Use of Virtual Reality in School-Aged Children with Developmental Coordination Disorder: A Novel Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
- Clinical outcome measures
- (a)
- “Visual Attention Subtest” of the Italian Version of Developmental Neuropsychological Assessment—Second Edition (NEPSY-II) was used to assess visual attention skills [26]. This subtest is a time trial of barrage that evaluates how well children can focus on visual target stimuli amidst distractor stimuli: it can be considered a dual-tasking test as subjects are required to split their attention to different elements simultaneously. The Scaled Score, standardised for age, from the test manual (range of 0–19, average score of 10) was obtained by converting the raw score (the number of correct answers minus errors).
- (b)
- To evaluate inhibition skills, the “Inhibition Subtest” from NEPSY-II [26] was administered. In this subtest, the child is asked to identify black-and-white shapes or arrows and name the shape, direction, or an alternate response based on the colour of the shape or arrow. Part A (Naming Condition) and Part B (Inhibition Condition) of the test were used for this study. The Naming Condition requires participants to name the shape of squares and circles or the up or down direction of arrows, so it evaluates, in particular, the capacity to assign the correct name to a figure rapidly; the Inhibition Condition requires participants to provide the opposite naming response on the same stimuli (e.g., if the child is shown a circle, s/he must say “square”; if s/he sees a square, s/he must say “circle”). Performance requires focused attention, verbal memory, resistance to interference, and rapid automatised naming. The Scaled Scores, standardised for the age, from the test manual (range of 0–19, average score of 10) were obtained based on time.
- (c)
- “Mazes Subtest” of the WISC-III [27] was included to evaluate planning abilities. It consists of 10 mazes of various sizes and complexity. The objective is for the child to draw a line from the centre to the outside of each maze without intersecting any lines representing walls. All items are timed. Based on age, the Standard Score is obtained from the test’s manual (range: 3–19) through a ratio between execution time and the number of errors allowed (range raw scores: 0–28).
- Technological outcome measures
- (a)
- Percentage of bonus targets (B%): it is evaluated as the ratio of bonus targets that the children hit (Bhit) by the total number of bonus targets spawned (Bspawned). A higher percentage of bonus hit is indicative of a better performance.
- (b)
- Percentage of malus targets (M%): it is evaluated as the ratio of malus targets that the children hit (Mhit) by the total number of malus targets spawned (Mspawned). A better performance is indicated by a lower percentage of malus targets hit.
- (c)
