Autonomic Responses Associated with Olfactory Preferences of Fragrance Consumers: Skin Conductance, Respiration, and Heart Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
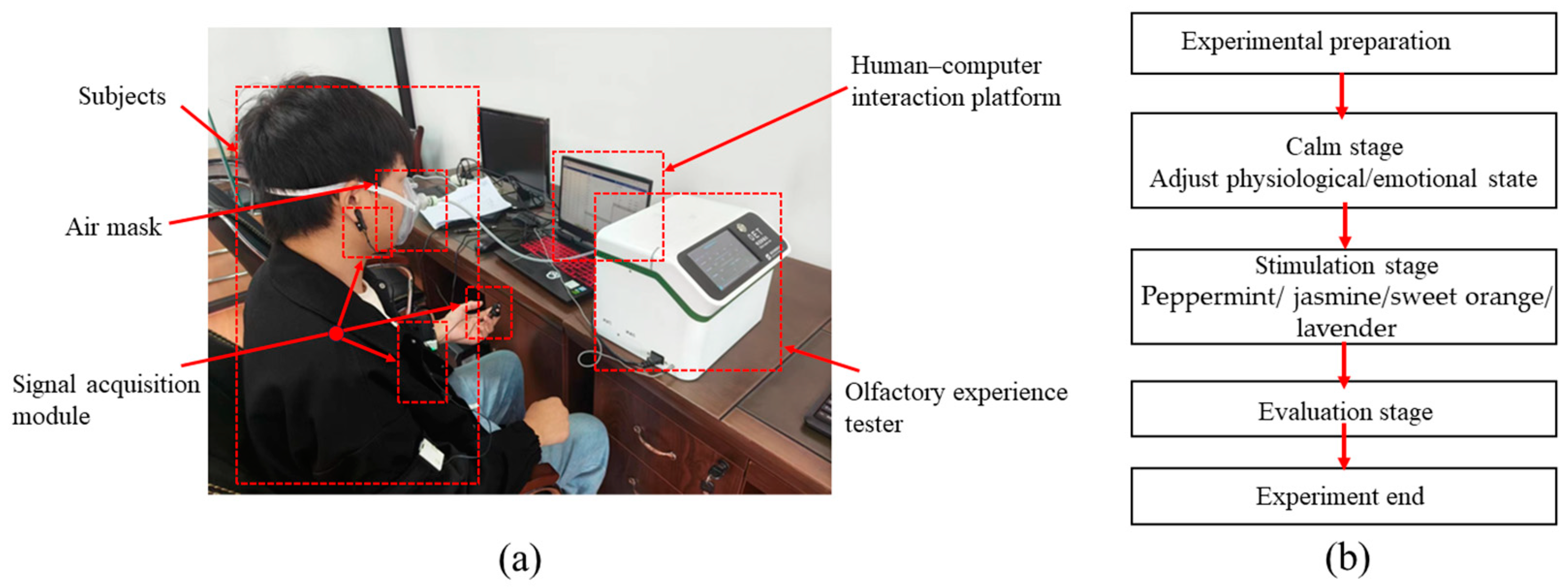
2.2. Equipment and Procedure
- (1)
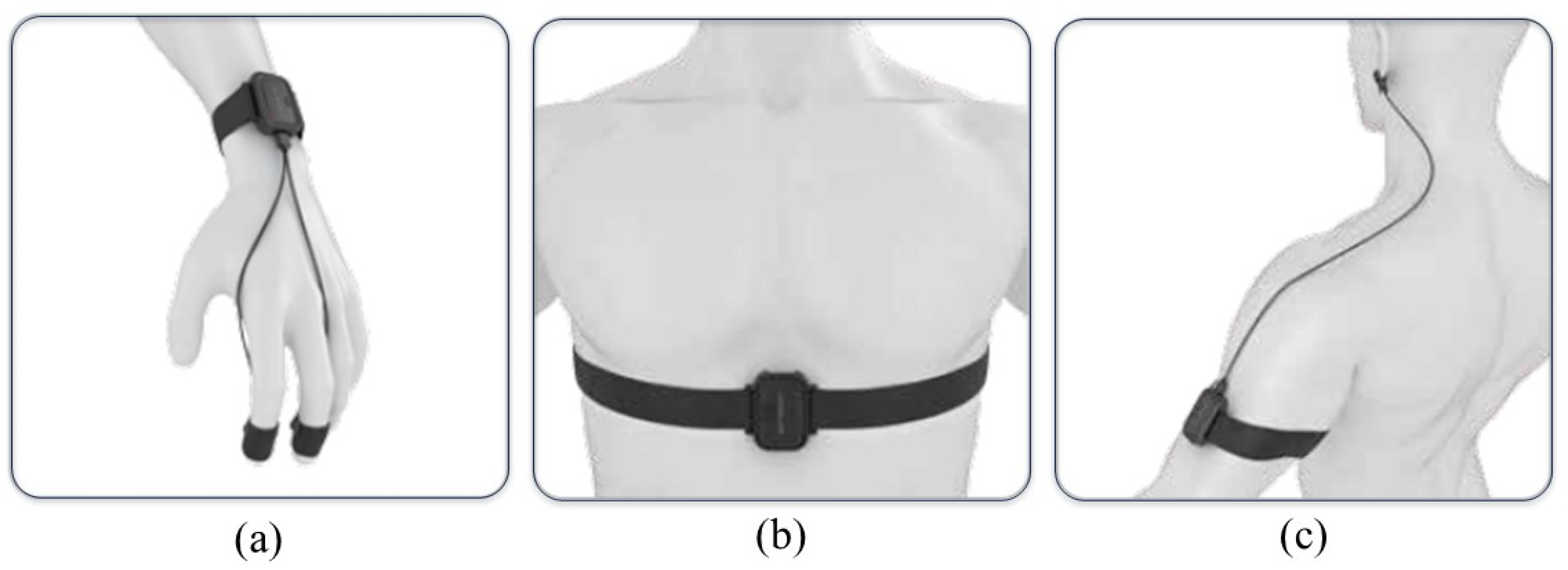
- ErgoLAB EDA wireless skin conductance sensor (sampling rate: 64 Hz, acquisition range: 0–30 μS). The two electrodes of the EDA sensor are fixed at the fingertips of the index finger and middle finger (as shown in Figure 1a).
- (2)
- ErgoLAB RESP wireless respiratory sensor (sampling rate: 64 Hz; acquisition range: 0–140 rpm). The belt of the RESP sensor is fixed between the chest and abdomen of the subject (as shown in Figure 1b).
- (3)
- ErgoLAB PPG wireless blood volume pulse sensor (sampling rate: 64 Hz; acquisition range: 0–240 bpm). The ear clip electrodes of the PPG sensor are fixed on the earlobe (as shown in Figure 1c).
