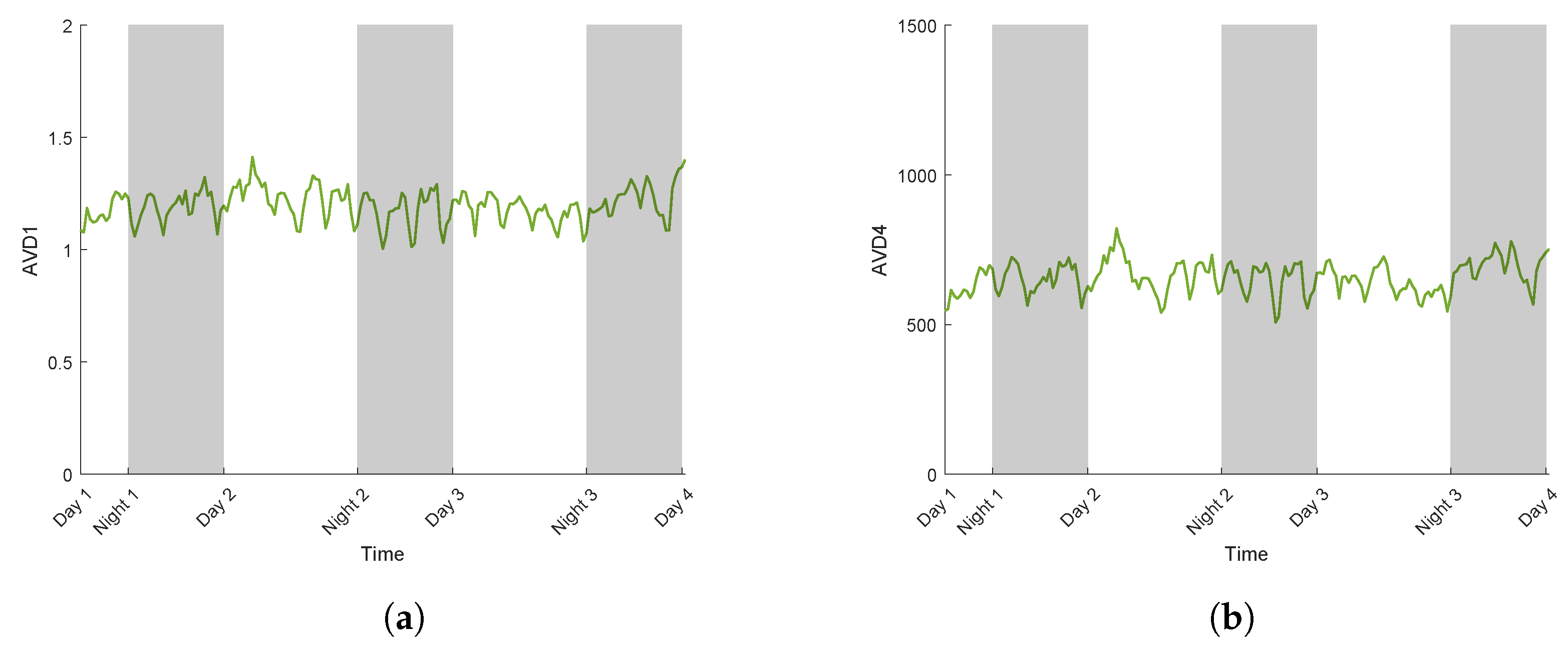
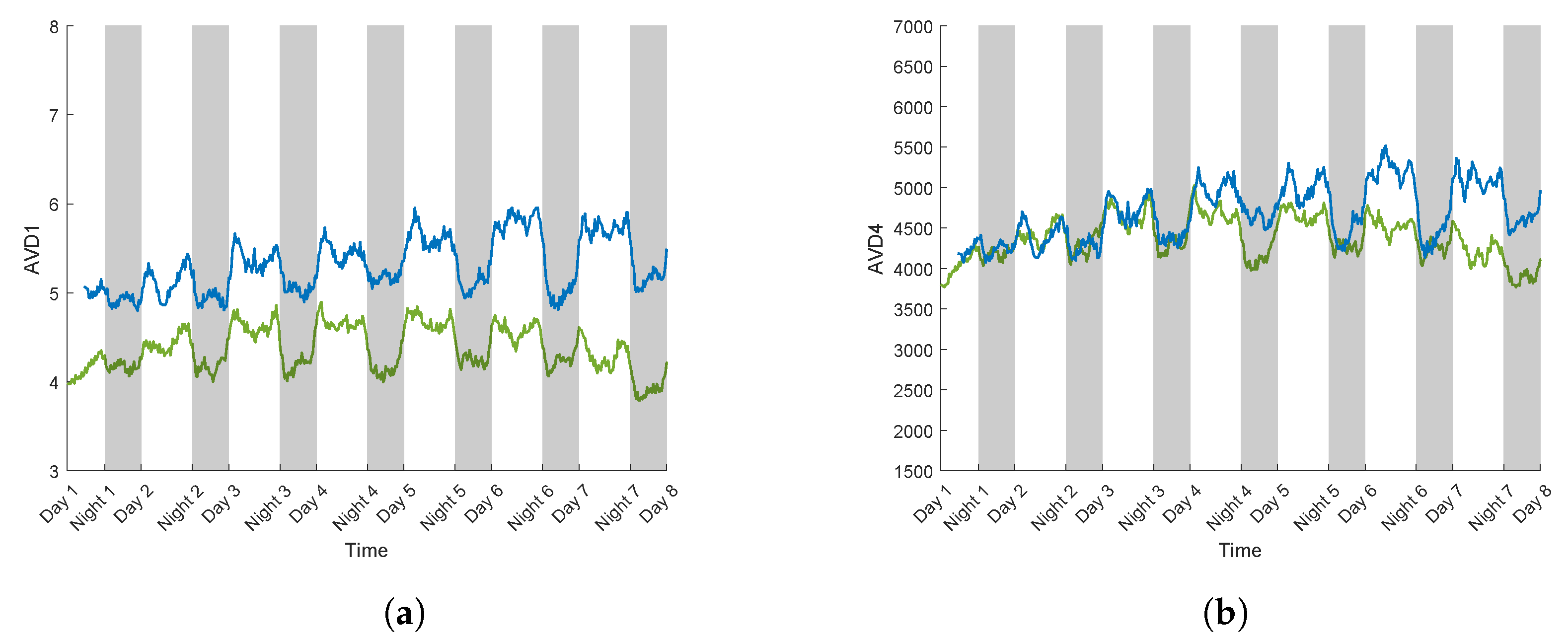
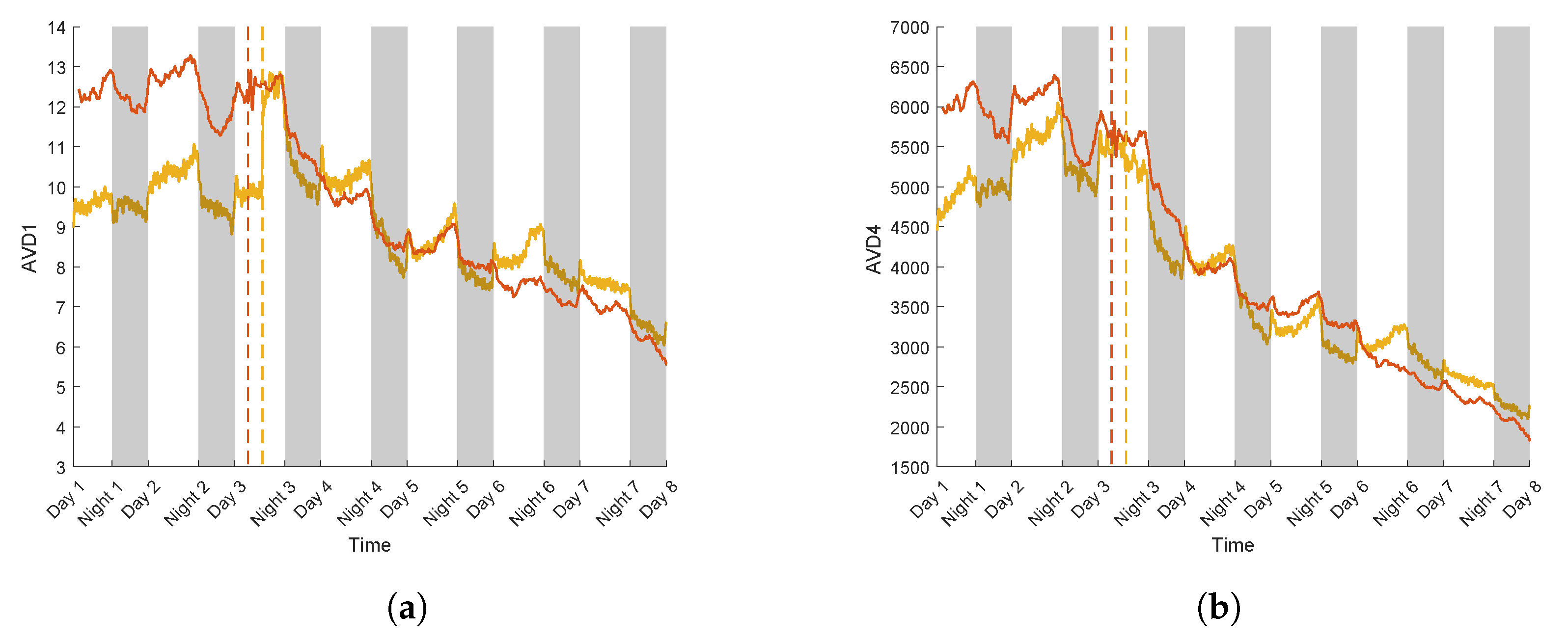
3.2.2. AVD Temporal Evolution
| Realisations | Days/Nights | SNR | |
|---|---|---|---|
| AVD1 | AVD4 | ||
| 5 | Night | 3.5 | 6.3 |
| Day | 3.7 | 6.9 | |
| 6 | Night | 4.3 | 6.7 |
| Day | 4.6 | 7.5 | |
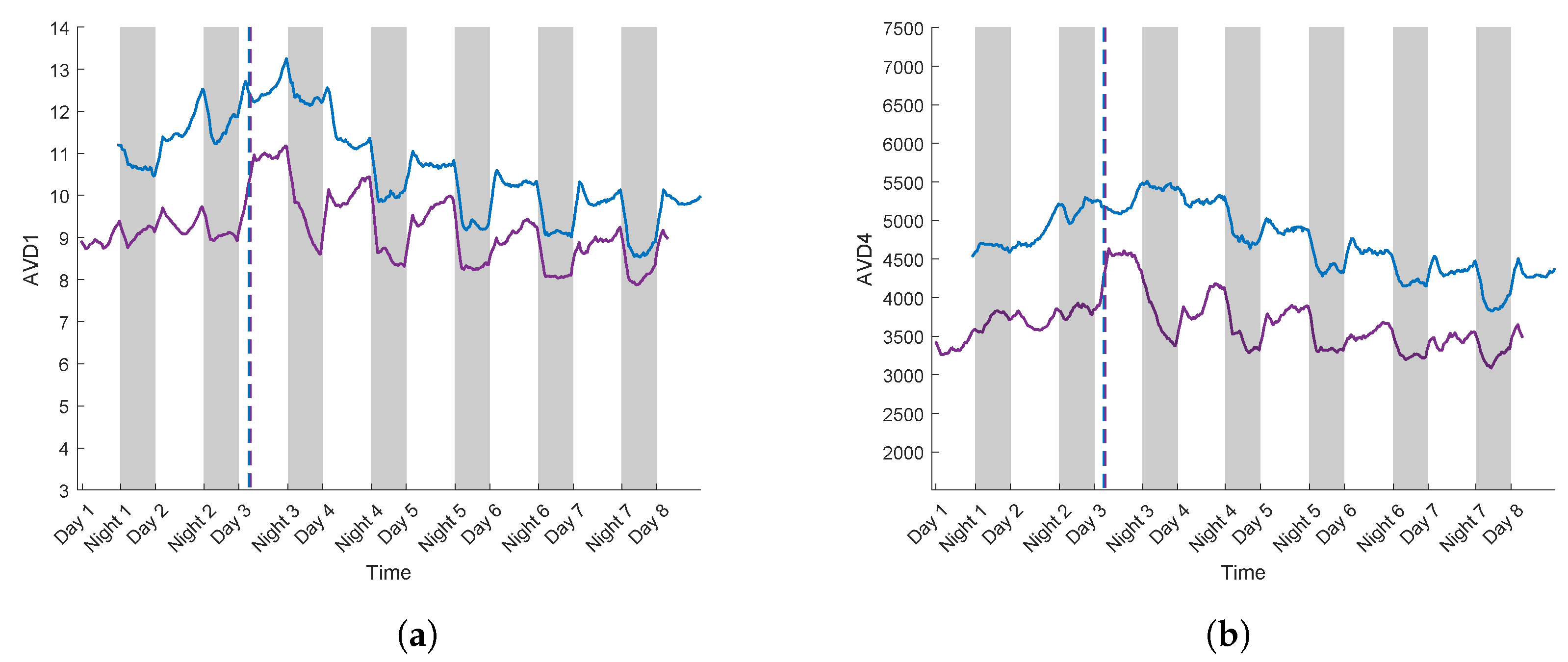
3.3. Case Study for Plant Breeding: Application to a Tolerant Variety
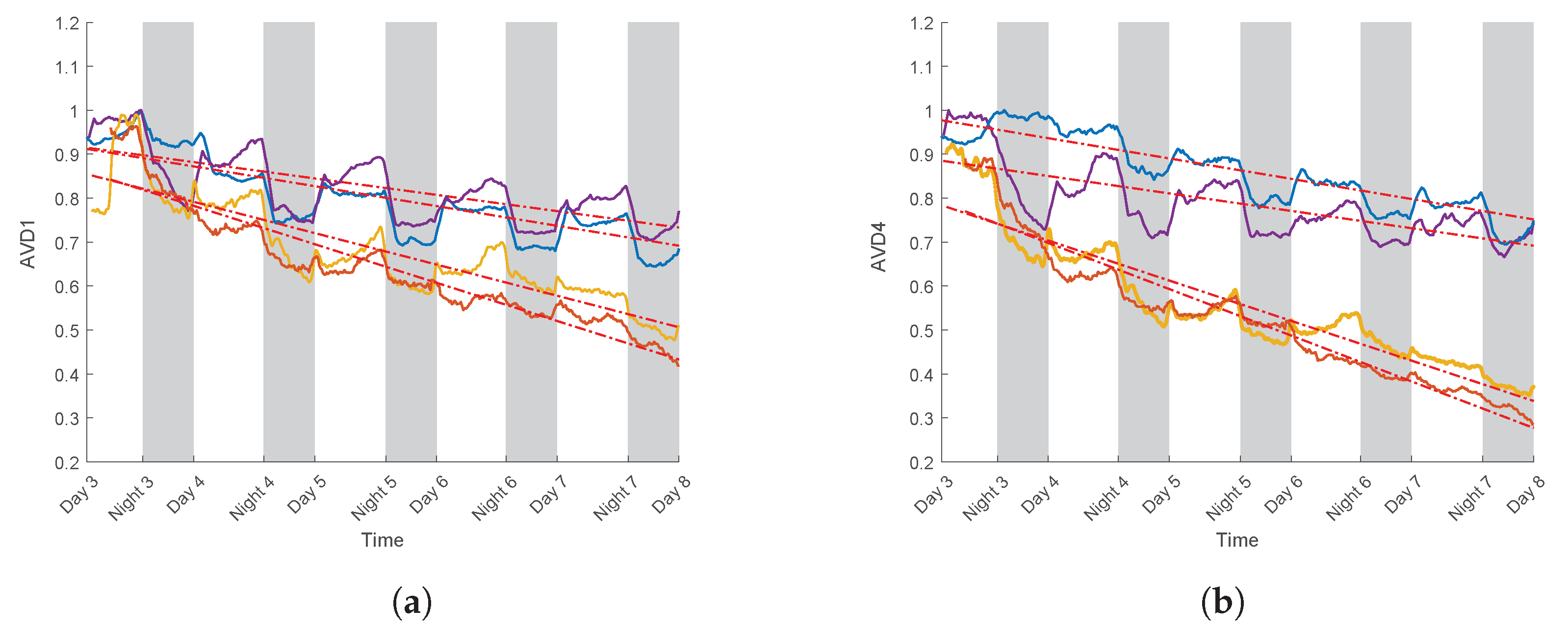
3.4. Linear Regression Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janssen, S.J.C.; Porter, C.H.; Moore, A.D.; Athanasiadis, I.N.; Foster, I.; Jones, J.W.; Antle, J.M. Towards a new generation of agricultural system data, models and knowledge products: Information and communication technology. Agric. Syst. 2017, 155, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Wei, S.; Zhang, M.; Li, H.; Sun, H.; Liu, G.; Li, M. Sensors for measuring plant phenotyping: A review. Int. J. Agric. Biol. Eng. 2018, 11, 1–17. [Google Scholar] [CrossRef]
- Chawade, A.; van Ham, J.; Blomquist, H.; Bagge, O.; Alexandersson, E.; Ortiz, R. High-Throughput Field-Phenotyping Tools for Plant Breeding and Precision Agriculture. Agronomy 2019, 9, 258. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Mas Garcia, S.; Ryckewaert, M.; Abdelghafour, F.; Metz, M.; Moura, D.; Feilhes, C.; Prezman, F.; Bendoula, R. Combination of multivariate curve resolution with factorial discriminant analysis for the detection of grapevine diseases using hyperspectral imaging. A case study: Flavescence dorée. Analyst 2021, 24, 7730–7739. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Steiner, U.; Hillnhütter, C.; Dehne, H.W.; Oerke, E.C. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.; González-Dugo, V.; Berni, J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Ryckewaert, M.; Héran, D.; Simonneau, T.; Abdelghafour, F.; Boulord, R.; Saurin, N.; Moura, D.; Mas-Garcia, S.; Bendoula, R. Physiological variable predictions using VIS–NIR spectroscopy for water stress detection on grapevine: Interest in combining climate data using multiblock method. Comput. Electron. Agric. 2022, 197, 106973. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S. Leaf Optical Properties; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Goodman, J.W. Speckle Phenomena in Optics: Theory and Applications, 2nd ed.; SPIE: Bellingham, WA, USA, 2020. [Google Scholar] [CrossRef]
- Arizaga, R.; Trivi, M.; Rabal, H. Speckle time evolution characterization by the co-occurrence matrix analysis. Opt. Laser Technol. 1999, 31, 163–169. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.; Kothakota, A.; Ramesh, S.; Thirumdas, R.; Juvvi, P. Biospeckle laser technique—A novel non-destructive approach for food quality and safety detection. Trends Food Sci. Technol. 2020, 97, 1–13. [Google Scholar] [CrossRef]
- Zdunek, A.; Adamiak, A.; Pieczywek, P.M.; Kurenda, A. The biospeckle method for the investigation of agricultural crops: A review. Opt. Lasers Eng. 2014, 52, 276–285. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Mujeeb, A.; Nirala, A.K. Assessment of biological leaf tissue using biospeckle laser imaging technique. Laser Phys. 2018, 28, 065608. [Google Scholar] [CrossRef]
