Increasing the Beam Width and Intensity with Refraction Power Effect Using a Combination of Beam Mirrors and Concave Mirrors for Surgical-Fluorescence-Emission-Guided Cancer Monitoring Method
Abstract
:1. Introduction
2. Analysis of Refraction Power Effect to Increase Beam Intensity and Beam Width
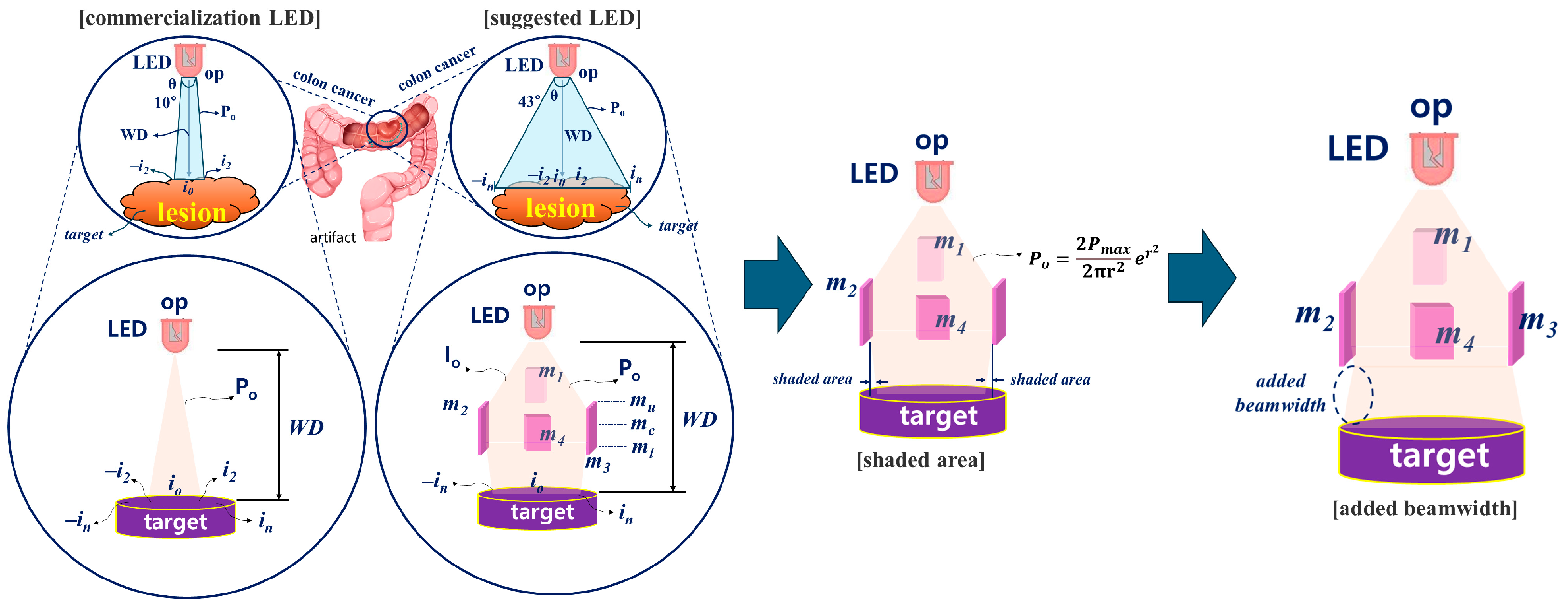
2.1. Improvement of Beam Width and Intensity Increase to Induce Fluorescence Emission Throughout the Lesion
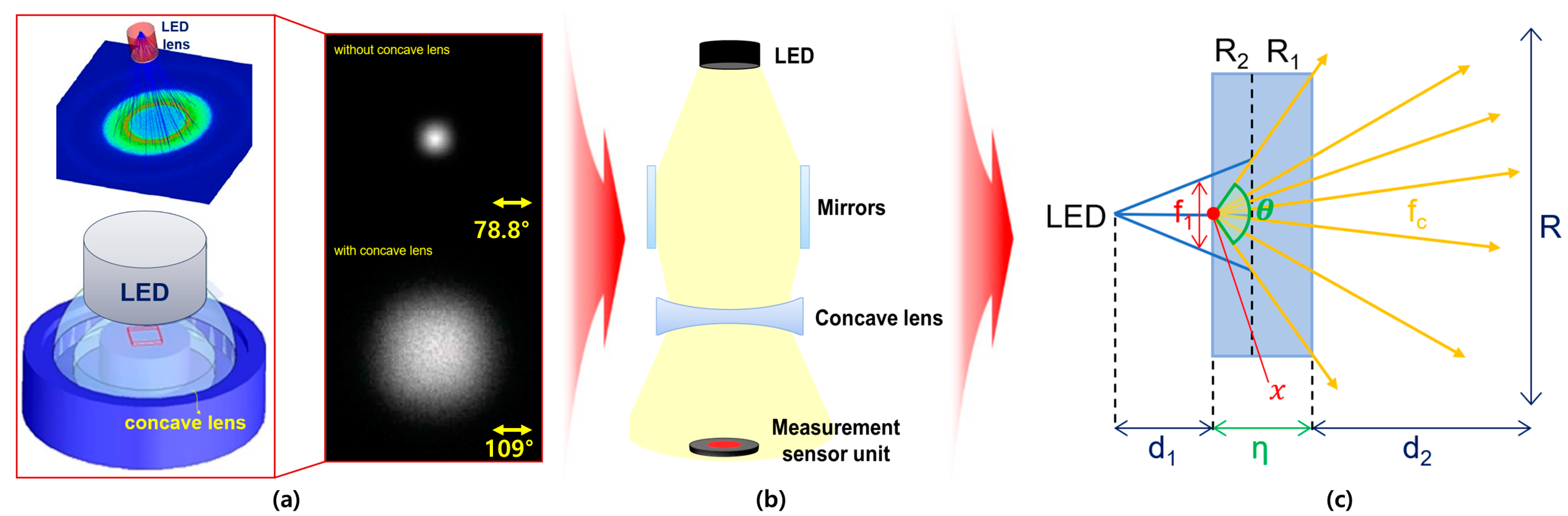
2.2. Method for Deriving Refractive Power Phenomenon
3. Experimental Methods and Results
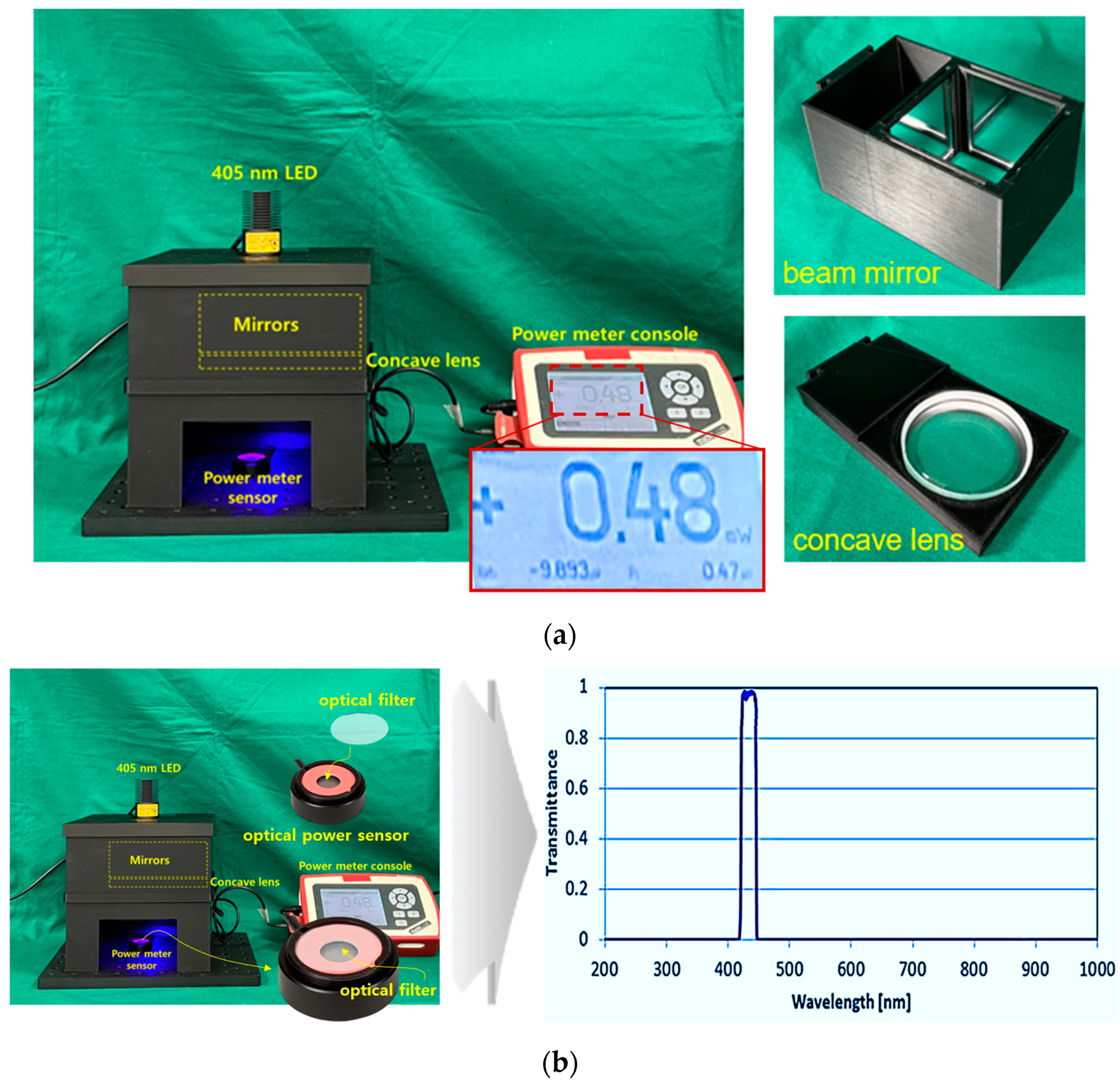
3.1. Experimental Environment Configuration
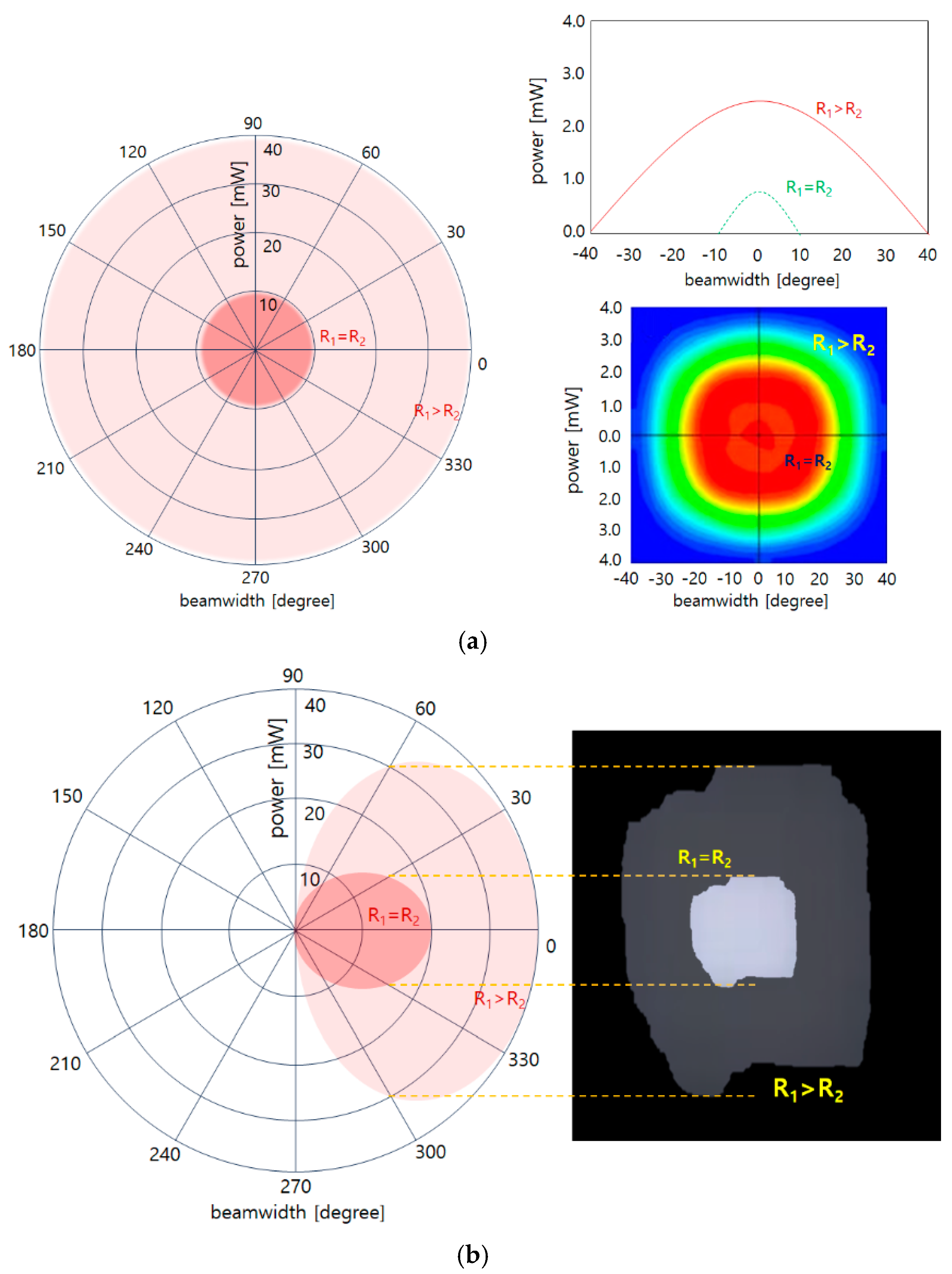
3.2. Experimental Method and Result Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almotiri, J.; Elleithy, K.; Elleithy, A. Retinal vessels segmentation techniques and algorithms: A survey. Appl. Sci. 2018, 8, 155. [Google Scholar] [CrossRef]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Song, K.D.; Kang, S.; Park, J.S.; Choi, W. Optical imaging featuring both long working distance and high spatial resolution by correcting the aberration of a large aperture lens. Sci. Rep. 2018, 8, 9165. [Google Scholar] [CrossRef]