2.3. Data Processing and Analysiss
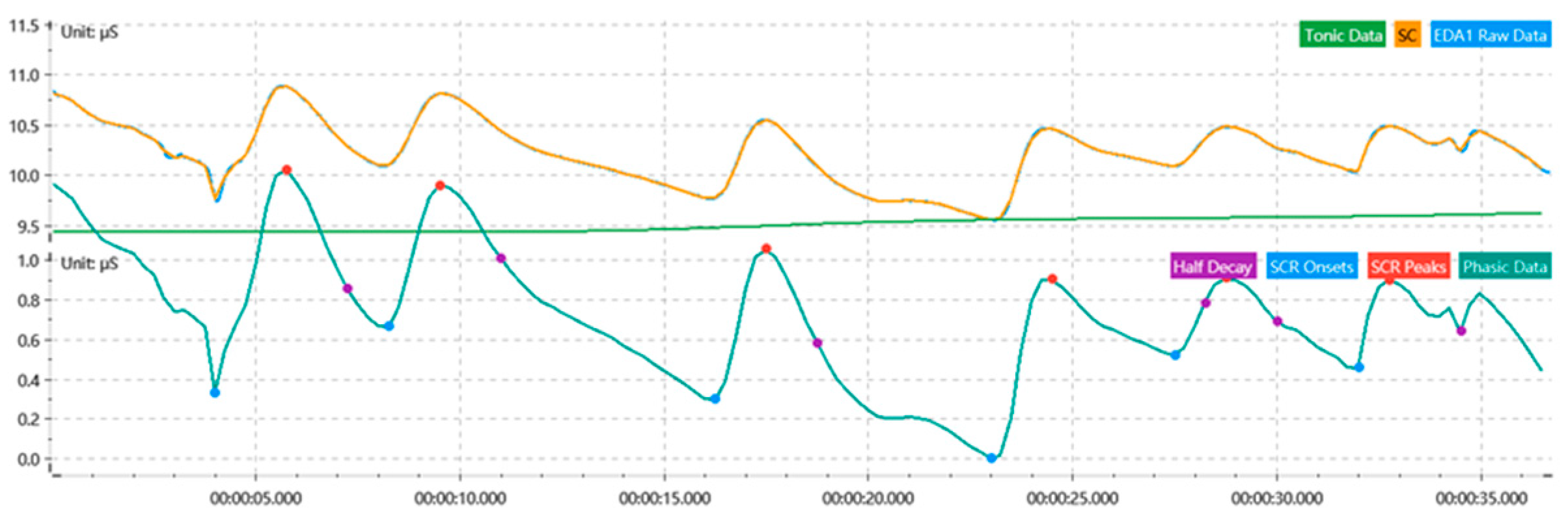
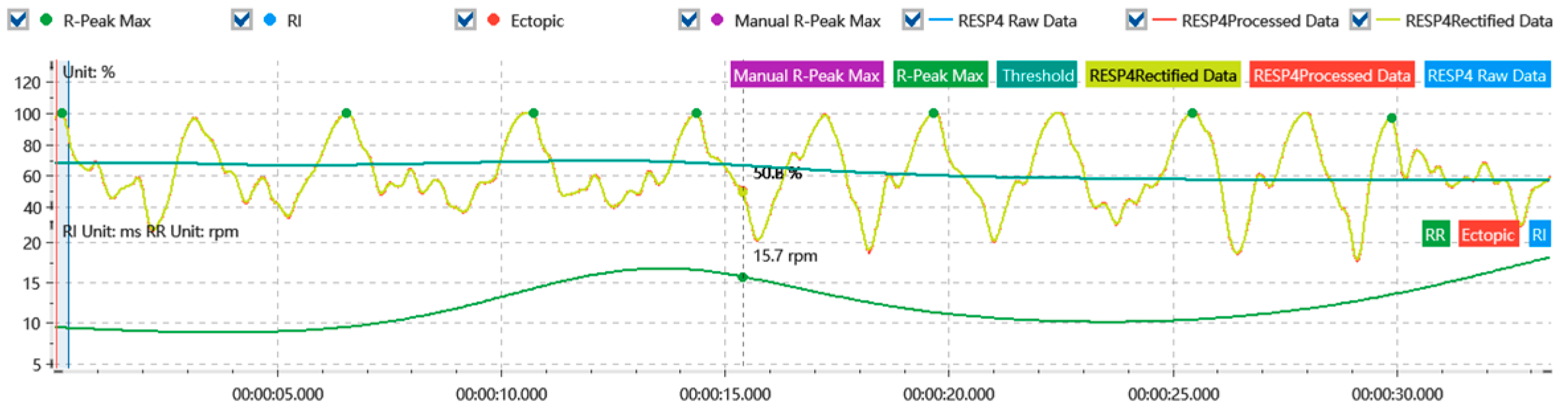
2.3.1. Indicator Extraction
2.3.2. Extraction of Physiological Signal Differences
2.3.3. Preference Comparative Analysis
2.3.4. Comparative Analysis of Male and Female
2.3.5. Prediction of Olfactory Perception Preference
3. Results
3.1. Comparative Analysis Results of Preferences
3.2. Comparative Analysis Results of Male and Female
3.3. Prediction Results of Olfactory Perception Preference
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laohakangvalvit, T.; Sripian, P.; Nakagawa, Y.; Feng, C.; Tazawa, T.; Sakai, S.; Sugaya, M. Study on the psychological states of olfactory stimuli using electroencephalography and heart rate variability. Sensors 2023, 23, 4026. [Google Scholar] [CrossRef] [PubMed]
- Colaferro, C.A.; Crescitelli, E. The contribution of neuromarketing to the study of consumer behavior. Braz. Bus. Rev. 2014, 11, 123–143. [Google Scholar] [CrossRef]
- Yang, W.; Chen, T.; He, R.; Goossens, R.; Huysmans, T. Autonomic responses to pressure sensitivity of head, face and neck: Heart rate and skin conductance. Appl. Ergon. 2024, 114, 104126. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Chabin, D.; Hummel, T.; Karwowski, M. Olfactory perception relates to food neophobia in adolescence. Nutrition 2022, 98, 111618. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Razavi-Termeh, S.V.; Sadeghi-Niaraki, A.; Choi, S.-M. People’s olfactory perception potential mapping using a machine learning algorithm: A Spatio-temporal approach. Sustain. Cities Soc. 2023, 93, 104472. [Google Scholar] [CrossRef]
- Klyuchnikova, M.A.; Kvasha, I.G.; Laktionova, T.K.; Voznessenskaya, V.V. Olfactory perception of 5α-androst-16-en-3-one: Data obtained in the residents of central Russia. Data Brief 2022, 45, 108704. [Google Scholar] [CrossRef]
- Fjaeldstad, A.W.; Nørgaard, H.J.; Fernandes, H.M. The impact of acoustic fMRI-noise on olfactory sensitivity and perception. Neuroscience 2019, 406, 262–267. [Google Scholar] [CrossRef]
- Lesur, M.R.; Stussi, Y.; Bertrand, P.; Delplanque, S.; Lenggenhager, B. Different armpits under my new nose: Olfactory sex but not gender affects implicit measures of embodiment. Biol. Psychol. 2023, 176, 108477. [Google Scholar] [CrossRef]
