Electrochemical Determination of Dopamine with a Carbon Paste–Lanthanum (III) Oxide Micro-Composite Electrode: Effect of Cetyl Trimethyl Ammonium Bromide Surfactanton Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Measurements
2.3. Sample Treatment
2.4. Preparation of CPE and La-OX/CPE
3. Results and Discussion
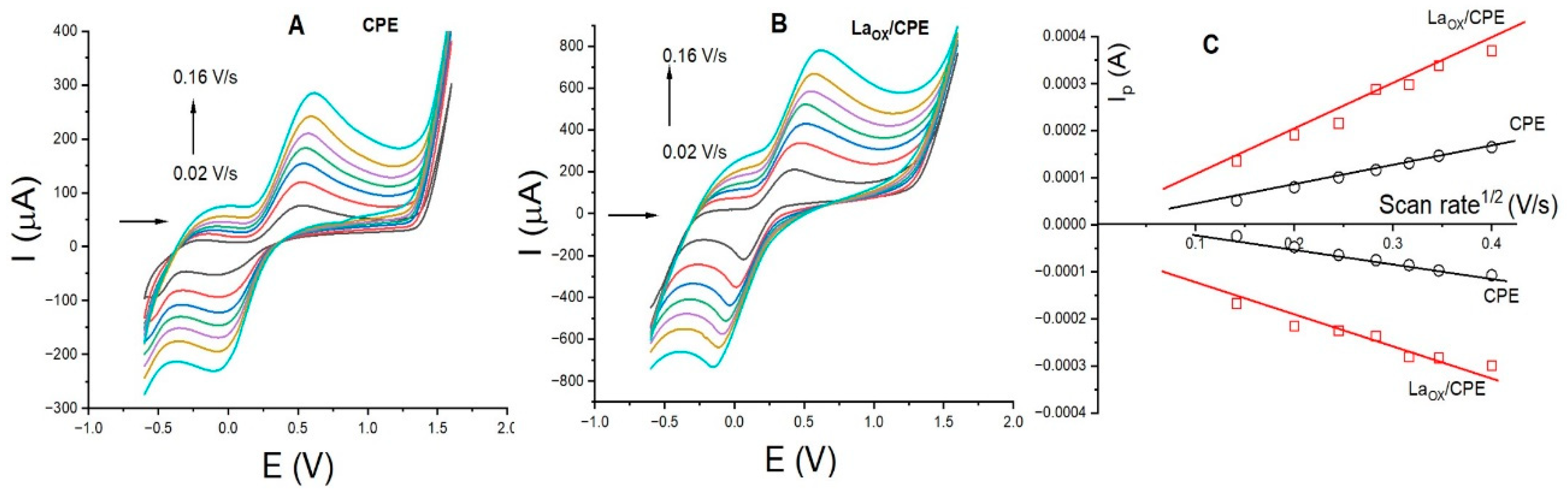
3.1. Electrode Characterization and Dopamine Interaction Study
3.2. pH Effect
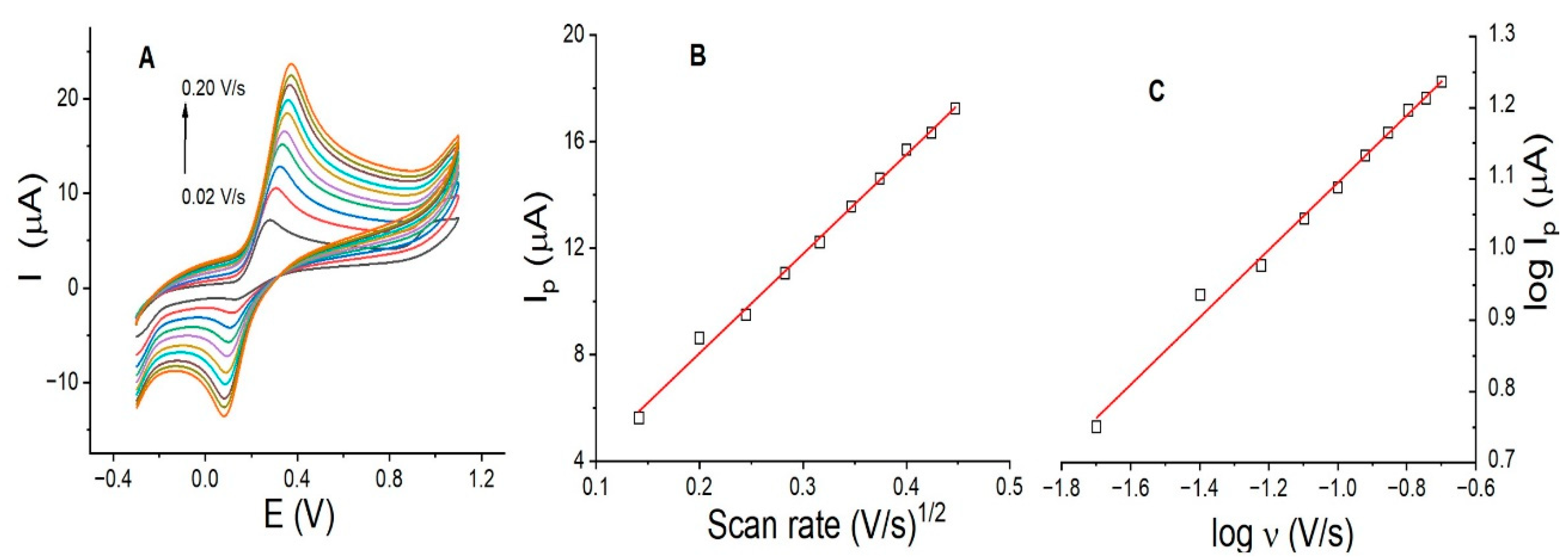
3.3. Scan Rate Effect on DP Using LaOX/CPE
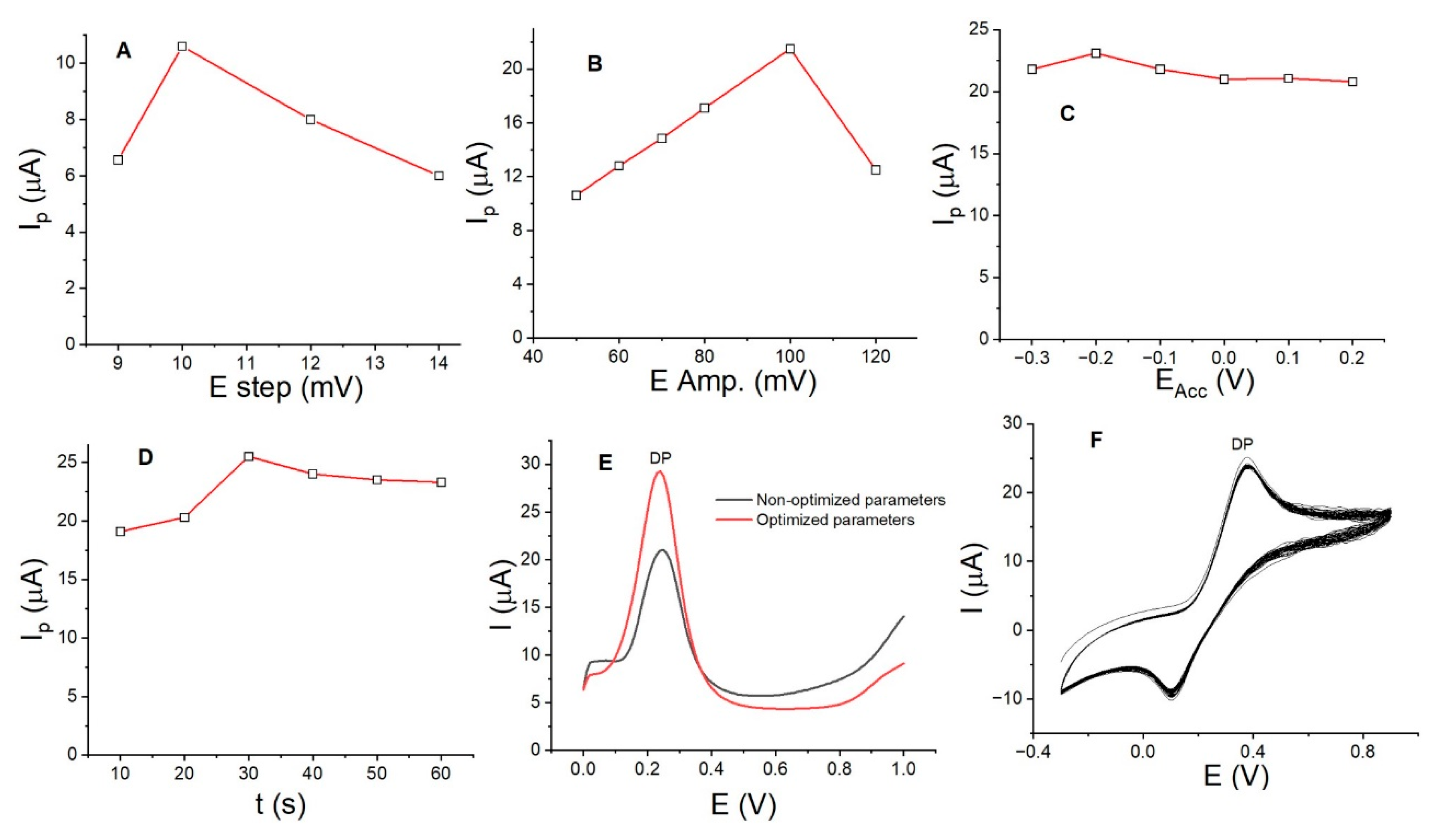
3.4. Optimization of DP Parameters and Assessment of the Lifespan of LaOX/CPE
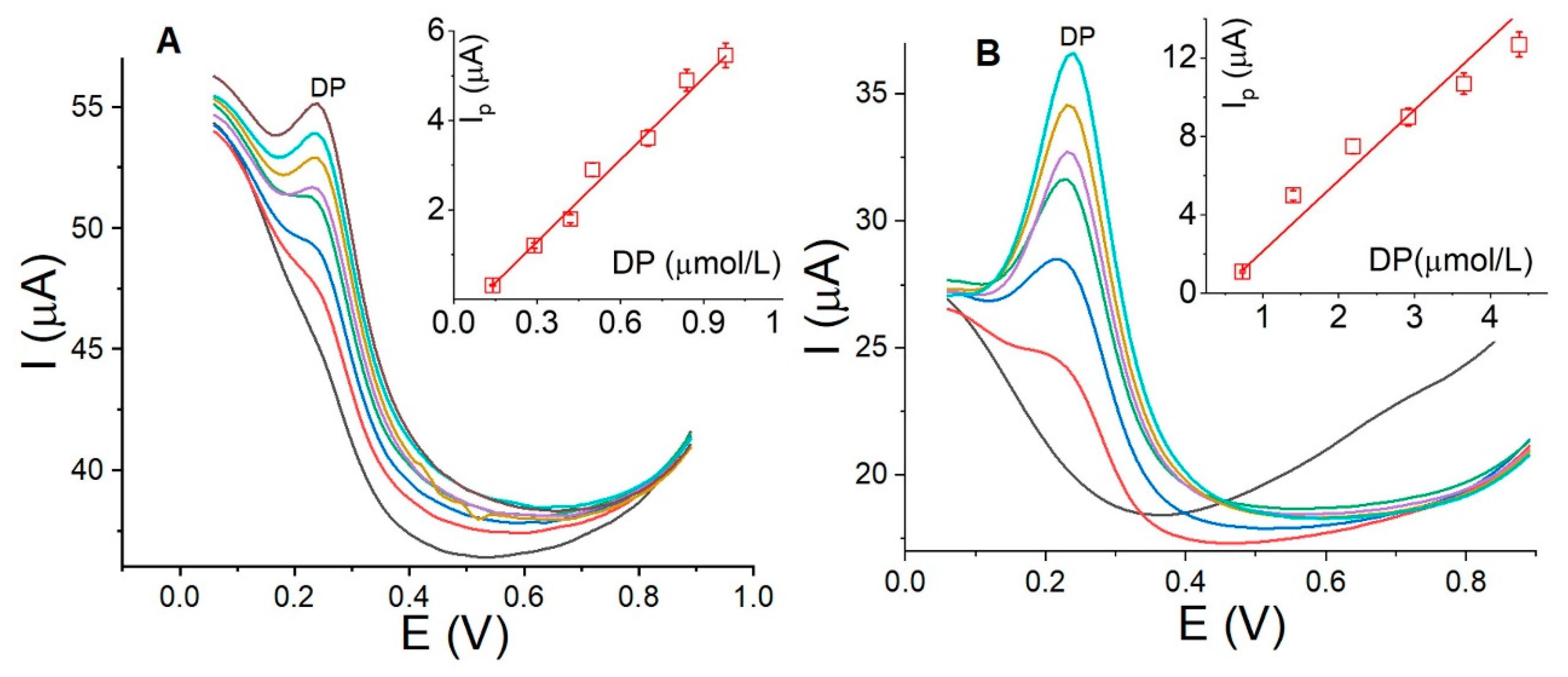
3.5. Calibration Curve, Detection Limits, and Reproducibility
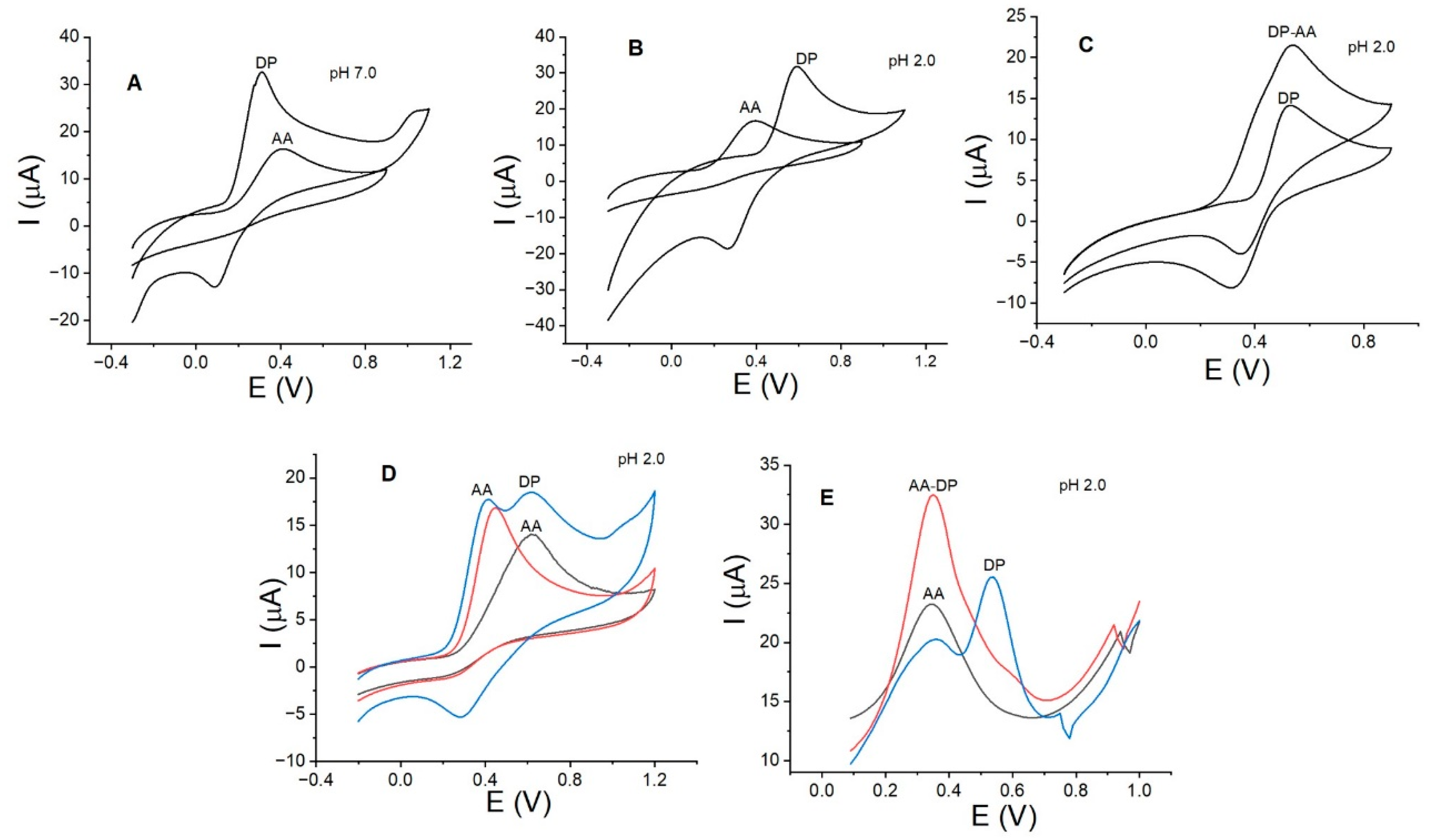
3.6. Interference Study with DP, AA, and CTAB
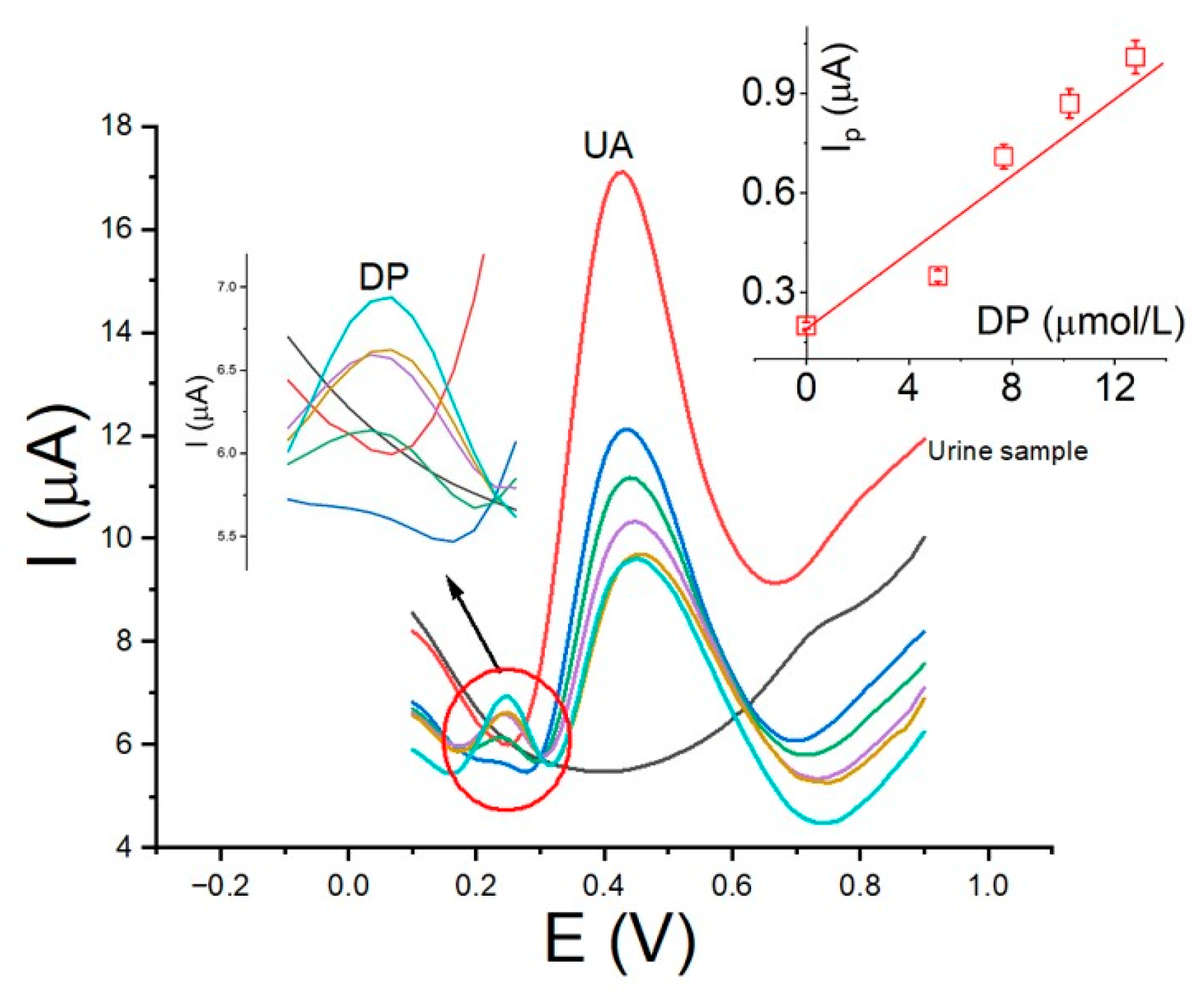
3.7. Analytical Application in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandikumar, A.; Soon How, A.G.T.; See, T.P.; Omar, F.S.; Jayabal, S.; Kamali, K.Z.; Yusoff, N.; Jamil, A.; Ramaraj, R.; John, A.A.; et al. Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RSC Adv. 2014, 4, 63296–63323. [Google Scholar] [CrossRef]
- Shafi, P.M.; Joseph, N.; Karthik, R.; Shim, J.J.; Chandra Bose, A.; Ganesh, V. Lemon juice-assisted synthesis of LaMnO3 perovskite nanoparticles for electrochemical detection of dopamine. Microchem. J. 2021, 164, 105945. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Enfermedad de Parkinson. 2022. Available online: https://www.who.int/es/news-room/fact-sheets/detail/parkinson-disease (accessed on 30 July 2024).