- Janaszek-Mańkowska, M.; Ratajski, A.; Słoma, J. Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura). Appl. Sci. 2022, 12, 763. [Google Scholar] [CrossRef]
- Braga, R.A.; Dupuy, L.; Pasqual, M.; Cardoso, R.R. Live biospeckle laser imaging of root tissues. Eur. Biophys. J. 2009, 38, 679–686. [Google Scholar] [CrossRef]
- Rabelo, G.F.; Enes, A.M.; Braga Junior, R.A.; Dal Fabbro, I.M. Frequency response of biospeckle laser images of bean seeds contaminated by fungi. Biosyst. Eng. 2011, 110, 297–301. [Google Scholar] [CrossRef]
- Zdunek, A.; Cybulska, J. Relation of Biospeckle Activity with Quality Attributes of Apples. Sensors 2011, 11, 6317–6327. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, X.; Cooley, N.; Farrell, P.; Moran, B. Taking the pulse of a plant: Dynamic laser speckle analysis of plants. Infrared Laser Eng. 2016, 45, 0902002. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Cooley, N.; Farrell, P.; Moran, B. Application of Laser Speckle Pattern Analysis for Plant Sensing. In Proceedings of the Third International Conference on Photonics and Image in Agriculture Engineering, Sanya, China, 5–8 December 2013; p. 876102. [Google Scholar] [CrossRef]
- Thakur, P.S.; Bhatia, V.; Rajput, L.S.; Rana, S.; Prakash, S. Laser Biospeckle Technique for Evaluating Biotic Stress on Seed Germination. In Proceedings of the 2022 Workshop on Recent Advances in Photonics (WRAP), Mumbai, India, 4–6 March 2022; pp. 1–2. [Google Scholar] [CrossRef]