- Apnea, O.S. The effect of olfactory stimulation on neuronal activity in dreaming during nrem 2 stage of sleep and sensory perception during dreams. Abstr. Sleep Med. 2019, 64, S257. [Google Scholar]
- Mu, S.; Liu, L.; Liu, H.; Shen, Q.; Luo, J. Characterization of the relationship between olfactory perception and the release of aroma compounds before and after simulated oral processing. J. Dairy Sci. 2021, 104, 2855–2865. [Google Scholar] [CrossRef]
- Zhou, L.; Qin, M.; Han, P. Olfactory metacognition and memory in individuals with different subjective odor imagery abilities. Conscious. Cogn. 2022, 105, 103416. [Google Scholar] [CrossRef] [PubMed]
- Naudon, L.; François, A.; Mariadassou, M.; Monnoye, M.; Philippe, C.; Bruneau, A.; Dussauze, M.; Rué, O.; Rabot, S.; Meunier, N. First step of odorant detection in the olfactory epithelium and olfactory preferences differ according to the microbiota profile in mice. Behav. Brain Res. 2020, 384, 112549. [Google Scholar] [CrossRef]
- Ryan, B.C.; Young, N.B.; Moy, S.S.; Crawley, J.N. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav. Brain Res. 2008, 193, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.S.; Carvalho, V.M.; Papes, F. From Synapse to Supper: A Food Preference Recipe with Olfactory Synaptic Ingredients. Neuron 2020, 107, 8–11. [Google Scholar] [CrossRef]
- Islam, S.; Ueda, M.; Nishida, E.; Wang, M.X.; Osawa, M.; Lee, D.; Itoh, M.; Nakagawa, K.; Tana; Nakagawa, T. Odor preference and olfactory memory are impaired in Olfaxin-deficient mice. Brain Res. 2018, 1688, 81–90. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirotsu, T.; Tagawa, T.; Oda, S.; Iino, Y.; Ishihara, T. Coordinated change of acting sensory neurons is important for olfactory preference change depending upon odor concentration. Neurosci. Res. 2011, 71, e174. [Google Scholar] [CrossRef]
- Xiao, K.; Kondo, Y.; Sakuma, Y. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm. Behav. 2004, 46, 356–361. [Google Scholar] [CrossRef]
- Gong, L.; Chen, W.; Li, M.; Zhang, T. Emotion recognition from multiple physiological signals using intra-and inter-modality attention fusion network. Digit. Signal Process. 2024, 144, 104278. [Google Scholar] [CrossRef]
- Altıntop, Ç.G.; Latifoğlu, F.; Akın, A.K. Can patients in deep coma hear us? Examination of coma depth using physiological signals. Biomed. Signal Process. Control 2022, 77, 103756. [Google Scholar] [CrossRef]