- Hsine, Z.; Milka, R.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Review—Recent Progress in Graphene Based Modified Electrodes for Electrochemical Detection of Dopamine. Chemosensors 2022, 10, 249. [Google Scholar] [CrossRef]
- Kaya, S.I.; Kurbanoglue, S.; Ozkan, S.A. Nanomaterials-Based Nanosensors for the Simultaneous Electrochemical Determination of Biologically Important Compounds: Ascorbic Acid, Uric Acid, and Dopamine. Crit. Rev. Anal. Chem. 2019, 49, 101–125. [Google Scholar] [CrossRef]
- Van Staden, J.F.; Van Staden, R.I.S. Flow-injection analysis systems with different detection devices and other related techniques for the in vitro and in vivo determination of dopamine as neurotransmitter. Talanta 2012, 102, 34–43. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, X.; Kong, J. Recent Progress on Uric Acid Detection: A Review. Crit. Rev. Anal. Chem. 2020, 50, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Nováková, L.; Solich, P.; Solichová, D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. TrAC Trends Anal. Chem. 2008, 27, 942–958. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; Kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective detection of dopamine in the presence of ascorbic acid and uric acid by a carbon nanotubes-ionic liquid gel modified electrode. Talanta 2005, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ali Kamyabi, M.; Ali Shafiee, M. Electrocatalytic oxidation of dopamine, ascorbic acid and uric acid at poly2,6diaminopyridine on the surface of carbon nanotubes/gc electrodes. J. Braz. Chem. Soc. 2012, 23, 593–601. [Google Scholar]