- O’Callaghan, F.E.; Neilson, R.; MacFarlane, S.A.; Dupuy, L.X. Dynamic biospeckle analysis, a new tool for the fast screening of plant nematicide selectivity. Plant Methods 2019, 15, 155. [Google Scholar] [CrossRef]
- D’Jonsiles, M.F.; Galizzi, G.E.; Dolinko, A.E.; Novas, M.V.; Ceriani Nakamurakare, E.; Carmarán, C.C. Optical study of laser biospeckle activity in leaves of Jatropha curcas L.: A non-invasive and indirect assessment of foliar endophyte colonization. Mycol. Prog. 2020, 19, 339–349. [Google Scholar] [CrossRef]
- Botega, J.V.L.; Braga, R.A.; Machado, M.P.P.; Lima, L.A.; Rabelo, G.F.; Cardoso, R.R. Biospeckle Laser Portable Equipment Monitoring Water Behavior at Coffee Tree Leaves. In Proceedings of the Speckle 2010: Optical Metrology, SPIE, Florianapolis, Brazil, 13 September 2010; Volume 7387, pp. 476–481. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Da Silva, E.R.; Muramatsu, M. Comparative study of analysis methods in biospeckle phenomenon. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2008; Volume 992, pp. 320–325. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dhanotiya, J.; Bhatia, V.; Prakash, S. Study of visual processing techniques for dynamic speckles: A comparative analysis. arXiv 2021, arXiv:2106.15507. [Google Scholar] [CrossRef]
- Júnior, R.A.B.; Rivera, F.P.; Moreira, J. A Practical Guide to Biospeckle Laser Analysis; Editora UFLA: Lavras, Brazil, 2016; p. 164. [Google Scholar]
- Bressan, R.; Hasegawa, P.; Handa, A.K. Resistance of cultured higher plant cells to polyethylene glycol-induced water stress. Plant Sci. Lett. 1981, 21, 23–30. [Google Scholar] [CrossRef]