- Umer, W.; Yu, Y.; Afari, M.F.A.; Anwer, S.; Jamal, A. Towards automated physical fatigue monitoring and prediction among construction workers using physiological signals: An on-site study. Saf. Sci. 2023, 166, 106242. [Google Scholar] [CrossRef]
- Shishavan, H.H.; Garza, J.; Henning, R.; Cherniack, M.; Hirabayashi, L.; Scott, E.; Kim, I. Continuous physiological signal measurement over 24-hour periods to assess the impact of work-related stress and workplace violence. Appl. Ergon. 2023, 108, 103937. [Google Scholar] [CrossRef]
- Parreira, J.D.; Chalumuri, Y.R.; Mousavi, A.S.; Modak, M.; Zhou, Y.; Sanchez-Perez, J.A.; Gazi, A.H.; Harrison, A.B.; Inan, O.T.; Hahn, J.-O. A proof-of-concept investigation of multi-modal physiological signal responses to acute mental stress. Biomed. Signal Process. Control 2023, 85, 105001. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Kang, Y.; Zhong, Z.; Chen, W. A quick identification model for assessing human anxiety and thermal comfort based on physiological signals in a hot and humid working environment. Int. J. Ind. Ergon. 2023, 94, 103423. [Google Scholar] [CrossRef]
- Hiroike, S.; Doi, S.; Wada, T.; Kobayashi, E.; Karaki, M.; Mori, N.; Kusaka, T.; Ito, S. Study of olfactory effect on individual driver under driving. In Proceedings of the 2009 ICME International Conference on Complex Medical Engineering, Tempe, AZ, USA, 9–11 April 2009; pp. 1–6. [Google Scholar]
- Doi, S.; Kamesawa, K.; Wada, T.; Kobayashi, E.; Karaki, M.; Mori, N. Basic study on individual preference for scents and the arousal level for brain activity using MNIRS. In Proceedings of the IEEE/ICME International Conference on Complex Medical Engineering, Gold Coast, Australia, 13–15 July 2010; pp. 119–124. [Google Scholar]
- Ohira, H.; Hirao, N. Analysis of skin conductance response during evaluation of preferences for cosmetic products. Front. Psychol. 2015, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.M.; Coppin, G.; Cayeux, I.; Porcherot, C.; Sander, D.; Delplanque, S. Sensitivity of physiological emotional measures to odors depends on the product and the pleasantness ranges used. Front. Psychol. 2015, 6, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sarid, O.; Zaccai, M. Changes in mood states are induced by smelling familiar and exotic fragrances. Front. Psychol. 