- Percentage of screen area the children cover during the task (A%): it is estimated using the concave hull method. The concave hull is an evolution of the convex hull, representing the smallest convex polygon containing all the input points. While only one convex hull exists per cloud of point, this is not generally true for the concave hull, as more than one solution can be accepted depending on the final application (for more details, see Ref. [28]). In our case, we estimated the concave hull points following the k-nearest method proposed by Moreira and Santos [28]. Given this set of points, we estimated the area of the hull with the Shoelace formula, also called Gauss’s area formula or Suveryor’s Area formula (Ashoelace) [29]. Finally, we divided this area by the total screen area (Ascreen).
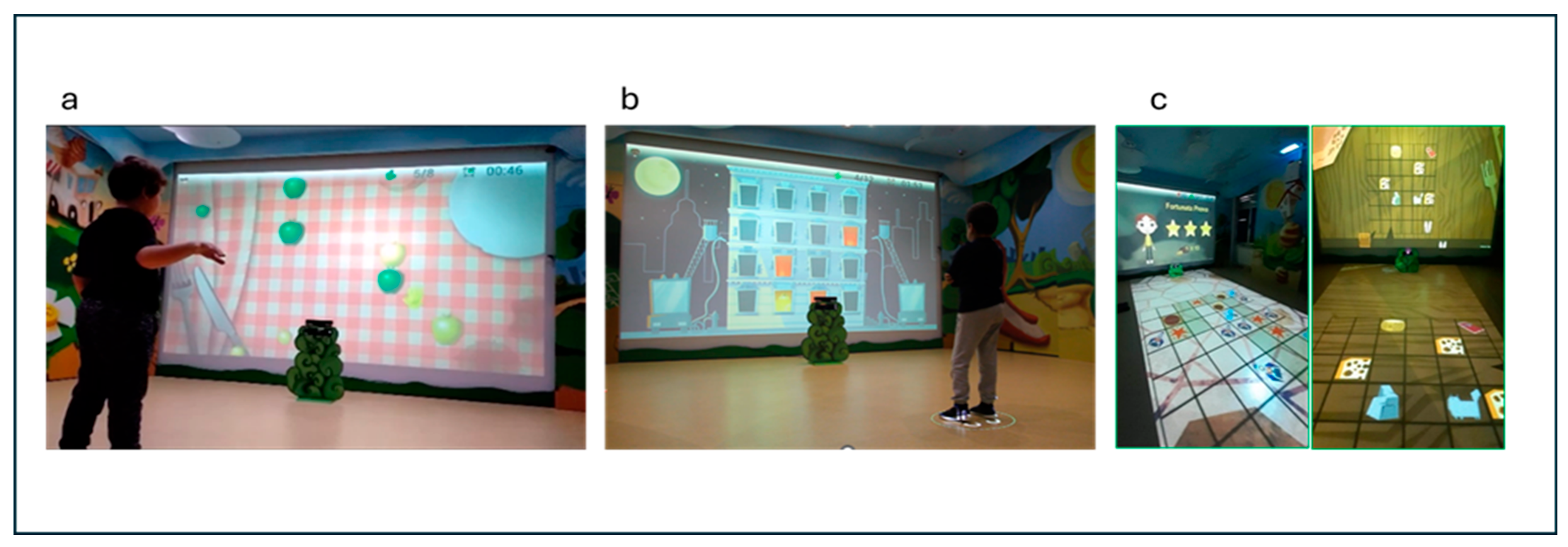
2.3. Computer-Assisted Rehabilitation Laboratory (CARE Lab)
- (a)
- “Gita al parco” (Italian for “Trip to the park”): This game offers moving targets and distractors on the screen that the subject must reach with the upper limb or avoid (for example, to reach the large green and small yellow apples and to avoid the large yellow apples). This game focuses on visual attention, inhibitory motor control, shifting, and upper limb quality movements (adduction–abduction in the frontal plane). A constant ability to plan and control the motor gesture is required since the targets appear randomly and are in continuous movement.
- (b)
- “Pronti, via!” (Italian for “Ready, go!”): In this game, a mixed sequence of targets (from a minimum of 2 to a maximum of 5), with some distractor (from a minimum of 0 to a maximum of 2) is proposed to the child on the screen. Children must memorise the sequence and hit the targets (for example, three princesses that appear in a specific spatial sequence on the screen) by a specific flexion and extension arm’s movements to reproduce the sequence in exact or reverse order (to exercise sequential and visuospatial memory). This game focuses on EFs like visual attention, working memory, and upper limb control movements.
- (c)
- “Passo, passo” (Italian for “Step by step”): during this game, the child is engaged in an activity in which he is moving within a grid projected on the floor. Its goal is to reach specific targets within the grid, following a path that is as direct and precise as possible. Along the way, the child must be careful to prevent unnecessary detours and maintain focus on the final goal. The grid consists of a series of boxes, some of which are active while others are passive. The active boxes present challenges, such as mini-games that require the child to complete exercises such as balancing on one leg, shifting body weight from side to side, or performing light squats, while other active boxes must be avoided because they include distractor targets that, if stepped on, force the child to re-start the game and re-plan the most suitable path. Passive boxes, on the other hand, do not contain additional challenges or exercises. These boxes serve as transit points, allowing the child to continue toward the target without interruption. This activity helps the child to develop motor, problem-solving, and planning skills.