- Li, Y.; Lin, X. Simultaneous electroanalysis of dopamine, ascorbic acid and uric acid by poly (vinyl alcohol) covalently modified glassy carbon electrode. Sens. Actuat. B 2006, 115, 134–139. [Google Scholar] [CrossRef]
- Cui, R.; Wang, X.; Zhang, G.; Wang, C. Simultaneous determination of dopamine, ascorbic acid, and uric acid using helical carbon nanotubes modified electrode. Sens. Actuators B Chem. 2012, 161, 1139–1143. [Google Scholar] [CrossRef]
- Ma, M.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuat. B 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Patella, B.; Sortino, A.; Mazzara, F.; Aiello, G.; Drago, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; Inguanta, R. Electrochemical detection of dopamine with negligible interference from ascorbic and uric acid by means of reduced graphene oxide and metals-NPs based electrodes. Anal. Chim. Acta 2021, 1187, 339124. [Google Scholar] [CrossRef]
- Sajid, M.; Khaled Nazal, M.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAC Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Salale Geleta, G. Recent advances in electrochemical sensors based on molecularly imprinted polymers and nanomaterials for detection of ascorbic acid, dopamine, and uric acid. Sens. Bio-Sens. Res. 2024, 43, 100610. [Google Scholar]
- Shakkthivel, P.; Chen, S.M. Simultaneous determination of ascorbic acid and dopamine in the presence of uric acid on ruthenium oxide modified electrode. Biosens. Bioelectron. 2007, 22, 1680–1687. [Google Scholar] [CrossRef]