2016, 7, 1724. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Mellado, J.; Serna, J.; Arbelaez-Garces, G.; Arrieta-Escobar, J.A.; Sadtler, V.; Kacha, M. Towards a Protocol for Preference Evaluation of Cosmetic Products Using Physiological Sensors. In Proceedings of the 2022 IEEE 28th International Conference on Engineering, Technology and Innovation (ICE/ITMC) & 31st International Association For Management of Technology (IAMOT) Joint Conference, Nancy, France, 19–23 June 2022; pp. 1–8. [Google Scholar]
- Karimi, N.; Hasanvand, S.; Beiranvand, A.; Gholami, M.; Birjandi, M. The effect of Aromatherapy with Pelargonium graveolens (P. graveolens) on the fatigue and sleep quality of critical care nurses during the COVID-19 pandemic: A randomized controlled trial. Explore 2024, 20, 82–88. [Google Scholar] [CrossRef]
- Diass, K.; Brahmi, F.; Mokhtari, O.; Abdellaoui, S.; Hammouti, B. Biological and pharmaceutical properties of essential oils of Rosmarinus officinalis L. and Lavandula officinalis L. Mater. Today Proc. 2021, 45, 7768–7773. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Al-Mijalli, S.H.; Mrabti, H.N.; Abdnim, R.; Abdallah, E.M.; Jeddi, M.; Bnouham, M.; Lee, L.-H.; Ardianto, C.; et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 2023, 164, 114937. [Google Scholar] [CrossRef]
- Tang, B.-B.; Wei, X.; Guo, G.; Yu, F.; Ji, M.; Lang, H.; Liu, J. The effect of odor exposure time on olfactory cognitive processing: An ERP study. J. Integr. Neurosci. 2019, 18, 87–93. [Google Scholar]
- Tang, B.; Cai, W.; Deng, L.; Zhu, M.; Chen, B.; Lei, Q.; Chen, H.; Wu, Y. Research on the effect of olfactory stimulus parameters on awakening effect of driving fatigue. In Proceedings of the 2022 6th CAA International Conference on Vehicular Control and Intelligence (CVCI), Nanjing, China, 28–30 October 2022; pp. 1–6. [Google Scholar]
- Zheng, H.; Qin, Y.; Du, Z. Atlas analysis of the impact of the interval changes in yellow light signals on driving behavior. IEEE Access 2021, 9, 46339–46347. [Google Scholar] [CrossRef]
- Qu, F.; Xie, Q. Effects of aircraft noise on psychophysiological feedback in under-route open spaces. In Proceedings of the INTER-NOISE and NOISE-CON Congress and Conference Proceedings, Glasgow, Scotland, 21–24 August 2023; pp. 5089–5094. [Google Scholar]