- Ryckewaert, M.; Metz, M.; Héran, D.; George, P.; Grèzes-Besset, B.; Akbarinia, R.; Roger, J.M.; Bendoula, R. Massive spectral data analysis for plant breeding using parSketch-PLSDA method: Discrimination of sunflower genotypes. Biosyst. Eng. 2021, 210, 69–77. [Google Scholar] [CrossRef]
- Fujii, H.; Nohira, K.; Yamamoto, Y.; Ikawa, H.; Ohura, T. Evaluation of blood flow by laser speckle image sensing. Part 1. Appl. Opt. 1987, 26, 5321–5325. [Google Scholar] [CrossRef]
- Fujii, H.; Asakura, T. Statistical properties of image speckle patterns in partially coherent light. Nouvelle Revue d’Optique 1975, 6, 5–14. [Google Scholar] [CrossRef]
- Oulamara, A.; Tribillon, G.; Duvernoy, J. Biological Activity Measurement on Botanical Specimen Surfaces Using a Temporal Decorrelation Effect of Laser Speckle. J. Mod. Opt. 1989, 36, 165–179. [Google Scholar] [CrossRef]
- Xu, Z.; Joenathan, C.; Khorana, B.M. Temporal and spatial properties of the time-varying speckles of botanical specimens. Opt. Eng. 1995, 34, 1487–1502. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Braga, R.A. Enhancement of the robustness on dynamic speckle laser numerical analysis. Opt. Lasers Eng. 2014, 63, 19–24. [Google Scholar] [CrossRef]
- Braga Júnior, R.A. A Practical Guide to Biospeckle Laser Analysis Theory and Software; Editora da UFLA: Lavras, Brazil, 2016. [Google Scholar]
- BSLTL-PROJECT-GROUP. Bio-Speckle Laser Tool Library. 2016. Available online: https://www.nongnu.org/bsltl/overview.html (accessed on 29 March 2016).
- Joarder, N.; Roy, A.; Sima, S.; Parvin, K. Leaf blade and midrib anatomy of two sugarcane cultivars of Bangladesh. J. Bio-Sci. 1970, 18, 66–73. [Google Scholar] [CrossRef]