- Rahman, M.; Lopa, N.S.; Ju, M.J.; Lee, J.J. Highly sensitive and simultaneous detection of dopamine and uric acid at graphene nanoplatelet-modified fluorine-doped tin oxide electrode in the presence of ascorbic acid. J. Electroanal. Chem. 2017, 792, 54–60. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; Calderón, J.A.; Nagles, E.; Hurtado, J. Voltammetric determination of thiomersal with a new modified electrode based on a carbon paste electrode decorated with La2O3. J. Electroanal. Chem. 2019, 833, 536–542. [Google Scholar] [CrossRef]
- Nagles, E.; Ceroni, M.; Hurtado, J. Simultaneous Detection of Tartrazine-Sunset Yellow in Food Samples Using Bioxide/Carbon Paste Microcomposite with Lanthanum and Titanium. J. Electrochem. Sci. Technol. 2020, 11, 421–429. [Google Scholar] [CrossRef]
- Vargas-Varela, A.; Cardenas-Riojas, A.A.; Nagles, E.; Hurtado, J. Amperometric Method for Detecting Paracetamol using a Carbon Paste Electrode Modified with Vanadium (V) Oxide. ChemistrySelect 2023, 8, e202204737. [Google Scholar]
- Zhang, W.; Yuan, R.; Chai, Y.; Zhang, Y.; Chen, S. A simple strategy based on lanthanum–multiwalled carbon nanotube nanocomposites for simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Sensor. Actuat. B-Chem. 2012, 166–167, 601–607. [Google Scholar] [CrossRef]
- Ye, F.; Feng, C.; Jiang, J.; Han, S. Simultaneous determination of dopamine, uric acid and nitrite using carboxylated graphene oxide/lanthanum modified electrode. Electrochim. Acta 2015, 182, 935–945. [Google Scholar] [CrossRef]