- Ju, M.; Yoon, K.C.; Lee, S.; Kim, K.G. Single quasi-symmetrical LED with high intensity and wide beam width using diamond-shaped mirror refraction method for surgical fluorescence microscope applications. Diagnostics 2023, 13, 2763. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, T.; Suzuki, T.; Shimoda, K. A prism anamorphic system for Gaussian beam expander. Appl. Phys. 1978, 17, 131–136. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, J.H.; Lee, W.B. A study on the development of a white light source module for a large-capacity searchlight using a blue laser diode. Electronics 2023, 12, 760. [Google Scholar] [CrossRef]
- Li, Y.N.Q.; Luo, Z.Y.; Wu, S.T. High-precision beam angle expander based on polymeric liquid crystal polarization lenses for LiDAR applications. Crystals 2022, 12, 349. [Google Scholar] [CrossRef]
- Oh, D.M.; Lee, J.K.; Kim, H.; Park, C.K.; Jung, J.K.; Kim, D.J.; Chung, Y.J.; Kim, T.H.; Park, M.I.; Park, J.P. Local recurrence and its risk factor after incomplete resection of colorectal advanced adenomas: A single center, Retrospective Study. Korean J. Gastroenterol. 2017, 70, 33–38. [Google Scholar] [CrossRef]
- Pohl, H.; Anderson, J.C.; Aguilera-Fish, A.; Calderwood, A.H.; Mackenzie, T.A.; Robertson, D.J. Recurrence of colorectal neoplastic polyps after incomplete resection. Ann. Intern. Med. 2021, 174, 1377–1384. [Google Scholar] [CrossRef]