- Sharma, K.C. The Leaf and Root System. 2007. Available online: https://nsdl.niscpr.res.in/handle/123456789/413 (accessed on 4 December 2007).
- Ansari, M.Z.; Nirala, A.K. Biospeckle assessment of torn plant leaf tissue and automated computation of leaf vein density (LVD). Eur. Phys. J. Appl. Phys. 2015, 70, 21201. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Nirala, A.K. Biospeckle numerical assessment followed by speckle quality tests. Optik 2016, 127, 5825–5833. [Google Scholar] [CrossRef]
- Rawson, H.; Constable, G. Carbon Production of Sunflower Cultivars in Field and Controlled Environments. I. Photosynthesis and Transpiration of Leaves, Stems and Heads. Funct. Plant Biol. 1980, 7, 555. [Google Scholar] [CrossRef]
- Connor, D.; Palta, J.; Jones, T. Response of sunflower to strategies of irrigation III. Crop photosynthesis and transpiration. Field Crop. Res. 1985, 12, 281–293. [Google Scholar] [CrossRef]
- Matsuo, T.; Hirabayashi, H.; Ishizawa, H.; Kanai, H.; Nishimatsu, T. Application of Laser Speckle Method to Water Flow Measurement in Plant Body. In Proceedings of the 2006 SICE-ICASE International Joint Conference, Busan, Republic of Korea, 18–21 October 2006; pp. 3563–3566. [Google Scholar] [CrossRef]
- Cohen, Y.; Huck, M.G.; Hesketh, J.D.; Frederick, J.R. Sap flow in the stem of water stressed soybean and maize plants. Irrig. Sci. 1990, 11, 45–50. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Yu, T.F.; Li, P.D. Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Snow, M.D.; Tingey, D.T. Evaluation of a System for the Imposition of Plant Water Stress. Plant Physiol. 1985, 77, 602–607. [Google Scholar] [CrossRef]