- Intervention procedures
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- APA—American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Biotteau, M.; Albaret, J.M.; Chaix, Y. Chapter 1—Developmental coordination disorder. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 174, pp. 3–20. [Google Scholar] [CrossRef]
- Geuze, R.H. Postural Control in Children with Developmental Coordination Disorder. Neural Plast. 2005, 12, 183–196; discussion 263–272. [Google Scholar] [CrossRef]
- Johnston, L.M.; Burns, Y.R.; Brauer, S.G.; A Richardson, C. Differences in postural control and movement performance during goal directed reaching in children with developmental coordination disorder. Hum. Mov. Sci. 2002, 21, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsson, H.; Whiting, H. Hand Preference in Children with Developmental Coordination Disorders: Cause and Effect? Brain Cogn. 2002, 49, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsson, H.; Ingvaldsen, R.P.; Whiting, H.T.A. Inter- and intra-sensory modality matching in children with hand-eye co-ordination problems. Exp. Brain Res. 1997, 114, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Grohs, M.N.; Hawe, R.L.; Dukelow, S.P.; Dewey, D. Unimanual and bimanual motor performance in children with developmental coordination disorder (DCD) provide evidence for underlying motor control deficits. Sci. Rep. 2021, 11, 5982. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Lee, C.-M. Motor skill learning in children with Developmental Coordination Disorder. Res. Dev. Disabil. 2013, 34, 2047–2055. [Google Scholar] [CrossRef]
- López, A.G.; Madrid, V.C.; Hidalgo-Robles, Á.; Gutiérrez-Ortega, M. Early signs of functioning and contextual factors in children 0 to 6 years of age at high risk of or with developmental coordination disorder: A scoping review. Child Care Health Dev. 2022, 49, 230–239. [Google Scholar] [CrossRef]
- Lust, J.M.; Steenbergen, B.; Diepstraten, J.E.M.; Wilson, P.H.; Schoemaker, M.M.; Poelma, M.J. The subtypes of developmental coordination disorder. Dev. Med. Child Neurol. 2022, 64, 1366–1374. [Google Scholar] [CrossRef]
- Van Dyck, D.; Baijot, S.; Aeby, A.; De Tiège, X.; Deconinck, N. Cognitive, perceptual, and motor profiles of school-aged children with developmental coordination disorder. Front. Psychol. 2022, 13, 860766. [Google Scholar] [CrossRef]
- Sartori, R.F.; Valentini, N.C.; Fonseca, R.P. A comparative study of executive function in children with and without developmental coordination disorder. Child Care Health Dev. 2019, 46, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.F.; Nobre, G.C.; Fonseca, R.P.; Valentini, N.C. Do executive functions and gross motor skills predict writing and mathematical performance in children with developmental coordination disorder? Appl. Neuropsychol. Child 2021, 11, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Querne, L.; Berquin, P.; Vernier-Hauvette, M.-P.; Fall, S.; Deltour, L.; Meyer, M.-E.; de Marco, G. Dysfunction of the attentional brain network in children with Developmental Coordination Disorder: A fMRI study. Brain Res. 2008, 1244, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Visser, J. Developmental coordination disorder: A review of research on subtypes and comorbidities. Hum. Mov. Sci. 2003, 22, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef] [PubMed]
- Michel, E.; Molitor, S.; Schneider, W. Differential changes in the development of motor coordination and executive functions in children with motor coordination impairments. Child Neuropsychol. 2016, 24, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Leonard, H.C. Internalising symptoms in Developmental Coordination Disorder: The indirect effect of everyday executive function. Res. Dev. Disabil. 2020, 109, 103831. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Ling, D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016, 18, 34–48. [Google Scholar] [CrossRef]
- Lino, F.; Arcangeli, V.; Chieffo, D.P.R. The Virtual Challenge: Virtual Reality Tools for Intervention in Children with Developmental Coordination Disorder. Children 2021, 8, 270. [Google Scholar] [CrossRef]