- Kumar, Y.; Pramanik, S.; Das, D.P. Lanthanum Ortho-Ferrite (LaFeO3) Nano-Particles Based Electrochemical Sensor for the Detection of Dopamine. Biointerface Res. Appl. Chem. 2020, 10, 6182–6188. [Google Scholar]
- Rajaitha, P.M.; Hajra, S.; Padhan, A.M.; Dubal, D.; Kim, H.J. Electrochemical detection of dopamine through hydrothermally prepared lanthanum metal–organic framework (La-BTC) /carbon nanotube nanohybrid. Mater. Sci. Eng B 2023, 296, 116638. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, G.; Singh, J.; Pandey, M.D. A highly efficient nanostructured Au@La2O3 based platform for dopamine detection. Mater. Lett. 2022, 308 Pt B, 131231. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, K.R.; Pandey, M.D.; Singh, J. Quantum-sized Ag nanoparticle conjugates on biofunctionalized La2O3–rGO ternary nanocomposite-based platform for the electrocatalytic determination of dopamine. New J. Chem. 2023, 47, 20866–20880. [Google Scholar] [CrossRef]
- Unal, D.N.; Yıldırım, S.; Kurbanoglu, S.; Uslu, B. Current trends and roles of surfactants for chromatographic and electrochemical sensing. TrAC Trends in Anal. Chem. 2021, 144, 116418. [Google Scholar] [CrossRef]
- Nagles, E.; Ceroni, M.; Hurtado-Murillo, J.J.; Hurtado, J.J. Electrochemical determination of paracetamol in a pharmaceutical dose by adsorptive voltammetry with a carbon paste/La2O3 microcomposite. Anal. Methods 2020, 12, 2608–2613. [Google Scholar] [CrossRef]
- Gaya, E.; Menendez, N.; Mazario, E.; Herrasti, P. Fe3O4-Nanoparticle-Modified Sensor for the Detection of Dopamine, Uric Acid and Ascorbic Acid. Chemosensors 2023, 11, 79. [Google Scholar] [CrossRef]