- Whitfield, D.; Connor, D.; Hall, A. Carbon dioxide balance of sunflower (Helianthus annuus) subjected to water stress during grain-filling. Field Crop. Res. 1989, 20, 65–80. [Google Scholar] [CrossRef]
- Human, J.J.; Du Toit, D.; Bezuidenhout, H.D.; De Bruyn, L.P. The Influence of Plant Water Stress on Net Photosynthesis and Yield of Sunflower (Helianthus annuus L.). J. Agron. Crop. Sci. 1990, 164, 231–241. [Google Scholar] [CrossRef]
- Fernández, J.; Cuevas, M.; Rodriguez-Dominguez, C.; Perez-Martin, A.; Torres-Ruiz, J.; Elsayed-Farag, S.; Diaz-Espejo, A.; Martín-Palomo, M. Sap flow response to olive water stress: A comparative study with trunk diameter VARIATIONS and leaf turgor pressure. Acta Hortic. 2012, 951, 101–110. [Google Scholar] [CrossRef]
- Nardini, A.; Tyree, M.T.; Salleo, S. Xylem Cavitation in the Leaf of Prunus laurocerasus and Its Impact on Leaf Hydraulics. Plant Physiol. 2001, 125, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.T.; Bao, Y. The influence of water stress on stem and leaf photosynthesis in glycine max and sparteum junceum (leguminosae). Am. J. Bot. 2001, 77, 1007–1015. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xie, Z.K.; Wang, Y.J.; Su, P.X.; An, L.P.; Gao, H. Effect of water stress on leaf photosynthesis, chlorophyll content, and growth of oriental lily. Russ. J. Plant Physiol. 2011, 58, 844–850. [Google Scholar] [CrossRef]

| Realisation | Experiment Conditions | Variety | Start of Measurements | End of Measurements | PEG Introduction |