- Seo, H.W.; Yoon, K.C.; Lee, S.; Lee, W.S.; Kim, K.G. Design of a miniature observation robot for light emitting diode irradiation and indocyanine green fluorescence-emission guided lymph node monitoring in operating rooms. Surg. Innov. 2023, 12, 349. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.F. Condenser-free contrast methods for transmitted-light microscopy. J. Microsc. 2015, 257, 8–22. [Google Scholar] [CrossRef]
- Williamson, D.E. Cone channel condenser optics. J. Opt. Soc. Am. 1952, 42, 712–715. [Google Scholar] [CrossRef]
- Yoon, K.C.; Kim, K.G. NIR irradiation based on low-power LED drive module design for fat reduction. Biomed Res. Int. 2021, 2021, 9992095. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Miyamoto, T. Optimization for compact and high output LED-based optical wireless power transmission system. Photonics 2022, 9, 14. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Zhang, Z. Fluorescence image-guided tumor surgery. Nat. Rev. Bioeng. 2023, 1, 161–179. [Google Scholar] [CrossRef]
- Liu, J.T.C.; Sanai, N. Trends and challenges for the clinical adoption of fluorescence-guided surgery. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 756–757. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the surgical benefit of utilizing 5-Aminolevulinic Acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Hoffnagle Jonhn, A.; Jefferson Michael, C. Design and performance of a refractive optical system that converts a Gaussian to a flattop beam. Appl. Opt. 2000, 39, 5488–5499. [Google Scholar] [CrossRef]
- Lee, S.M.; Yoon, K.C.; Lee, S.; Ryu, S.Y.; Kim, K.G. Multi-asymmetric irradiation method using a ring array to obtain an emission-capable LED beam power effect to observe cancer removal status in a surgical microscope. Diagnostics 2023, 13, 3482. [Google Scholar] [CrossRef]
- Yoon, K.C.; Kim, K.G.; Lee, S. A surgical pen-type probe design for real-time optical diagnosis of tumor status using 5-aminolevulinic acid. Diagnostics 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.T.; Iu, R.S.; Shi, Y.Q.; Xia, R.X. Optical design of color light-emitting diode ring light for machine vision inspection. Opt. Eng. 2011, 50, 043001. [Google Scholar] [CrossRef]
- Ma, L.; Fei, B. Comprehensive review of surgical microscopes: Technology development and medical applications. J. Biomed. Opt. 2021, 26, 010901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Wang, H. Comparison of Beam Profiles and Propagation Characteristics between LEDs and Lasers. Appl. Sci. 2023, 13, 4567. [Google Scholar]