- Liu, B.; Lian, Z.; Brown, R.D. Effect of landscape microclimates on thermal comfort and physiological wellbeing. Sustainability 2019, 11, 5387. [Google Scholar] [CrossRef]
- Sie, J.-H.; Chen, Y.-H.; Shiau, Y.-H.; Chu, W.-C. Gender- and age-specific differences in resting-state functional connectivity of the central autonomic network in adulthood. Front. Hum. Neurosci. 2019, 13, 369. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable sensors for assessing the role of olfactory training on the autonomic response to olfactory stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]
- Walla, P.; Brenner, G.; Koller, M. Objective measures of emotion related to brand attitude: A new way to quantify emotion-related aspects relevant to marketing. PLoS ONE 2011, 6, e26782. [Google Scholar] [CrossRef] [PubMed]
- Gatti, E.; Calzolari, E.; Maggioni, E.; Obrist, M. Emotional ratings and skin conductance response to visual, auditory and haptic stimuli. Sci. Data 2018, 5, 180120. [Google Scholar] [CrossRef]
- Chung, J.W.Y.; So, H.C.F.; Choi, M.M.T.; Yan, V.C.M.; Wong, T.K.S. Artificial Intelligence in education: Using heart rate variability (HRV) as a biomarker to assess emotions objectively. Comput. Educ. Artif. Intell. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Shi, H.; Yang, L.; Zhao, L.; Su, Z.; Mao, X.; Zhang, L.; Liu, C. Differences of heart rate variability between happiness and sadness emotion states: A pilot study. J. Med. Biol. Eng. 2017, 37, 527–539. [Google Scholar] [CrossRef]
- Zou, B.; Wang, Y.; Zhang, X.; Lyu, X.; Ma, H. Concordance between facial micro-expressions and physiological signals under emotion elicitation. Pattern Recognit. Lett. 2022, 164, 200–209. [Google Scholar] [CrossRef]
- Ayata, D.; Yaslan, Y.; Kamasak, M.E. Emotion recognition from multimodal physiological signals for emotion aware healthcare systems. J. Med. Biol. Eng. 2020, 40, 149–157. [Google Scholar] [CrossRef]
- Macey, P.M.; Rieken, N.S.; Kumar, R.; Ogren, J.A.; Middlekauff, H.R.; Wu, P.; Woo, M.A.; Harper, R.M. Sex differences in insular cortex gyri responses to the Valsalva maneuver. Front. Neurol. 2016, 7, 87. [Google Scholar] [CrossRef]





| Test Project | Agree | Disagree |
|---|---|---|
| I don’t have any neurological diseases. | ||
| I don’t have rhinitis. | ||
| I can accurately perceive smells. | ||
| I am able to clearly express what I want. | ||