|---|---|---|---|---|---|
| 1 | PEG | T | 04-Aug-2023 09:43:16 | 11-Aug-2023 10:28:28 | 06-Aug-2023 10:28:40 |
| 2 | PEG | T | 15-May-2023 20:11:34 | 25-May-2023 10:10:34 | 18-May-2023 10:02:38 |
| 3 | PEG | S | 16-Mar-2021 10:15:36 | 23-Mar-2021 08:51:16 | 18-Mar-2021 14:45:48 |
| 4 | PEG | S | 29-Jun-2021 11:45:38 | 06-Jul-2021 09:01:48 | 01-Jul-2021 10:45:42 |
| 5 | Control | S | 24-Feb-2023 10:41:00 | 03-Mar-2023 10:21:00 | None |
| 6 | Control | S | 16-Feb-2023 15:20:00 | 23-Feb-2023 15:00:12 | None |
| 7 | Reference | Inert object | 27-Jan-2023 16:12:42 | 30-Jan-2023 07:32:46 | None |
| Realisations | Variety | Slope after PEG | |
|---|---|---|---|
| AVD1 | AVD4 | ||
| 1 | T | −0.82 | −0.89 |
| 2 | T | −0.70 | −0.72 |
| 3 | S | −0.89 | −0.93 |
| 4 | S | −0.95 | −0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzaouia, S.; Ryckewaert, M.; Héran, D.; Ducanchez, A.; Bendoula, R. Using Dynamic Laser Speckle Imaging for Plant Breeding: A Case Study of Water Stress in Sunflowers. Sensors 2024, 24, 5260. https://doi.org/10.3390/s24165260
Bouzaouia S, Ryckewaert M, Héran D, Ducanchez A, Bendoula R. Using Dynamic Laser Speckle Imaging for Plant Breeding: A Case Study of Water Stress in Sunflowers. Sensors. 2024; 24(16):5260. https://doi.org/10.3390/s24165260
Chicago/Turabian StyleBouzaouia, Sherif, Maxime Ryckewaert, Daphné Héran, Arnaud Ducanchez, and Ryad Bendoula. 2024. "Using Dynamic Laser Speckle Imaging for Plant Breeding: A Case Study of Water Stress in Sunflowers" Sensors 24, no. 16: 5260. https://doi.org/10.3390/s24165260