- Setoudeh, N.; Jahani, S.; Kazemipour, M.; Foroughi, M.M.; Nadiki, H.H. Zeolitic imidazolate frameworks and cobalt-tannic acid nanocomposite modified carbon paste electrode for simultaneous determination of dopamine, uric acid, acetaminophen and tryptophan: Investigation of kinetic parameters of surface electrode and its analytical performance. J. Electroanal. Chem. 2020, 863, 114045. [Google Scholar]
- Thiagarajan, S. Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta 2007, 74, 212–222. [Google Scholar] [CrossRef]
- Nagles, E.; Ceroni, M.; Villanueva Huerta, C.; Hurtado, J.J. Simultaneous Electrochemical Determination of Paracetamol and Allura Red in Pharmaceutical Doses and Food Using a Mo(VI) Oxide-Carbon Paste Microcomposite. Electroanalysis 2021, 33, 2335–2344. [Google Scholar] [CrossRef]
- Nagles, E.; Bello, M.; Hurtado, J.J. Electrochemical Determination of Morin in Natural Food Using a Chitosan–Graphene Glassy Carbon Modified Electrode. Sensors 2022, 22, 7780. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Sensitive and selective detection of dopamine using electrochemical microfluidic paper-based analytical nanosensor. Sens. Bio-Sens. Res. 2019, 23, 100270. [Google Scholar] [CrossRef]
- Nagles, E.; García-Beltrán, O.; Calderón, J.A. Evaluation of the usefulness of a novel electrochemical sensor in detecting uric acid and dopamine in the presence of ascorbic acid using a screen-printed carbon electrode modified with single walled carbon nanotubes and ionic liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Nagles, E.; Ibarra, L.; Llanos, J.P.; Hurtado, J.; Garcia-Beltrán, O. Development