- Smith, R.; Jones, T.; Green, D. High-Precision Beam Expansion for Laser Systems: Techniques and Applications. Photonics 2022, 11, 234. [Google Scholar]
- Smith, R.; Brown, D. Design and Performance Analysis of Beam Expanders for Laser Systems. Opt. Express 2021, 29, 6789–6802. [Google Scholar]
- Schebesch, K.M.; Proescholdt, M.; Höhne, J.; Hohenberger, C.; Hansen, E.; Riemenschneider, M.J.; Ullrich, W.; Doenitz, C.; Schlaier, J.; Lange, M.; et al. Sodium fluorescein–guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—A feasibility study. Acta Neurochir. 2013, 155, 693–699. [Google Scholar] [CrossRef]
- Miller, A.R.; Davis, G.L.; Oden, Z.M.; Razavi, M.R.; Fateh, A.; Ghazanfari, M.; Abdolrahimi, F.; Poorazar, S.; Sakhaie, F.; Olsen, R.J.; et al. Portable, battery-operated, low-cost, bright field and fluorescence microscope. PLoS ONE 2010, 5, e11890. [Google Scholar] [CrossRef]
- Wang, X. LED ring array light source design and uniform illumination properties analysis. Optik 2017, 140, 273–281. [Google Scholar] [CrossRef]
- Tyrer, H.E.; Pratten, J.; Wilson, M. The potential of visible blue light (405 nm) as a novel decontamination strategy for carbapenemase-producing enterobacteriaceae (CPE). Antimicrob. Resist. Infect. Control 2022, 11, 75. [Google Scholar]