- Adams, I.L.; Lust, J.M.; Wilson, P.H.; Steenbergen, B. Compromised motor control in children with DCD: A deficit in the internal model?—A systematic review. Neurosci. Biobehav. Rev. 2014, 47, 225–244. [Google Scholar] [CrossRef]
- Wang, M.; Reid, D. Virtual Reality in Pediatric Neurorehabilitation: Attention Deficit Hyperactivity Disorder, Autism and Cerebral Palsy. Neuroepidemiology 2010, 36, 2–18. [Google Scholar] [CrossRef]
- Holden, M.K. Virtual Environments for Motor Rehabilitation: Review. CyberPsychol. Behav. 2005, 8, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Roopchand-Martin, S.; Gregg, A. Potential of the Nintendo Wii™ as a rehabilitation tool for children with cerebral palsy in a developing country: A pilot study. Physiotherapy 2012, 98, 238–242. [Google Scholar] [CrossRef]
- Di Giusto, V.; Purpura, G.; Zorzi, C.F.; Blonda, R.; Brazzoli, E.; Meriggi, P.; Reina, T.; Rezzonico, S.; Sala, R.; Olivieri, I.; et al. Virtual reality rehabilitation program on executive functions of children with specific learning disorders: A pilot study. Front. Psychol. 2023, 14, 1241860. [Google Scholar] [CrossRef]
- Urgesi, C.; Campanella, F.; Fabbro, F. La Versione Italiana Della NEPSY-II per la Valutazione Neuropsicologica ad Ampio Raggio del Bambino da 3 a 16 Anni; Giunti: Florence, Italy, 2011. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children—Third Edition (WISC-III); The Psychological Corporation: San Antonio, TX, USA, 1991. [Google Scholar]
- Moreira, A.; Santos, M.Y. Concave Hull: A k-nearest neighbours approach for the computation of the region occupied by a set of points. In Proceedings of the 2nd International Conference on Computer Graphics Theory and Applications (VISIGRAPP 2007), Barcelona, Spain, 8–11 March 2007; SciTePress: Setúbal, Portugal, 2007; Volume 2, pp. 61–68, ISBN 978-972-8865-71-9, ISSN 2184-4321. [Google Scholar] [CrossRef]
- Braden, B. The Surveyor’s Area Formula. Coll. Math. J. 1986, 17, 326–337. [Google Scholar] [CrossRef]
- Olivieri, I.; Meriggi, P.; Fedeli, C.; Brazzoli, E.; Castagna, A.; Roidi, M.L.R.; Angelini, L. Computer Assisted REhabilitation (CARE) Lab: A novel approach towards Pediatric Rehabilitation 2.0. J. Pediatr. Rehabil. Med. 2018, 11, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, P.; Mandalà, M.; Randazzo, M.; Brazzoli, E.; Castagna, A.; Di Giusto, V.; Cavallini, A.; Marzegan, A.; Lencioni, T.; Olivieri, I. Non-immersive virtual reality based treatment for children with unilateral cerebral palsy: Preliminary results. J. Pediatr. Rehabil. Med. 2024, 17, 107–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serdarevic, F.; van Batenburg-Eddes, T.; Mous, S.E.; White, T.; Hofman, A.; Jaddoe, V.W.; Verhulst, F.C.; Ghassabian, A.; Tiemeier, H. Relation of infant motor development with nonverbal intelligence, language comprehension and neuropsychological functioning in childhood: A population-based study. Dev. Sci. 2015, 19, 790–802. [Google Scholar] [CrossRef]
- Asonitou, K.; Koutsouki, D.; Kourtessis, T.; Charitou, S. Motor and cognitive performance differences between children with and without developmental coordination disorder (DCD). Res. Dev. Disabil. 2012, 33, 996–1005. [Google Scholar] [CrossRef]
- Asonitou, K.; Koutsouki, D. Cognitive process-based subtypes of developmental coordination disorder (DCD). Hum. Mov. Sci. 2016, 47, 121–134. [Google Scholar] [CrossRef]
- Roebers, C.M.; Kauer, M. Motor and cognitive control in a normative sample of 7-year-olds. Dev. Sci. 2008, 12, 175–181. [Google Scholar] [CrossRef]
- Dewey, D.; Kaplan, B.J.; Crawford, S.G.; Wilson, B.N. Developmental coordination disorder: Associated problems in attention, learning, and psychosocial adjustment. Hum. Mov. Sci. 2002, 21, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Kadesjö, B.; Gillberg, C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev. Med. Child Neurol. 1998, 40, 796–804. [Google Scholar] [CrossRef]
- Gillberg, C.; Kadesjö, B. Why Bother About Clumsiness? The Implications of Having Developmental Coordination Disorder (DCD). Neural Plast. 2000, 10, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lino, F.; Chieffo, D.P.R. Developmental Coordination Disorder and Most Prevalent Comorbidities: A Narrative Review. Children 2022, 9, 1095. [Google Scholar] [CrossRef]