of a novel electrochemical sensor based on cobalt(II) complex useful in the detection of dopamine in presence of ascorbic acid and uric acid. J. Electroanal. Chem. 2017, 788, 38. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Qian, W.; Chen, H.; Feng, C.; Han, S.; Lin, H.; Ye, F.Y. Electrochemical investigation of da and ua on carboxylated graphene oxide/lanthanum electrodes with sundry content of CTAB. Surf. Rev. Lett. 2017, 24, 1750097. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Kiewlicz, J. Study of interaction between cationic surfactant (CTAB) and ascorbic acid/ ascorbic acids derivatives by tensiometric and spectroscopic methods. J. Mol. Liq. 2022, 354, 118917. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Zare-Mehrjardi, H.R. Cobalt salophen-modified carbon-paste electrode incorporating a cationic surfactant for simultaneous voltammetric detection of ascorbic acid and dopamine. Sens. Actuators B Chem. 2007, 121, 530. [Google Scholar] [CrossRef]
- Wen, X.L.; Jia, Y.H.; Liu, Z.L. Micellar effects on the electrochemistry of dopamine and its selective detection in the presence of ascorbic acid. Talanta 1999, 50, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
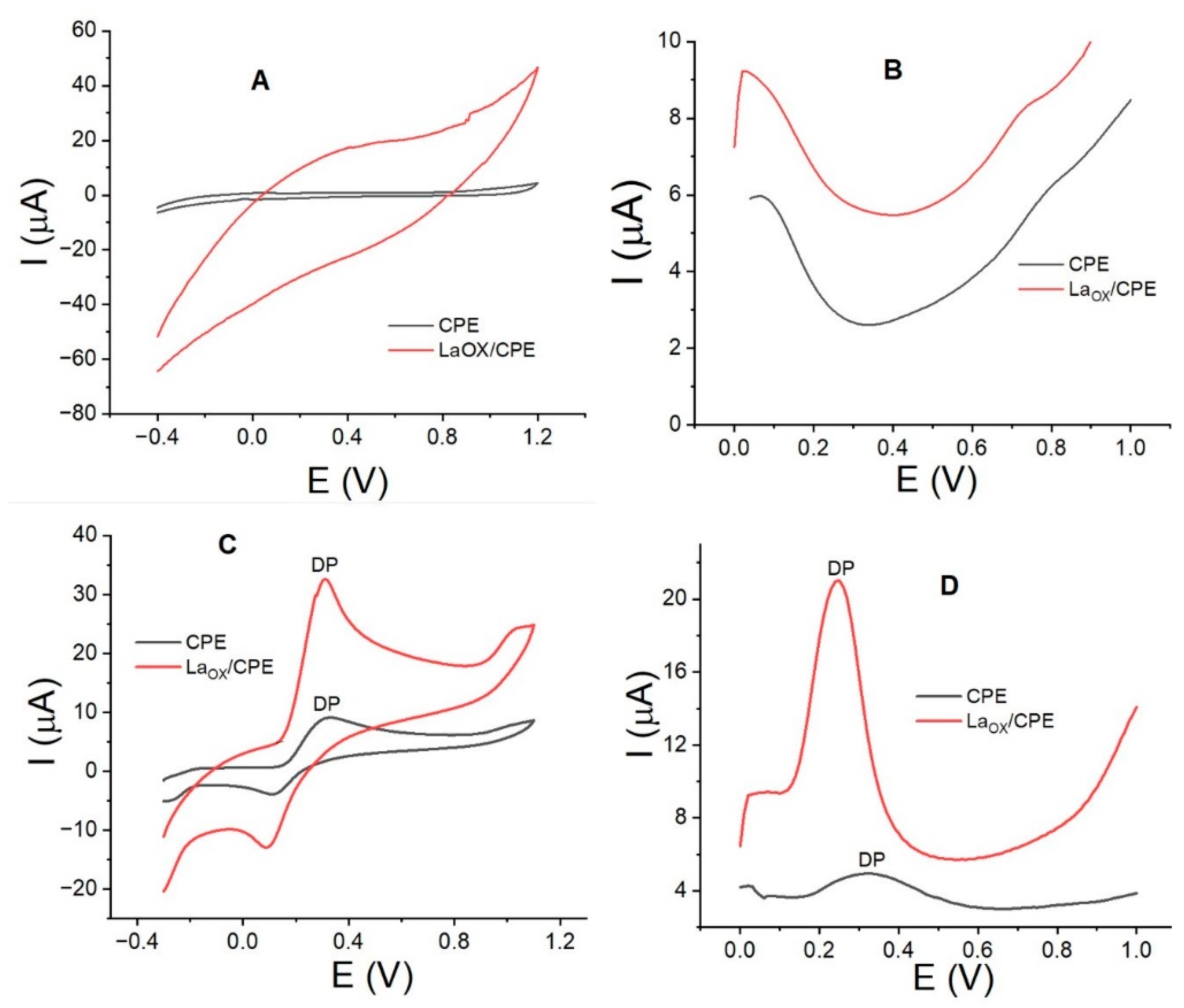


| Electrode | CV | % Current Increased | SWV | % Current Increased | ||
|---|---|---|---|---|---|---|
| Ep (V) | Ip/(μA) | Ep (V) | Ip/(μA) | |||
| CPE | 0.31 | 5.0 | 0.32 | 1.03 | - | |
| LaOX/CPE | 0.32 | 22 | 400 | 0.24 | 11.2 | 900 |