- Thurman Colleen, E.; Muthuswamy, A.; Klinger Mark, M.; Roble Gordon, S. Safety evaluation of a 405-nm LED device for direct antimicrobial treatment of the murine brain. Comp Med. 2019, 69, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Gu, Y.; Freyssinier-Nova, J.P.; Zhu, Y. Thermal Management of High Power LEDs. IEEE Trans. Device Mater. Reliab. 2005, 5, 701–705. [Google Scholar]
- Piprek, J. Heat Generation and Transport in Light-Emitting Diodes. IEEE J. Quantum Electron. 2003, 38, 1253–1259. [Google Scholar] [CrossRef]
- Meneghini, M.; Trivellin, N.; Mura, G.; Buffolo, M.; Pavesi, M.; Zanoni, E. Thermal Effects in High-Power AlGaInN LEDs: Junction Temperature and Reliability Impact. IEEE Trans. Electron. Devices 2008, 55, 180–186. [Google Scholar]




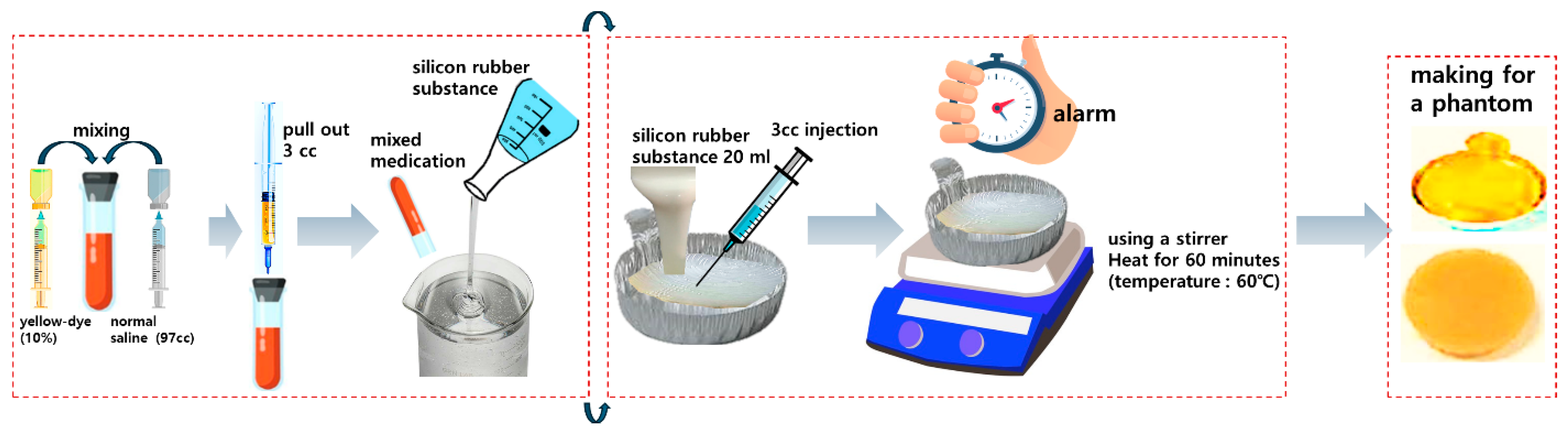
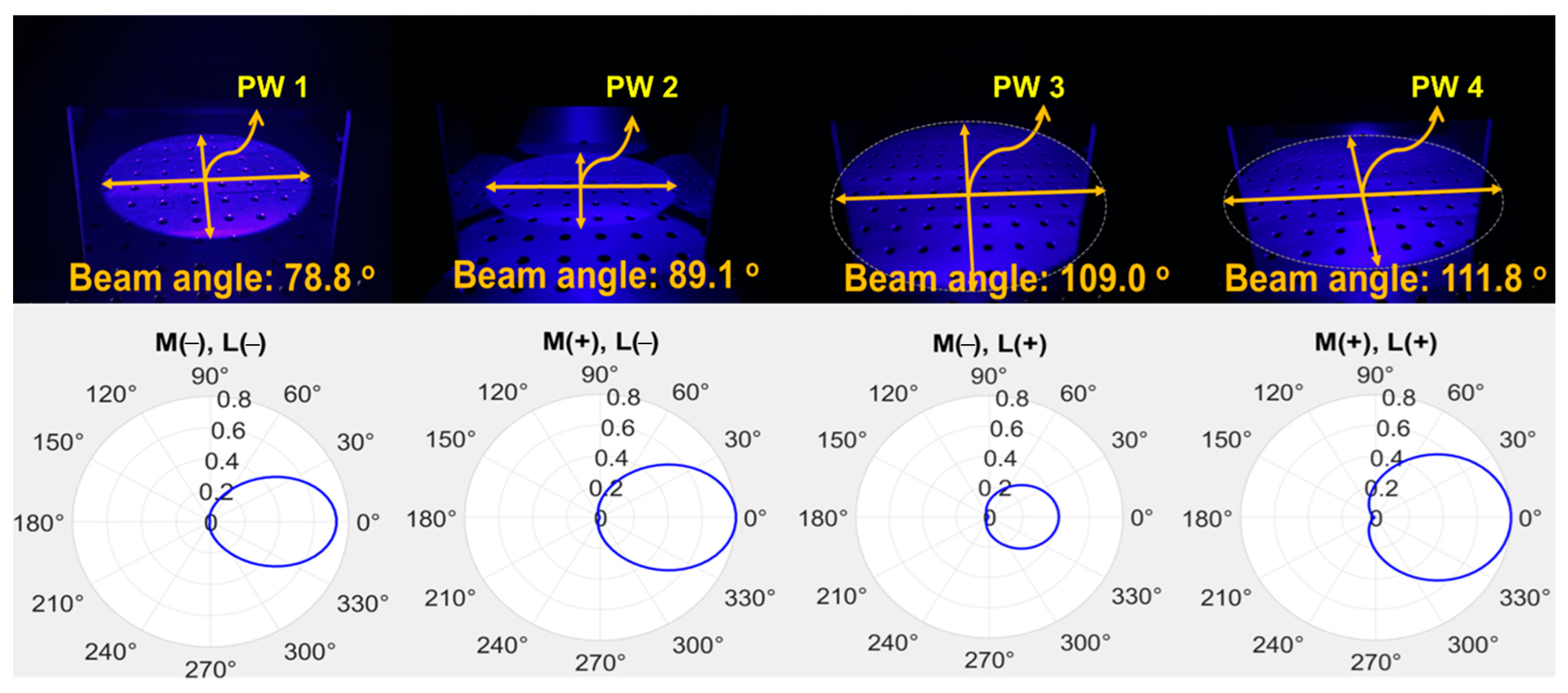

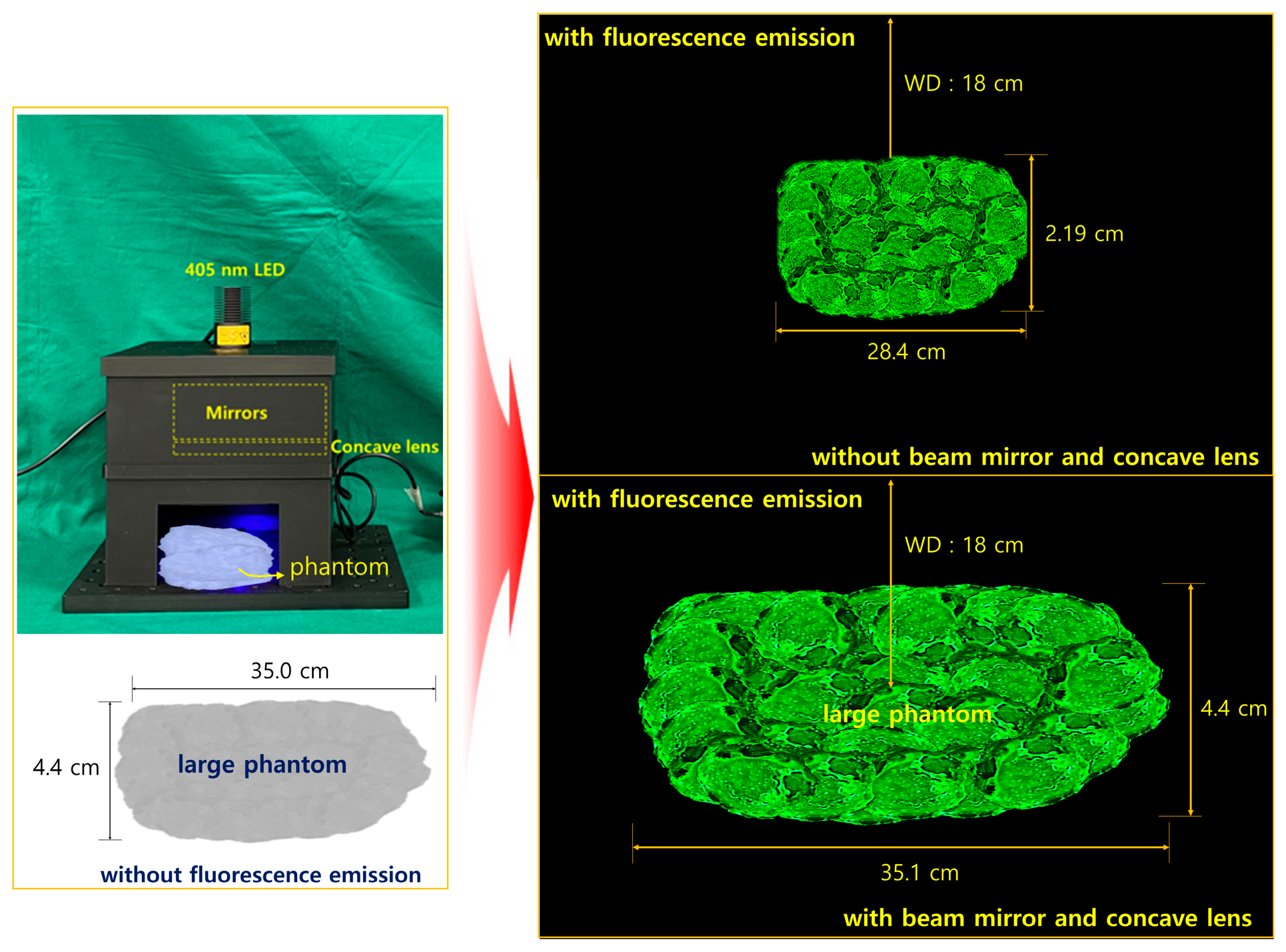
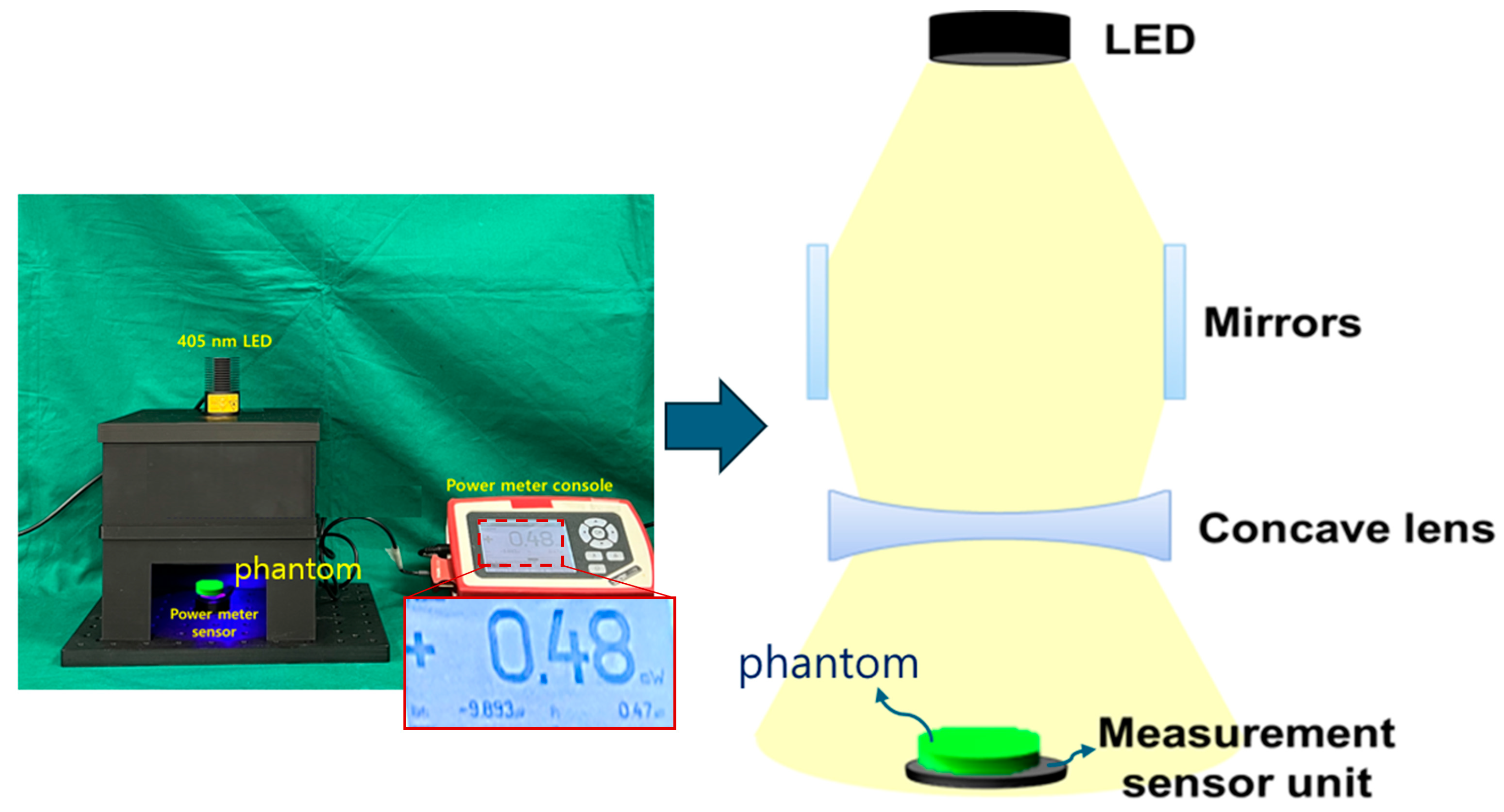
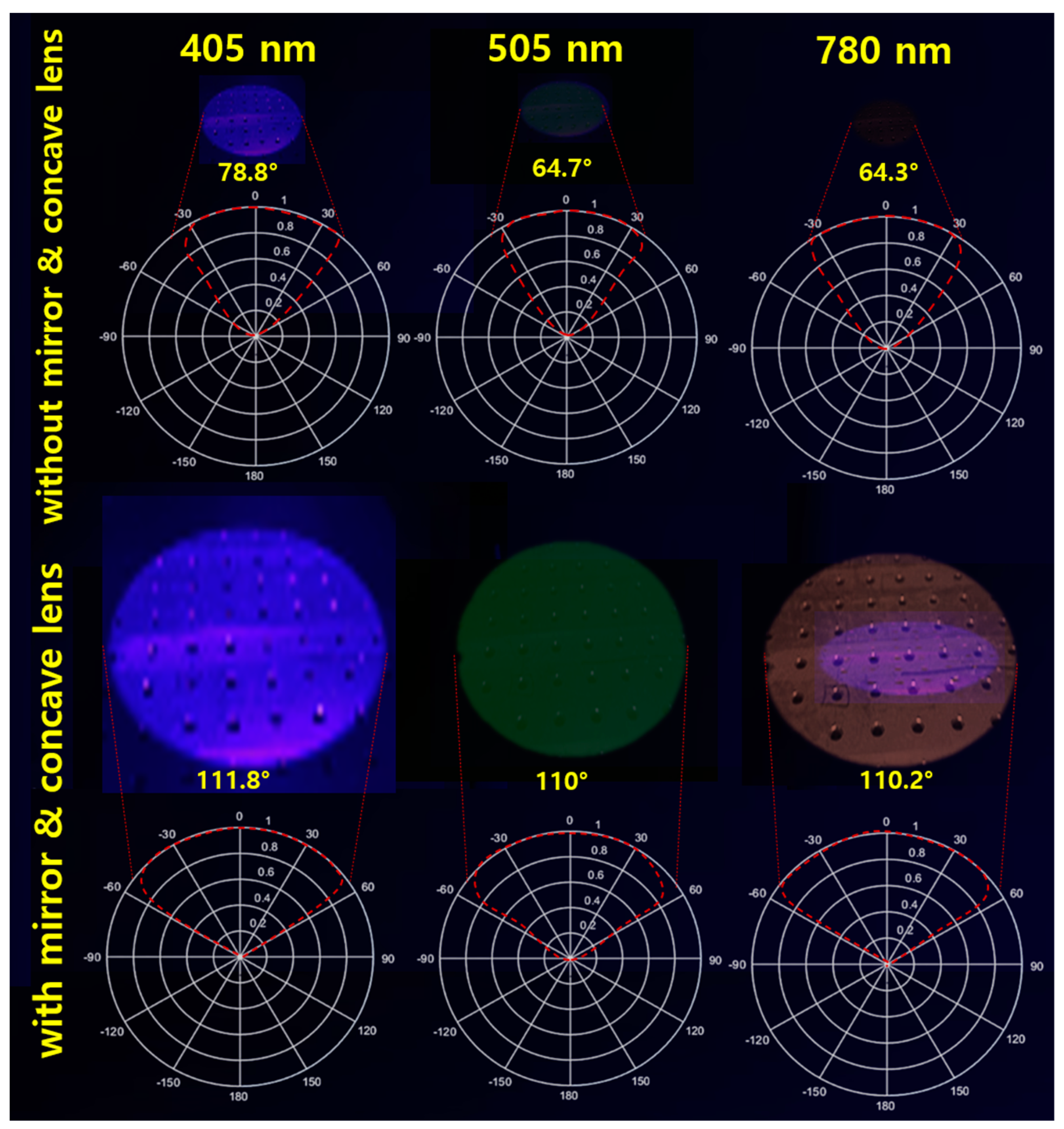
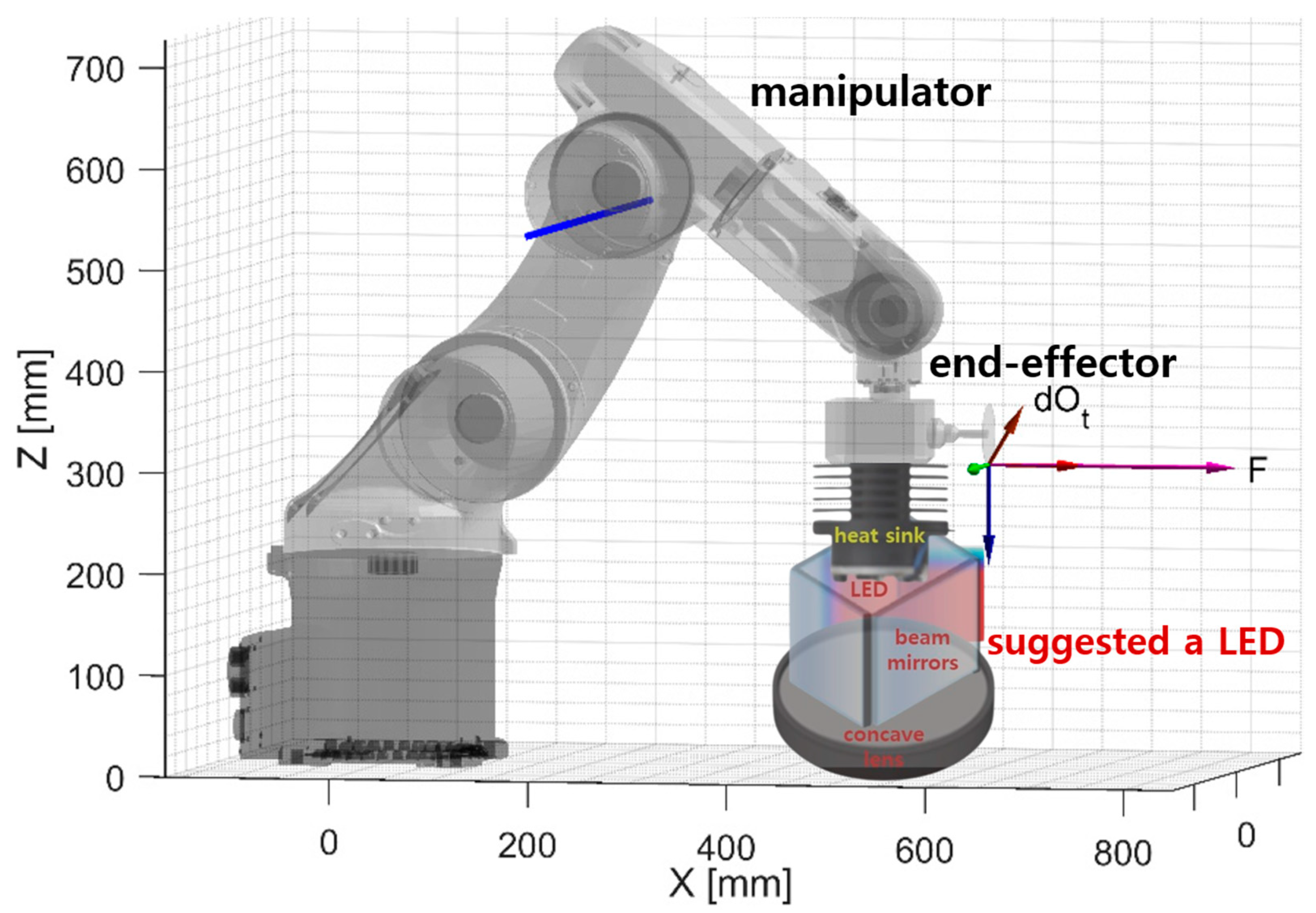
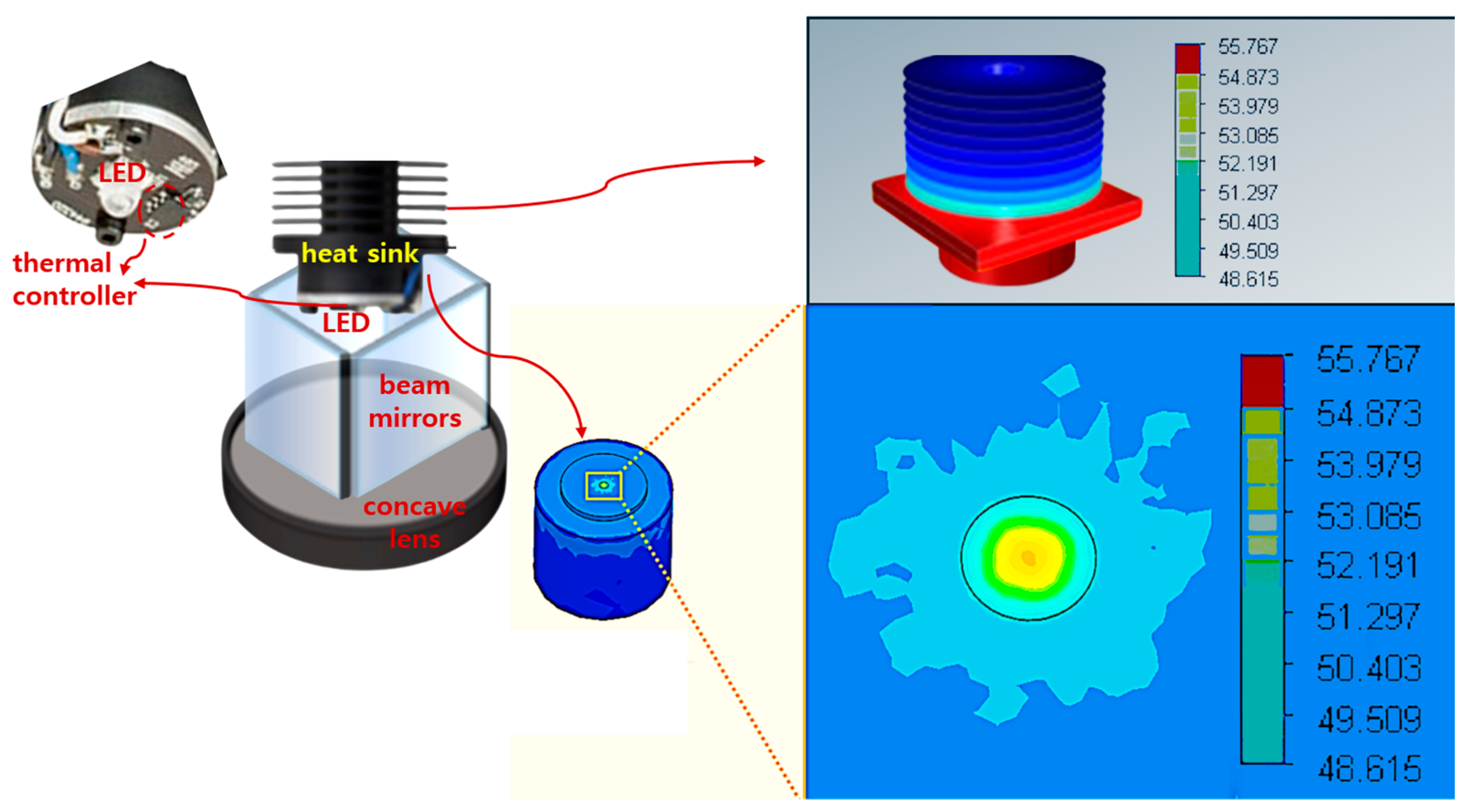
| Unit | Target Power [mW] | Beam Width | Irradiation Area [cm] | Fluorescence Emission Power [mW] | Applications | |
|---|---|---|---|---|---|---|
| Mirrors (M) | Concave Lens (L) | |||||