| I often use fragrance products in my daily life (more than twice a week). | ||
| I’m not allergic to any smell. |
| Change Value | Calm Stage (Like) | Stimulation Stage (Like) | t | p |
|---|---|---|---|---|
| SC_Mean | 5.736 | 5.523 | 1.997 | <0.01 |
| AVRESP | 12.480 | 10.851 | 1.997 | <0.01 |
| HR_Mean | 79.030 | 81.924 | 1.997 | <0.01 |
| Change Value | Calm Stage (Disgust) | Stimulation Stage (Disgust) | t | p |
| SC_Mean | 6.570 | 6.348 | 1.997 | <0.01 |
| AVRESP | 12.436 | 12.881 | 1.997 | 0.286 |
| HR_Mean | 78.924 | 76.742 | 1.997 | <0.01 |
| Change Value | Like (Male) | Like (Female) | Difference (Absolute Value) | t | p |
|---|---|---|---|---|---|
| SC_Mean’ | −0.142 | −0.253 | 0.111 | 2.028 | 0.382 |
| AVRESP’ | −1.877 | −1.487 | 0.39 | 1.997 | 0.614 |
| HR_Mean’ | 3.75 | 2.404 | 1.346 | 2.030 | 0.209 |
| Change Value | Disgust (Male) | Disgust (Female) | Difference (Absolute Value) | t | p |
| SC_Mean’ | −0.046 | −0.264 | 0.218 | 2.015 | 0.141 |
| AVRESP’ | 0.036 | 0.935 | 0.899 | 1.997 | 0.283 |
| HR_Mean’ | −1.777 | −2.666 | 0.889 | 1.997 | 0.350 |
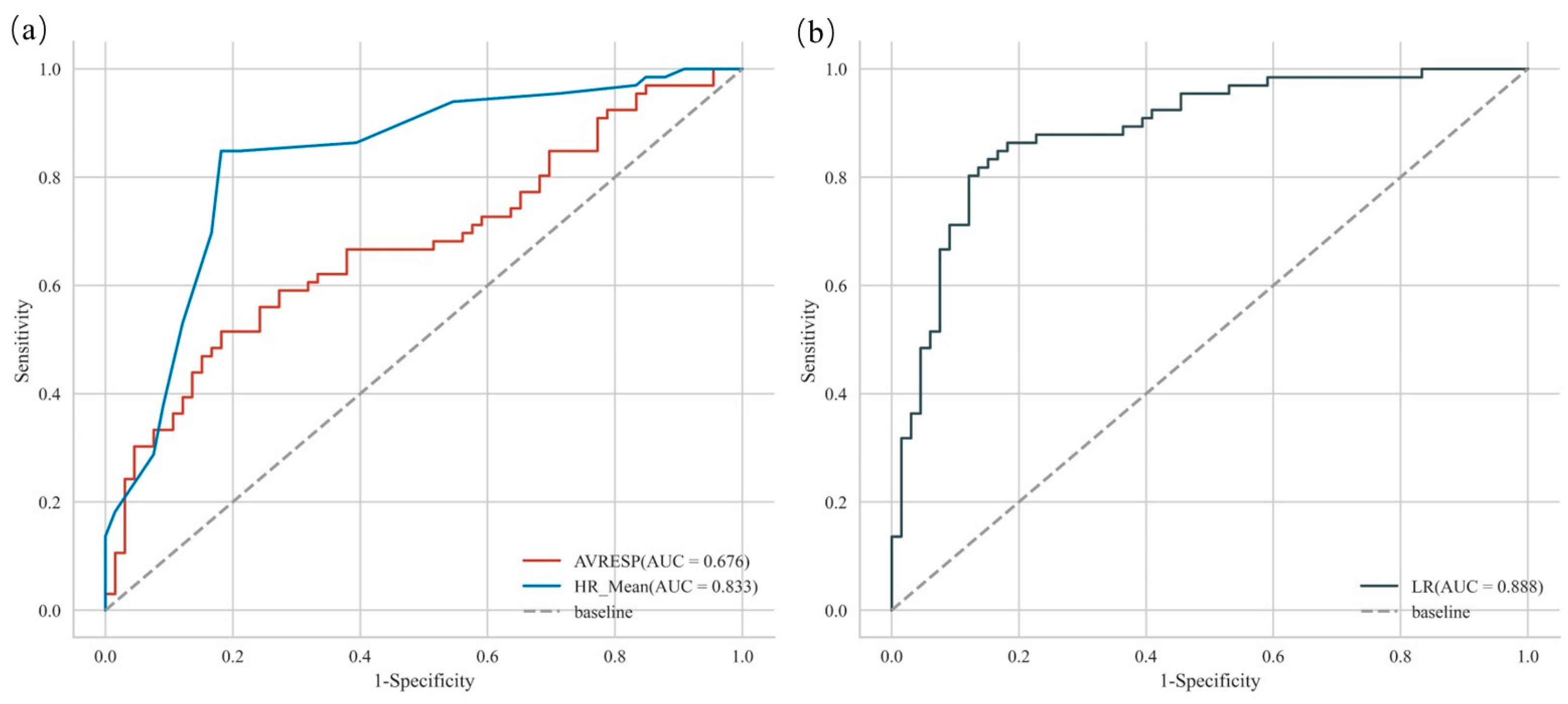
| Prediction Model | Independent Variable | β | p | AUC |
|---|---|---|---|---|
| Logistic regression | Intercept | −0.329 | 0.190 | \ |
| SC_Mean’ | 0.039 | 0.921 | \ | |
| AVRESP’ | −0.335 | <0.01 | 0.676 | |
| HR_Mean’ | 0.474 | <0.01 | 0.833 |
| Observation/Forecast | Forecast | ||
|---|---|---|---|
| Observation | Like | Disgust | Correct Percentage |
| Like | 55 | 11 | 83.3% |
| Disgust | 10 | 56 | 84.8% |
| Overall percentage | 84.1% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Zhu, M.; Wu, Y.; Guo, G.; Hu, Z.; Ding, Y. Autonomic Responses Associated with Olfactory Preferences of Fragrance Consumers: Skin Conductance, Respiration, and Heart Rate. Sensors 2024, 24, 5604. https://doi.org/10.3390/s24175604
Tang B, Zhu M, Wu Y, Guo G, Hu Z, Ding Y. Autonomic Responses Associated with Olfactory Preferences of Fragrance Consumers: Skin Conductance, Respiration, and Heart Rate. Sensors. 2024; 24(17):5604. https://doi.org/10.3390/s24175604
Chicago/Turabian StyleTang, Bangbei, Mingxin Zhu, Yingzhang Wu, Gang Guo, Zhian Hu, and Yongfeng Ding. 2024. "Autonomic Responses Associated with Olfactory Preferences of Fragrance Consumers: Skin Conductance, Respiration, and Heart Rate" Sensors 24, no. 17: 5604. https://doi.org/10.3390/s24175604