| Electrode | Slope (Ip/ ʋ ½) | Area (cm2) | Total Area (cm2) | ||
|---|---|---|---|---|---|
| Ipa | Ipc | Ipa | Ipc | ||
| CPE | 0.0004 | 0.0003 | 0.054 | 0.040 | 0.094 |
| LaOX/CPE | 0.0009 | 0.0005 | 0.121 | 0.067 | 0.188 |
| Electrode | Application | LoD (µmol/L) | Ref. |
|---|---|---|---|
| LaMnO3 NPs/GCE | Drugs | 0.032 | [1] |
| Au NPs Pt NPs | Urine | 0.075 0.062 | [15] |
| RuOx·nH2O/GCE | 0.080 | [19] | |
| GNP/FTO | Human serum | 0.220 | [22] |
| La–MWCNTs/CGE GO-COOLa/GCE LaFeO3/CPE La-BTC-CNT Tyr/Au-La2O3/ITO | Drugs Human serum and urine | 0.013 0.058 0.600 0.073 0.258 | [24] [25] [26] [27] [28] |
| CTAB/GO-COOLa/GCE | 0.036 | [40] | |
| LaOX/CPE | Urine | 0.030 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagles, E.; Riesco, F.; Roldan-Tello, L. Electrochemical Determination of Dopamine with a Carbon Paste–Lanthanum (III) Oxide Micro-Composite Electrode: Effect of Cetyl Trimethyl Ammonium Bromide Surfactanton Selectivity. Sensors 2024, 24, 5420. https://doi.org/10.3390/s24165420
Nagles E, Riesco F, Roldan-Tello L. Electrochemical Determination of Dopamine with a Carbon Paste–Lanthanum (III) Oxide Micro-Composite Electrode: Effect of Cetyl Trimethyl Ammonium Bromide Surfactanton Selectivity. Sensors. 2024; 24(16):5420. https://doi.org/10.3390/s24165420
Chicago/Turabian StyleNagles, Edgar, Fernando Riesco, and Luz Roldan-Tello. 2024. "Electrochemical Determination of Dopamine with a Carbon Paste–Lanthanum (III) Oxide Micro-Composite Electrode: Effect of Cetyl Trimethyl Ammonium Bromide Surfactanton Selectivity" Sensors 24, no. 16: 5420. https://doi.org/10.3390/s24165420