| PW1 | 0.73 | 78.8° | 24.8 | 0.12 | not applicable (M−) | not applicable (L−) |
| PW2 | 10.1 | 89.1° | 28.0 | 4.00 | Applicable (M+) | not applicable (L−) |
| PW3 | 10.9 | 109° | 34.2 | 5.30 | not applicable (M−) | Applicable (L+) |
| PW4 | 13.6 | 111.8° | 35.1 | 6.20 | Applicable (M+) | Applicable (L+) |
| Irradiation Wavelength [nm] | Emission Wavelength [nm] | Mirror & Concave Lens | Beam Width | Irradiation Area [cm] | Received Power [mW] | Emission Power [mW] |
|---|---|---|---|---|---|---|
| 405 | 530-560 | without | 78.8 | 24.8 | 0.73 | 0.12 |
| with | 111.8 | 35.1 | 13.6 | 6.20 | ||
| 505 | 550-580 | without | 64.7 | 22.7 | 0.69 | 0.11 |
| with | 110.0 | 34.4 | 12.7 | 5.86 | ||
| 780 | 830-860 | without | 64.3 | 22.2 | 0.56 | 0.092 |
| with | 110.2 | 34.8 | 11.6 | 5.65 |
| Ref. [#] | λext [nm] | WD [cm] | Beam Width [cm2] | Pmax [mW] | Target Received Power [mW] | LED Quantity [ea] |
|---|---|---|---|---|---|---|
| this work | 405 | 18.0 | 35.1 | 200 | 13.6 | 1.00 (LED) |
| [23] | 405 | 0.25 | 0.027 | 40.0 | 12.3 | 1.0 (laser) |
| [27] | 550 | 56.75 | 5.72 | 7920 | 0.196 | 9.00 (LED) |
| [28] | 625 | 30.00 | 3.24 | 300,300 | 26.55 | 130 (LED) |