- Martini, G.; Beani, E.; Filogna, S.; Menici, V.; Cioni, G.; Battini, R.; Sgandurra, G. New Technological Approach for the Evaluation of Postural Control Abilities in Children with Developmental Coordination Disorder. Children 2022, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Dan, B. Gamification of therapy: The fun factor in rehabilitation. Dev. Med. Child Neurol. 2022, 64, 276. [Google Scholar] [CrossRef]
- Ashkenazi, T.M.; Weiss, P.L.P.; Orian, D.; Laufer, Y.D. Low-Cost Virtual Reality Intervention Program for Children with Developmental Coordination Disorder: A pilot feasibility study. Pediatr. Phys. Ther. 2013, 25, 467–473. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; FitzGerald, T.L.; Clark, R.A.; Bower, K.J.; Denehy, L.; Spittle, A.J. Do video game interventions improve motor outcomes in children with developmental coordination disorder? A systematic review using the ICF framework. BMC Pediatr. 2019, 19, 22. [Google Scholar] [CrossRef]
- Ferguson, G.; Jelsma, D.; Jelsma, J.; Smits-Engelsman, B. The efficacy of two task-orientated interventions for children with Developmental Coordination Disorder: Neuromotor Task Training and Nintendo Wii Fit training. Res. Dev. Disabil. 2013, 34, 2449–2461. [Google Scholar] [CrossRef]
- Cavalcante Neto, J.L.C.; Steenbergen, B.; Wilson, P.; Zamunér, A.R.; Tudella, E. Is Wii-based motor training better than task-specific matched training for children with developmental coordination disorder? A randomized controlled trial. Disabil. Rehabil. 2019, 42, 2611–2620. [Google Scholar] [CrossRef]
- EbrahimiSani, S.; Sohrabi, M.; Taheri, H.; Agdasi, M.T.; Amiri, S. Effects of virtual reality training intervention on predictive motor control of children with DCD—A randomized controlled trial. Res. Dev. Disabil. 2020, 107, 103768. [Google Scholar] [CrossRef]

| Gender | Age | Dominant Hand * | IQ | Comorbidities ** | |
|---|---|---|---|---|---|
| S1 | M | 7 | R | 106 | DG |
| S2 | M | 8 | R | 102 | DG |
| S3 | M | 9 | R | 90 | DG + DC |
| S4 | M | 8 | L | 101 | DG |
| S5 | M | 9 | R | 71 | DL + DO + DG + DC |
| S6 | F | 7 | R | 83 | DL + DG |
| S7 | F | 9 | R | 102 | DG + DO |
| S8 | F | 7 | L | 92 | DL + DO + DG |
| S9 | F | 8 | R | 100 | DG + DO |
| S10 | M | 7 | R | 82 | DG |
| Indexes | Pre-Training Mean (SD) | Post-Training Mean (SD) | p-Value (Wilcoxon Test) | Effect Size | Mean of Δelta (%of Amelioration) |
|---|---|---|---|---|---|
| Bonus (%)—Dominant Hand | 70.42 (13.5) | 84.53(12.1) | 0.010 * | large | 21.7 |
| Malus (%)—Dominant Hand | 26.16 (16.8) | 10.0 (8.4) | 0.014 * | large | −45.8 |
| Covered Area (%)—Dominant Hand | 65.89 (5.5) | 62.0 (7.9) | 0.333 | small | −4.6 |
| Bonus (%)—Non-Dominant Hand | 67.01 (13.8) | 83.85 (10.5) | 0.006 ** | large | 28.5 |
| Malus (%)—Non-Dominant Hand | 23.45 (12.5) | 14.8 (11.6) | 0.020 * | large | −36.4 |
| Covered Area (%)—Non-Dominant Hand | 65.50 (9.7) | 65.69 (8.5) | 1.000 | null | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purpura, G.; Di Giusto, V.; Zorzi, C.F.; Figliano, G.; Randazzo, M.; Volpicelli, V.; Blonda, R.; Brazzoli, E.; Reina, T.; Rezzonico, S.; et al. Use of Virtual Reality in School-Aged Children with Developmental Coordination Disorder: A Novel Approach. Sensors 2024, 24, 5578. https://doi.org/10.3390/s24175578
Purpura G, Di Giusto V, Zorzi CF, Figliano G, Randazzo M, Volpicelli V, Blonda R, Brazzoli E, Reina T, Rezzonico S, et al. Use of Virtual Reality in School-Aged Children with Developmental Coordination Disorder: A Novel Approach. Sensors. 2024; 24(17):5578. https://doi.org/10.3390/s24175578
Chicago/Turabian StylePurpura, Giulia, Valentina Di Giusto, Carla Fulvia Zorzi, Giusi Figliano, Mattia Randazzo, Valentina Volpicelli, Rosanna Blonda, Elena Brazzoli, Tarjn Reina, Silvia Rezzonico, and et al. 2024. "Use of Virtual Reality in School-Aged Children with Developmental Coordination Disorder: A Novel Approach" Sensors 24, no. 17: 5578. https://doi.org/10.3390/s24175578