| [29] | 467 | 6.17 | 3.31 | 100 | 6.10 | 52 (LED) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Seo, J.; Yoon, K.; Lee, S.; Kim, M.; Ryu, S.Y.; Kim, K.G. Increasing the Beam Width and Intensity with Refraction Power Effect Using a Combination of Beam Mirrors and Concave Mirrors for Surgical-Fluorescence-Emission-Guided Cancer Monitoring Method. Sensors 2024, 24, 5503. https://doi.org/10.3390/s24175503
Park J, Seo J, Yoon K, Lee S, Kim M, Ryu SY, Kim KG. Increasing the Beam Width and Intensity with Refraction Power Effect Using a Combination of Beam Mirrors and Concave Mirrors for Surgical-Fluorescence-Emission-Guided Cancer Monitoring Method. Sensors. 2024; 24(17):5503. https://doi.org/10.3390/s24175503
Chicago/Turabian StylePark, Jina, Jeongmin Seo, Kicheol Yoon, Sangyun Lee, Minchan Kim, Seung Yeob Ryu, and Kwang Gi Kim. 2024. "Increasing the Beam Width and Intensity with Refraction Power Effect Using a Combination of Beam Mirrors and Concave Mirrors for Surgical-Fluorescence-Emission-Guided Cancer Monitoring Method" Sensors 24, no. 17: 5503. https://doi.org/10.3390/s24175503
APA StylePark, J., Seo, J., Yoon, K., Lee, S., Kim, M., Ryu, S. Y., & Kim, K. G. (2024). Increasing the Beam Width and Intensity with Refraction Power Effect Using a Combination of Beam Mirrors and Concave Mirrors for Surgical-Fluorescence-Emission-Guided Cancer Monitoring Method. Sensors, 24(17), 5503. https://doi.org/10.3390/s24175503







